Enzalutamide alleviates anxiety and depression as well as improves quality of life compared to bicalutamide in metastatic castration-resistant prostate cancer patients: a cohort study
Introduction
Prostate cancer is the second most frequently diagnosed cancer and the fifth leading cause of cancer death in men all around the world, with approximately 1,600,000 newly diagnosed cases and 366,000 prostate cancer-related deaths each year (1-6). Metastatic castration-resistant prostate cancer (mCRPC), the advanced stage of prostate cancer, happens in about 10–20% prostate cancer patients within 5 years after initial diagnosis (5,7-9). Recently, improved survival in mCRPC patients has been achieved partly owing to the application of common therapeutic agents (such as bicalutamide), whereas there are still a large portion of patients complicated with psychological disorders including anxiety and depression due to the fast disease progression, intolerable clinical symptoms or other severe complications, which directly decrease their quality of life (QoL) and indirectly cause poor prognosis (10-13). Therefore, exploring additional and convincing treatment agents that not only delay disease progression but also alleviate psychological disorders is pivotal for improving QoL and prognosis in mCRPC patients.
Enzalutamide, a second-generation nonsteroidal antiandrogen (NSAA) approved by United States food and drug administration (FDA) in 2012 for the mCRPC treatment, suppresses proliferation of LNCaP/AR human prostate cancer cells through inhibiting translocation of androgen receptor (AR) to cell nucleus (14,15). In clinical practices, enzalutamide presents with better efficacy in delaying prostate specific antigen (PSA) progression and improving median progression-free survival (PFS) as well as overall survival (OS) compared with bicalutamide in mCRPC patients. However, limited information related to its efficacy on psychological disorders (especially on anxiety and depression) in mCRPC patients is discovered (5,14,16-18). Therefore, the objective of the current study was to evaluate the effect of enzalutamide on anxiety, depression and QoL in treating mCRPC patients compared with bicalutamide.
Methods
Patients
One hundred and thirty-four mCRPC patients underwent enzalutamide or bicalutamide treatment between 2015/6/30 and 2016/12/30 at The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology were consecutively enrolled in this prospective cohort study. Patients were included according to the following inclusion criteria: (I) diagnosed as prostate cancer by pathological findings; (II) documented metastases; (III) testosterone concentration equal or below 50 ng/dL; (IV) disease progression by androgen deprivation therapy (ADT) treatment. Disease progression was defined by the presence of at least one of the following criteria: (I) PSA progression: the increment of PSA values ≥2 ng/mL at an interval ≥1 week between determinations; if PSA values ≥2 and <5 ng/mL, then PSA doubling time ≤10 months; (II) soft tissue disease progression: defined by Response Evaluation Criteria for Solid Tumors (RECIST) (version 1.1 criteria); (III) bone disease progression: defined by at least two new lesions on bone scan; (IV) about to receive enzalutamide or bicalutamide treatment. While patients with the following conditions were excluded: (I) life expectancy less than 60 weeks; (II) cognitive impairment or other mental diseases that affects the evaluation of anxiety, depression or QoL; (III) unable to be followed up regularly as the protocol; (IV) secondary prostate cancer patients. This study was approved by the Ethics Review Board of The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology with the ethical approval ID of No. 2015-2, and all patients provided written informed consents.
Baseline data collection
Comprehensive baseline data of all patients were documented including: (I) demographic features: age, body mass index (BMI), smoke and drink; (II) tumor features: disease duration, disease localization, prostate-specific antigen (PSA) level and Gleason score at diagnosis; (III) previous treatments for prostate cancer; (IV) common complications: hypertension, hyperlipidemia, diabetes, chronic kidney disease (CKD); (V) education status.
Treatments
Patients received enzalutamide or bicalutamide treatment and were correspondingly categorized into Enzalutamide group (N=53) and Bicalutamide group (N=81). In Enzalutamide group, patients received 160 mg enzalutamide capsule orally per day, while in Bicalutamide group, patients received 50 mg bicalutamide tablet orally per day. Patients in both groups received combined ADT treatment.
