Radiotherapy in elderly patients with breast cancer: a literature review of acute and late toxicity
Introduction
Breast cancer is the most common neoplasm and the leading cause of cancer death in female gender worldwide, with the highest incidence in aged women (1). In fact, it is estimated that one-third of breast cancers are diagnosed in women older than 70 years of age. Despite the increasing incidence of breast cancer, death rates show a progressive reduction due to earlier diagnosis and development of more effective systemic therapies. However, significant improvement in the elderly is not well documented (2).
Although data on standard therapeutic approach are well consolidated, under-treatment of elderly patients is not unusual in clinical practice, particularly the omission of adjuvant radiotherapy (RT). This choice is generally justified by evidences of improved local control after adjuvant RT in patients treated with breast conservative surgery (BCS) but not overall survival (OS) in elderly patients, as reported in a recent meta-analysis (3).
Moreover, in clinical practice, RT is often omitted due to fear about its impact on quality of life (QoL), produced by toxicity and logistical issues, and due to concerns about increased toxicity rates in elderly patients (4). However, a worse RT-induced toxicity in elderly patients is not well documented in the available literature due to the inhomogeneous and fragmentary data.
Therefore, the aim of this review was to analyze data on acute and late toxicity in elderly breast cancer patients treated with RT, to evaluate toxicity rates and to provide a decision-making support in clinical practice.
Methods
The primary endpoint of this review was RT-related acute and late toxicity in elderly breast cancer patients. The secondary endpoint was RT interruption rate in this patients’ population.
We included in the analysis all the studies (retrospective or prospective, observational or interventional) reporting data on acute and/or late toxicity during/after RT in elderly women with breast cancer. We considered all the studies where patients’ age was at least 60 years, to be as inclusive as possible, because studies considering specific age ranges are extremely rare. All types of RT settings were included and no restriction was applied regarding other primary/adjuvant associated treatment (surgery, chemotherapy, targeted therapies, endocrine therapy).
A bibliographic search was performed on PubMed. The following search strategy was used: (((breast cancer[Title/Abstract]) AND (radiotherapy[Title/Abstract] OR radiation therapy[Title/Abstract])) AND (elderly[Title/Abstract] OR older[Title/Abstract] OR old[Title/Abstract])) AND (toxicity[Title/Abstract] OR side effects[Title/Abstract]). Only articles in English were considered while no chronological limitation was applied.
Results
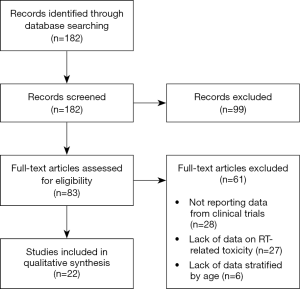
From the literature search as described above, 182 papers were identified and screened at title and abstract level. Figure 1 describes the process of paper selection. Eighty-three articles were retrieved for full text reading and 22 met the inclusion criteria. Twenty-eight papers were excluded because they did not report data on RT-related toxicity, 6 papers because data on toxicity were not stratified by age, and 27 papers because they did not report data from clinical trials. Finally, 22 papers were included in the descriptive analysis.
Table 1 summarize the characteristics of studies included in the review. Twelve studies were retrospective (5-16), 5 were prospective observational trials (17-21), 1 phase III trial sub analysis (22) and 4 phase I-II trials (23-26).
Table 1
| First author, year | Design | Patients (n) | Enrollment period | Median age [range], (years) | Stage | RT technique | Surgical treatment | Systemic treatment (%) |
|---|---|---|---|---|---|---|---|---|
| Ortholan, 2005 | Retrospective | 150 | 1987–1999 | 78 [42–90]* | Non metastatic | EBRT (WBI) | BCS: 71.5%; mastectomy: 28.5% | ET: 76.2; CT: 2.7 |
| Courdi, 2006 | Retrospective | 115 | 1987–1999 | 83 [64–95] | Non metastatic | EBRT (WBI) | No surgery | ET: 91.0; CT: 10.0 |
| Fiorica, 2012 | Retrospective | 131 | 2000–2007 | 78.3 [75–88] | EBC | EBRT (WBI) | BCS | ET: 62.6; CT: 22.9 |
| Khan, 2013 | Observational prospective | 537 | 2002–2004 | 78 [70–94] | EBC | BRT | BCS | ET: 49.7; CT: 1.9; ET+CT: 1.3 |
| Lemanski, 2013 | Phase II | 42 | 2004–2007 | 72 [66–80] | EBC | IORT | BCS | ET: when indicated; CT: 0.0 |
| Tuschy, 2013 | Retrospective | 54 | 2002–2007 | 76 [70–95] | EBC | IORT | BCS | NR |
| Méry, 2014 | Retrospective | 44 | 2003–2013 | 92 | Any | EBRT (WBI) | 59.0% breast surgery†; 41.0% no surgery | ET: 9.0; CT: 0.0 |
| Smith, 2014 | Registry study | 1,310 | 2002–2007 | 75 | EBC | BRT | BCS | ET: NR; CT: 8.2 |
| 26,383 | EBRT (WBI) | ET: NR; CT: 16.6 | ||||||
| Cante, 2015 | Observational prospective | 83 | 2005–2012 | 70–80: 93.0%; >80: 7.0% | EBC | EBRT (WBI) | BCS | ET: 88.0; CT: 16.0 |
| Doré, 2015 | Retrospective | 205 | 2004–2012 | 81 [52–91]; 94%>70 | Non metastatic | EBRT (WBI) | BCS: 57.0%; mastectomy: 43.0% | ET: 75.0; CT: 24.0 |
| Rovea, 2015 | Retrospective | 298 | 2007–2013 | 80 [57–89] | Non metastatic | EBRT (WBI) | BCS | ET: 77.9; CT: 2.8 |
| Giugliano, 2016 | Retrospective | 36 | 2011–2013 | <75 | EBC | EBRT (WBI) | BCS | ET: 93.3; CT: 8.3 |
| 24 | ≥75 | |||||||
| Monten, 2017 | Phase I–II | 95 | NR | 73.6 | Non metastatic | EBRT (WBI) | BCS: 75.8%; mastectomy: 24.2% | ET: 80.0; CT: 10.5; trastuzumab: 10.5 |
| Sayan, 2017 | Phase I–II | 40 | 2006–2013 | 73 [65–88] | EBC | EBRT (APBI) | BCS | ET when indicated; CT: 0.0 |
| Cao, 2018 | Retrospective | 752 | 2003–2009 | 75 [70–93.3] | non metastatic | EBRT (WBI) | BCS: 86.0%; mastectomy: 14.0% | ET: 8.9; CT: 11.7 |
| De Santis, 2018 | Observational prospective | 752 | 2009–2017 | 74 [65–92]; 78.9%>70 | EBC | EBRT (WBI) | BCS | ET: 79.5; CT: 16.9; CT+ trastuzumab: 6.7 |
| Fiorentino, 2018 | Retrospective | 40 IMRT | 2011–2015 | 75 [70–83] | EBC | EBRT (WBI) | BCS | ET: 90.0; CT: 10.0 |
| 40 VMAT | 72 [65–87] | ET: 92.5; CT: 7.5 | ||||||
| Jacobs, 2018 | Observational prospective | 267 | 2011–2016 | 68 [59–90] | EBC | IORT | BCS | ET: 34.0; CT: 1.9; ET+CT: 5.2 |
| 206 | 67 [59–86] | EBRT (APBI) | ET: 29.0; CT: 2.5; ET+CT: 6.0 | |||||
| Kinj, 2018 | Retrospective | 48 | 2012–2015 | 77.7 [65–92] | EBC | BRT | BCS | ET/CT when indicated |
| Meattini, 2018 | Phase III (subgroup analysis) | 58 | 2005–2013 | 74.1 [70–83.2] | EBC | EBRT (WBI) | BCS | ET: 79.3; CT: 0.0; ET+CT: 3.5 |
| 59 | 74.4 [70–85.3] | EBRT (APBI) | ET: 72.9; CT: 0.0; ET+CT: 1.7 | |||||
| Sanz, 2018 | Observational prospective | 486 | 1992–2016 | 79 [58–97] | non metastatic | EBRT (WBI) | BCS: 78.6%; mastectomy: 20.0% | ET: 78.7; CT: 13.4 |