Assessments of anxiety, depression and QoL
Hospital Anxiety and Depression Scale (HADS) and Zung Self-Rating Anxiety/Depression Scale (SAS/SDS) were used for measurement of anxiety and depression, and Functional Assessment of Cancer Therapy-General (FACT-G) as well as Functional Assessment of Cancer Therapy-Prostate (FACT-P) scores were used for measurement of QoL at W0 (baseline), W12, W24, W36, W48 and W60. Anxiety assessed by HADS was defined as HADS-anxiety (HADS-A) score ≥8 points while anxiety assessed by SAS was defined as SAS score ≥50 points; Similarly, depression assessed by HADS was defined as HADS-depression (HADS-D) score ≥8 points while depression assessed by SDS was defined as SDS score ≥50 points.
Statistics
Statistical analysis was performed using SPSS 21.0 software (IBM, USA) and graphs were made using GraphPad 6.01 software (GraphPad Int, USA). Intent-to-treat (ITT) method was used for statistical analysis and patients who withdrew during the study were analyzed based on the data at last follow-up visit. Data were presented as mean ± standard deviation, median (range) or count (percentage). Comparison was determined by t test, Wilcoxon rank sum test or Chi-square test. P<0.05 was considered as significant.
Results
Study flow
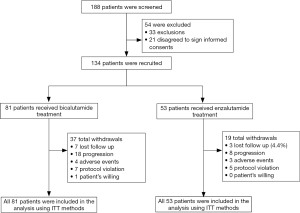
As depicted in Figure 1, 188 mCRPC patients were screened for eligibility, among whom 33 patients didn’t meet inclusion criteria and 21 patients disagreed to sign informed consents. Subsequently, 134 patients remained, among whom 53 patients received enzalutamide and assigned into Enzalutamide group, while 81 patients received bicalutamide and assigned into Bicalutamide group. In Enzalutamide group, 19 patients withdrew, including that: 3 patients lost follow up, 8 patients progressed, 3 patients were intolerant to adverse events and 5 patients violated protocol. In Bicalutamide group, 37 patients withdrew, including that: 7 patients lost follow up, 18 patients progressed, 4 patients were intolerant to adverse events, 7 patients violated protocol and 1 patient decided to quit. This study adopted ITT method that patients who withdrew during the study were analyzed based on the data at last visit. Therefore, all 53 patients in Enzalutamide group and 81 patients in Bicalutamide group were included in the analysis.
Baseline characteristics of mCRPC patients in Enzalutamide group and Bicalutamide group
There was no difference of demographic characteristics between Enzalutamide group and Bicalutamide group, including age, BMI, smoke and drink (all P>0.05, Table 1). The mean age in Enzalutamide group and Bicalutamide group was 65.94±9.19 and 65.85±9.61 years, respectively. For tumor features, disease duration was longer in Enzalutamide group compared with Bicalutamide group (P=0.028), while no difference in disease localization, PSA level or Gleason score at diagnosis was observed between two groups (all P>0.05). Furthermore, the percentage of patients with previous treatment history of antiandrogen (P=0.044) or radiation therapy (P=0.031) was greater in Enzalutamide group than that in Bicalutamide group. There was no difference in other baseline characteristics between two groups (all P>0.05, Table 1).
Table 1
| Parameters | Bicalutamide (N=81) | Enzalutamide (N=53) | P value |
|---|---|---|---|
| Demographic features | |||
| Age (years) | 65.85±9.61 | 65.94±9.19 | 0.956 |
| BMI (kg/m2) | 25.93±2.74 | 26.32±2.82 | 0.424 |
| Smoke (n/%) | 35 (43.2) | 27 (50.9) | 0.380 |
| Drink (n/%) | 32 (39.5) | 16 (30.2) | 0.271 |
| Tumor features | |||
| Disease duration (years) | 3 (0–19) | 6 (0–21) | 0.028 |
| Disease localization (n/%) | 0.256 | ||
| Bone only | 43 (53.1) | 27 (50.9) | |
| Soft tissue only | 21 (25.9) | 9 (17.0) | |
| Both bone and soft tissue | 17 (21.0) | 17 (32.1) | |
| PSA level (ug/L) | 18 [2–4,226] | 34 [2–4,192] | 0.226 |
| Gleason score at diagnosis (n/%) | 0.306 | ||
| ≥8 | 37 (45.7) | 29 (54.7) | |
| ≤7 | 44 (54.3) | 24 (45.3) | |
| Previous treatments (n/%) | |||
| Antiandrogen use | 36 (44.4) | 33 (62.3) | 0.044 |
| Radiation therapy | 22 (27.2) | 24 (45.3) | 0.031 |
| Prostatectomy | 19 (23.5) | 15 (28.3) | 0.529 |
| Orchiectomy | 10 (12.3) | 4 (7.5) | 0.375 |
| TURP | 4 (4.9) | 2 (3.8) | 0.750 |
| Cryoablation | 2 (2.5) | 1 (1.9) | 0.824 |
| Pelvic lymph node dissection | 4 (4.9) | 3 (5.7) | 0.854 |
| Common complications (n/%) | |||
| Hypertension | 41 (50.6) | 24 (45.3) | 0.546 |
| Hyperlipidemia | 32 (39.5) | 13 (24.5) | 0.073 |
| Diabetes | 12 (14.8) | 6 (11.3) | 0.562 |
| CKD | 9 (11.1) | 3 (5.7) | 0.280 |
| Education status (n/%) | |||
| Highest education | 0.599 | ||
| Primary school or less | 40 (49.4) | 20 (37.7) | |
| High school | 22 (27.2) | 19 (35.8) | |
| Undergraduate | 15 (18.5) | 11 (20.8) | |
| Graduate or above | 4 (4.9) | 3 (5.7) |
Data were presented as mean ± standard deviation, median (range) or count (percentage). Comparison was determined by t test, Wilcoxon rank sum test or Chi-square test. mCRPC, metastatic castration-resistant prostate cancer; BMI, body mass index; PSA, prostate-specific antigen; TURP, transurethral resection of the prostate; CKD, chronic kidney disease.
Baseline anxiety, depression as well as QoL status in Enzalutamide group and Bicalutamide group
Comparison of baseline anxiety, depression as well as QoL status between Enzalutamide group and Bicalutamide group was performed, which revealed that no difference was observed in anxiety status (assessed by HADS-A and SAS), depression status (assessed by HADS-D and SDS) or QoL status (assessed by FACT-G and FACT-P) between Enzalutamide group and Bicalutamide group at W0 (baseline) (all P>0.05, Table 2 ), which indicated that these assessments were comparable between two groups.
Table 2
| Parameters | Bicalutamide (N=81) | Enzalutamide (N=53) | P value |
|---|---|---|---|
| Anxiety status | |||
| HADS-A score (n/%) | 6.49±3.96 | 6.77±3.52 | 0.677 |
| Anxiety patients (HADS-A ≥8) | 30 (37.0) | 17 (32.1) | 0.556 |
| SAS score (n/%) | 49.43±10.82 | 49.00±10.92 | 0.822 |
| Anxiety patients (SAS ≥50) | 38 (46.9) | 22 (41.5) | 0.538 |
| Depression status | |||
| HADS-D score (n/%) | 6.42±4.02 | 7.23±3.23 | 0.223 |
| Depression patients (HADS-D ≥8) | 30 (37.0) | 25 (47.2) | 0.244 |
| SDS score (n/%) | 47.83±11.79 | 49.04±11.40 | 0.557 |
| Depression patients (SDS ≥50) | 34 (42.0) | 24 (45.3) | 0.706 |
| QoL status | |||
| FACT-G score | 82.7±14.2 | 85.1±14.9 | 0.350 |
| FACT-P score | 112.8±19.3 | 118.4±20.6 | 0.112 |
Data were presented as mean ± standard deviation or count (percentage). Comparison was determined by t test or Chi-square test. QoL, quality of life; mCRPC, metastatic castration-resistant prostate cancer; HADS-A, Hospital Anxiety and Depression Scale-Anxiety; HADS-D, Hospital Anxiety and Depression Scale-Depression; SAS, Zung Self-Rating Anxiety Scale; SDS, Zung Self-Rating Depression Scale; FACT-G, Functional Assessment of Cancer Therapy-General; FACT-P, Functional Assessment of Cancer Therapy-Prostate.