| Vinante, 2019 | Phase II | 80 | 2008–2012 | 68 [60–83] | EBC | EBRT (APBI) | BCS | ET: 62.0; CT: 9.0; ET+CT: 2.0 |
*, 11.3% patients <70 years; †, type of surgery not reported. APBI, accelerated partial breast irradiation; BCS, breast conservative surgery; BRT, brachytherapy; CT, chemotherapy; EBC, early breast cancer; EBRT, external beam radiotherapy; ET, endocrine therapy; IMRT, intensity modulated radiotherapy; IORT, intraoperative radiotherapy; NR, not reported; RT, radiotherapy; VMAT, Volumetric Modulated Arc Therapy; WBI, whole breast irradiation;
Thirteen studies reported results about whole breast irradiation (WBI) delivered by external beams (EB) RT ± boost (simultaneous or consecutive) on the tumor bed (5-7,9,10,12,14-17,19,20,24). The majority of the studies analyzed different hypofractionated (HF) RT regimens (5,7,10,15-17,19,20,24), while Fiorica et al. retrospectively analyzed 131 patients treated with conventional fractionation (CF) (14). Cao et al. (12) and Fiorentino et al. (6) reported results on both patients treated with CF or HF. In the study of Méry et al. (9), RT techniques were not specified since the analysis focused on nonagenarian patients rather than on RT techniques.
Nine studies reported results about accelerated partial breast irradiation (APBI): 2 focused on EB APBI (23,25), 2 on intraoperative RT (IORT) (13,26), and 2 on brachytherapy (BRT) (8,21). Jacobs et al. compared IORT and EB-APBI (18), Smith et al. compared BRT and EB-RT (11), and Meattini et al. compared WBI and EB-APBI (22).
Four studies reported data on only acute toxicity (13,18,21,24) while in 4 studies (9,11,13,21) RT technique details and/or doses were not described. Toxicity assessment was considered as the primary endpoint only in 5 studies (6,13,23-25). Acute and late toxicity were reported using different criteria in 1 study (14) and not specified in 2 studies (11,21). Acute toxicity was mainly categorized using the RTOG/EORTC in 9 studies (5-7,10,15,19,20,22,23) and CTCAE criteria in 10 studies (8,9,12,13,16-18,24-26). Late toxicity was reported using CTCAE, RTOG/EORTC, SOMA-LENT criteria in 6 studies (5,8,16,17,25,26), 6 studies (6,14,19,20,22,23), and 4 studies (5,7,12,15), respectively.
APBI delivered with EB
As shown in Table 2, Sayan et al. (23) reported in 2017 the results of a phase I-II trial testing a new schedule of APBI (40 Gy in 10 daily fractions) after BCS in early breast cancer (EBC) patients. APBI was delivered using IMRT technique. Forty-two patients aged 65 years or more (median: 73 years; range, 65–88) were enrolled from 2006 to 2013. Acute toxicity was reported by patients without severity specification. Erythema and skin pigmentation, breast edema, subcutaneous toxicity and dry desquamation were reported by 69.0%, 61.5%, 51.0%, and 7.7% of patients, respectively. Physicians reported late toxicities were as follows: pigmentation defects in 43.0% of patients, breast edema in 30.0%, subcutaneous toxicity in 70.0%, telangiectasia in 19.0%, retraction or contour defect in 51.0%. There were few severe (≥ G3) toxicities: G3 breast edema was seen in 3.0% of patients and G3 subcutaneous toxicity in 10.0%.