Comparison of anxiety assessed by HADS-A between Enzalutamide group and Bicalutamide group
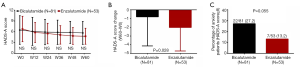
No difference of HADS-A score was found between two groups at W12, W24, W36, W48 or W60 (all P>0.05, Figure 2A). However, the reduction of HADS-A score (W60–W0) was increased in Enzalutamide group compared to Bicalutamide group (P=0.028, Figure 2B). Meanwhile, percentage of anxiety patients was numerically decreased in Enzalutamide group compared to Bicalutamide group, but no statistical significance (P=0.055, Figure 2C).
Comparison of anxiety assessed by SAS between Enzalutamide group and Bicalutamide group
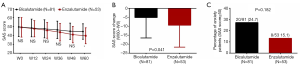
SAS score was decreased in Enzalutamide group compared with Bicalutamide group at W60 (P<0.05), while the score was of no difference between two groups at W12, W24, W36 or W48 (all P>0.05, Figure 3A). Decrement of SAS score (W60–W0) in Enzalutamide group was greater compared with Bicalutamide group (P=0.041, Figure 3B), whereas percentage of anxiety patients between two groups was similar (P=0.182, Figure 3C).
Comparison of depression assessed by HADS-D between Enzalutamide group and Bicalutamide group
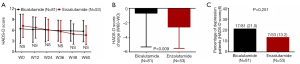
HADS-D score was of no difference between Enzalutamide group and Bicalutamide group at each visit (all P>0.05, Figure 4A), whereas HADS-D score reduction (W60–W0) in Enzalutamide group was larger than that in Bicalutamide group (P=0.009, Figure 4B). As to percentage of depression patients, no difference was found between two groups (P=0.251, Figure 4C).
Comparison of depression assessed by SDS between Enzalutamide group and Bicalutamide group
At W60 (P<0.05), SDS score was reduced in Enzalutamide group compared to Bicalutamide group, while it was similar between two groups at W12, W24, W36 and W48 (all P>0.05, Figure 5A). What’s more, Enzalutamide group presented with more SDS score reduction (W60–W0) compared with Bicalutamide group (P=0.033, Figure 5B). Additionally, percentage of depression patients between two groups was similar (P=0.238, Figure 5C).
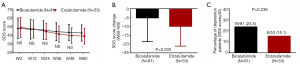
Comparison of QoL assessed by FACT-G and FACT-P between Enzalutamide group and Bicalutamide group
FACT-G score between Enzalutamide group and Bicalutamide group was similar at each visit (all P>0.05, Figure 6A), whereas the decrement of FACT-G score (W60–W0) in Enzalutamide group was larger compared with Bicalutamide group (P=0.009, Figure 6B). As for FACT-P score, it was also similar between two groups at each visit (all P>0.05, Figure 6C). However, it was reduced more obviously (W60–W0) in Enzalutamide group compared with Bicalutamide group (P<0.001, Figure 6D). In addition, subgroup analysis in Enzalutamide group between patients with good response and bad response were shown in Table S1.
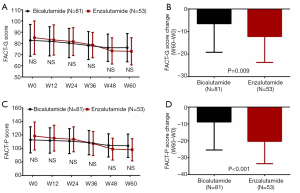
Table S1
| Parameters | Good response patients (PSA decline ≥50% from baseline, N=34) | Bad response patients (PSA decline <50% from baseline, N=19) | P value |
|---|---|---|---|
| HADS-A Score | |||
| W0 | 7.4±3.4 | 5.7±3.6 | 0.218 |
| W60 | 5.0±2.2 | 4.3±2.3 | 0.568 |
| Change (W60–W0) | –2.4±2.8 | –1.4±2.4 | 0.232 |
| Anxiety patients (W60) (HADS-A Score ≥8, n/%) | 5 (14.7) | 2 (10.5) | 0.666 |
| SAS Score | |||
| W0 | 51.3±11.8 | 44.8±7.7 | 0.074 |
| W60 | 38.4±9.3 | 41.6±8.1 | 0.400 |
| Change (W60–W0) | –12.9±12.9 | –3.2±7.8 | 0.004 |
| Anxiety patients (W60) (SAS Score ≥50, n/%) | 5 (14.7) | 3 (15.8) | 0.916 |
| HADS-D Score | |||
| W0 | 7.8±3.4 | 6.2±2.8 | 0.174 |
| W60 | 4.6±2.8 | 4.6±2.2 | 0.999 |
| Change (W60–W0) | –3.2±3.1 | –1.5±2.4 | 0.047 |
| Depression patients (W60) (HADS-D Score ≥8, n/%) | 6 (17.6) | 1 (5.3) | 0.202 |
| SDS Score | |||
| W0 | 49.7±11.2 | 47.8±12.0 | 0.999 |
| W60 | 36.8±7.5 | 43.1±10.3 | 0.028 |
| Change (W60–W0) | –12.9±11.7 | –4.7±8.1 | 0.009 |
| Depression patients (W60) (SDS Score ≥50, n/%) | 2 (5.9) | 6 (31.6) | 0.012 |
| FACT-G Score | |||
| W0 | 85.5±13.8 | 84.4±14.2 | 0.999 |
| W60 | 69.4±10.1 | 79.2±11.3 | 0.004 |
| Change (W60–W0) | –16.1±12.0 | –5.2±9.2 | 0.001 |
| FACT-P Score | |||
| W0 | 119.5±21.1 | 116.4±19.4 | 0.924 |
| W60 | 92.7±19.8 | 107.5±20.6 | 0.026 |
| Change (W60–W0) | –26.8±14.4 | –8.9±13.8 | <0.001 |
Data were presented as mean ± standard deviation or count (percentage). Comparison was determined by t-test (Boneferroni correction) or Chi-square test. HADS-A, Hospital Anxiety and Depression Scale-Anxiety; SAS, Zung Self-Rating Anxiety Scale; HADS-D, Hospital Anxiety and Depression Scale-Depression; SDS, Zung Self-Rating Depression Scale; FACT-G, Functional Assessment of Cancer Therapy-General; FACT-P, Functional Assessment of Cancer Therapy-Prostate.
Discussion
In the current study we found that (I) enzalutamide presented with better efficacy in alleviating anxiety and depression compared with bicalutamide; (II) enzalutamide was more effective on improving QoL compared to bicalutamide in mCRPC patients.
Enzalutamide is a novel NSAA that binds to AR with 5–8 folds higher affinity than bicalutamide and displays no agonistic effect to mutant AR protein (W741C) in castration-resistant LNCaP/AR human prostate cancer cells (19,20). According to several studies, enzalutamide is capable of delaying initial time to chemotherapy, reducing risk of first skeletal-related event and prolonging PFS as well as OS in mCRPC patients (16-18). Whereas limited information is discovered related to the influence of enzalutamide on mental status, just one interesting study discloses that mCRPC patients who received enzalutamide present with better mental states compared to patients who received bicalutamide at W13 (21). Considering that previous study evaluates patients’ psychological states via European Quality of Life 5-Domain Scale (EQ-5D), but not specific assessment tools (such as HADS or SAS/SDS), the efficacy of enzalutamide on mental status in mCRPC patients remains to be further explored. In the current study, we used HADS-A/HADS-D and SAS/SDS specifically to assess the anxiety and depression in mCRPC patients received enzalutamide or bicalutamide at each visit, and validated that enzalutamide relieved anxiety and depression more effectively compared with bicalutamide in mCRPC patients. The possible reason might be that: (I) enzalutamide presented with better anti-tumor effect compared with bicalutamide, thereby indirectly alleviated anxiety and depression than bicalutamide in mCRPC patients; (II) enzalutamide might directly improve anxiety and depression via greatly regulating androgen which resulted in dysregulated homeostasis, while this hypothesis should be further verified in future studies. To our knowledge, this is the first study to specifically and comprehensively compare the efficacy on anxiety as well as depression between enzalutamide and bicalutamide in mCRPC patients, which might provide more information for the utilization of enzalutamide in clinical practices.
As for QoL, it is the general well-being of individuals’ daily life, and its assessment is commonly conducted in mCRPC patients via different self-reports (such as FACT-G and FACT-P) from different aspects (including emotional, social and physical aspects) (17,19,22). Based on a limited number of studies, enzalutamide is disclosed to contribute to the improvement of QoL in mCRPC patients (23,24). For instance, a clinical study illustrates that enzalutamide presents with higher rate of QoL improvement and longer median time to QoL deterioration (assessed by FACT-P questionnaire) compared to placebo group in mCRPC patients who have previously received chemotherapy (docetaxel) (24). In addition, increased percentage of patients achieving QoL improvement and median time to QoL deterioration (assessed by FACT-P and EQ-5D questionnaires) is also observed in enzalutamide group compared with placebo group in chemotherapy-native mCRPC patients (23). However, the comparison of QoL between enzalutamide and bicalutamide is seldom illuminated. In our study, we found that enzalutamide better improved QoL assessed by FACT-G and FACT-P compared with bicalutamide in mCRPC patients. This result might be explained by that: Firstly, enzalutamide presents with better efficacy in relieving clinical symptoms and improving survival, thereby directly improving QoL in mCRPC patients. Secondly, enzalutamide might reduce mental stress and elevate confidence through its good treatment efficacy on delaying disease progression, thereby resulting in the remission of anxiety and depression, which indirectly improved QoL in mCRPC patients (16,17,19,20)
There were some limitations in this study: (I) since this was a cohort study, several baseline characteristics including disease duration, previous history of antiandrogen therapy or radiation therapy between Enzalutamide group and Bicalutamide group were different, which might cause confounding bias. However, we separately compared baseline anxiety status, depression status and QoL between the two groups, and we observed that no difference existed, which indicated these assessments were comparable between two groups; (II) the relative small sample size in this study with 53 patients in Enzalutamide group and 81 patients in Bicalutamide group might decrease statistical power. And considering that quite a few patients withdrew during the studies in both groups, we utilized intention to treat analysis to avoid decreasing statistical power although this was an observational study. However, randomized trials with larger sample size needed to be conducted in the future.
In conclusion, enzalutamide presents with better efficacy on alleviating anxiety and depression, as well as improving QoL in mCRPC patients compared to bicalutamide.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.09.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Review Board of The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology with the ethical approval ID of No. 2015-2, and all patients provided written informed consents.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sakamoto S. Editorial Comment to Epidemiology of prostate cancer in Asian countries. Int J Urol 2018;25:531-2. [Crossref] [PubMed]
- Pernar CH, Ebot EM, Wilson KM, et al. The Epidemiology of Prostate Cancer. Cold Spring Harb Perspect Med 2018; [Crossref] [PubMed]
- Mirzaei-Alavijeh M, Ahmadi-Jouybari T, Vaezi M, et al. Prevalence, Cognitive and Socio-Demographic Determinants of Prostate Cancer Screening. Asian Pac J Cancer Prev 2018;19:1041-6. [PubMed]
- Kimura T, Egawa S. Epidemiology of prostate cancer in Asian countries. Int J Urol 2018;25:524-31. [Crossref] [PubMed]
- Bhattacharya S, Hirmand M, Phung D, et al. Development of enzalutamide for metastatic castration-resistant prostate cancer. Ann N Y Acad Sci 2015;1358:13-27. [Crossref] [PubMed]
- Damber JE, Aus G. Prostate cancer. Lancet 2008;371:1710-21. [Crossref] [PubMed]
- Wang Y, Zhang H, Shen W, et al. Effectiveness and tolerability of targeted drugs for the treatment of metastatic castration-resistant prostate cancer: a network meta-analysis of randomized controlled trials. J Cancer Res Clin Oncol 2018;144:1751-68. [Crossref] [PubMed]
- Salem S, Komisarenko M, Timilshina N, et al. Impact of Abiraterone Acetate and Enzalutamide on Symptom Burden of Patients with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer. Clin Oncol (R Coll Radiol) 2017;29:601-8. [Crossref] [PubMed]
- Sade JP, Baez CAV, Greco M, et al. Optimizing the treatment of metastatic castration-resistant prostate cancer: a Latin America perspective. Med Oncol 2018;35:56. [Crossref] [PubMed]
- Watts S, Leydon G, Birch B, et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open 2014;4:e003901. [Crossref] [PubMed]
- Marzouk K, Assel M, Ehdaie B, et al. Long-Term Cancer Specific Anxiety in Men Undergoing Active Surveillance of Prostate Cancer: Findings from a Large Prospective Cohort. J Urol 2018;200:1250-5. [Crossref] [PubMed]
- Armengol C, Sarrias MR, Sala M. Hepatocellular carcinoma: Present and future. Med Clin (Barc) 2018;150:390-7. [Crossref] [PubMed]
- Cucchetti A, Trevisani F, Cappelli A, et al. Cost-effectiveness of doxorubicin-eluting beads versus conventional trans-arterial chemo-embolization for hepatocellular carcinoma. Dig Liver Dis 2016;48:798-805. [Crossref] [PubMed]
- Bambury RM, Scher HI. Enzalutamide: Development from bench to bedside. Urol Oncol 2015;33:280-8. [Crossref] [PubMed]
- Ciccarese C, Nobili E, Grilli D, et al. The safety and efficacy of enzalutamide in the treatment of advanced prostate cancer. Expert Rev Anticancer Ther 2016;16:681-96. [Crossref] [PubMed]
- Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide Versus Bicalutamide in Castration-Resistant Prostate Cancer: The STRIVE Trial. J Clin Oncol 2016;34:2098-106. [Crossref] [PubMed]
- Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol 2016;17:153-63. [Crossref] [PubMed]
- Siemens DR, Klotz L, Heidenreich A, et al. Efficacy and Safety of Enzalutamide vs Bicalutamide in Younger and Older Patients with Metastatic Castration Resistant Prostate Cancer in the TERRAIN Trial. J Urol 2018;199:147-54. [Crossref] [PubMed]
- Ito Y, Sadar MD. Enzalutamide and blocking androgen receptor in advanced prostate cancer: lessons learnt from the history of drug development of antiandrogens. Res Rep Urol 2018;10:23-32. [Crossref] [PubMed]
- Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009;324:787-90. [Crossref] [PubMed]
- Devlin N, Herdman M, Pavesi M, et al. Health-related quality of life effects of enzalutamide in patients with metastatic castration-resistant prostate cancer: an in-depth post hoc analysis of EQ-5D data from the PREVAIL trial. Health Qual Life Outcomes 2017;15:130. [Crossref] [PubMed]
- Luo J, Graff JN. Impact of enzalutamide on patient-related outcomes in metastatic castration-resistant prostate cancer: current perspectives. Res Rep Urol 2016;8:217-24. [Crossref] [PubMed]
- Loriot Y, Miller K, Sternberg CN, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol 2015;16:509-21. [Crossref] [PubMed]
- Fizazi K, Scher HI, Miller K, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol 2014;15:1147-56. [Crossref] [PubMed]