Table 2
| First author, year | Patients (n) | Median age [range], years | RT details | RT dose | Median FU [range], months | Toxicity evaluative scale | Acute toxicity (%) | Late toxicity (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Khan, 2013 | 537 | 78 [70–94] | BRT: balloon brachytherapy catheter | NR | 55 | NR | Seroma: 28.9; fat necrosis: 2.4; infection: 8.5 | Telangiectasia: 7.9; retraction: 6.8 | NR |
| Smith, 2014 | 1,310 | 75 | BRT | NR | 42 | NR | Post-operative infections: 16.5; non-infectious post-operative complications: 18.7 | Fat necrosis: 15.3; rib fracture: 4.2; rad pneumonitis: 0.4 | |
| 26,383 | EBRT | NR | Post-operative infections: 11.4; non-infectious post-operative complications: 9.5 | Fat necrosis: 7.7; rib fracture: 4.0; rad pneumonitis: 0.9 | |||||
| Kinj, 2018 | 48 | 78 [65–92] | BRT: MIB | Single fraction: 16 Gy to 100% isodose | 40 [36–42] | CTCAE | G1: 66.7; G2: 22.3; G3: 6.7 | G1: 86.7; G2: 13.3; G3: 0.0 | |
| Lemanski, 2013 | 42 | 72 [66–80] | IORT: dedicated LINAC, 6–9 MeV electron beam | 23 Gy to 100% isodose | 72 [66–74] | CTCAE | Wound complications: 7.1; wound infection: 2.4; hematoma: 11.9 | G1 hyperpigmentation: 6.9; G1 scar fibrosis: 48.3; G2 scar fibrosis: 10.3; rib fracture: 2.4 | |
| Tuschy, 2013 | 54 | 76 [70–95] | IORT: miniature X-ray source (50 kV photon beam) | NR* | NR | CTCAE | G1–2 erythema: 13; mastitis: 5.6; fever: 5.6; antibiotic treatment: 31.5; Tumor bed induration: 11.1; scar retraction: 1.9 | Seroma breast: 3.7; seroma axilla: 22.2; hematoma breast: 24.1; hematoma axilla: 18.5; inconspicuous wound healing: 48.1 | NR |
| Jacobs, 2018 | 267 | 68 [59–90] | IORT: mobile LINAC, 6–12 MeV beam | 23.3 Gy to 100% isodose | At least 3 months | CTCAE | Wound infection: G2: 5.2; G3: 1.9 | Other: G2: 7.0; G3: 3.3 | NR |
| 206 | 67 [59–86] | EB-APBI: 3D-CRT or IMRT, photon beam | 38. Gy (3.85 Gy/fr)† | Wound infection: G2: 1.0; G3: 1.5 | Other: G2: 3.4; G3: 1.5 | ||||
| Meattini,2015 | 58 | 74 [70–83] | IMRT | EB-APBI: 30 Gy‡ | 60 [40–84] | RTOG/EORTC | G1: 20.3; G2: 1.7; G3: 1.7 | G1: 3.4; G2: 0.0; G3: 0.0 | |
| 59 | 74 [70–85] | WBI+boost: 50+10 Gy | G1: 32.8; G2: 25.9; G3: 5.1 | G1: 13.8; G2: 0.0; G3: 0.0 | |||||
| Sayan, 2017 | 40 | 73 [65–88] | IMRT | 40Gy† | 54 | RTOG/EORTC | erythema and skin pigmentation: 69breast edema: 61.5subcutaneous toxicities: 51 dry desquamation: 7.7 | G3 edema: 3.0; G3 subcutaneous toxicity: 10.0; moderate/severe contour defect: 13.0; pigmentation: 43.0; breast edema: 30.0; subcutaneous toxicity: 70.0; telangiectasia: 19.0; volume loss: 84.0; retraction or contour defect: 51.0 | |
| Vinante, 2019 | 80 | 68 [60–83] | 3D–CRT6 MV photon beam | 40 Gy† | 67 [41–97] | CTCAE | G1–2 skin toxicity: 8.0 | G1 fibrosis: 23.0; G2 fibrosis: 5.0; fat necrosis: 5.0; hyperpigmentation/telangiectasia: 3.8 | |
*, 25.9% IORT alone; 74.1% IORT as a boost; †, in 10 daily fractions; ‡, in 5 non-consecutive daily fractions. 3D-CRT, three dimensional conformal radiotherapy; APBI, accelerated partial breast irradiation; BRT, brachytherapy; CTCAE, common terminology criteria for adverse events; CTC-EORTC, common toxicity criteria-European Organisation for Research and Treatment of Cancer; EBRT, external beam radiotherapy; FU, follow-up; IMRT, intensity modulated radiotherapy; IORT, intraoperative radiotherapy; LINAC, linear accelerator; NR, not reported; MIB, multicatheter interstitial HDR-BRT; RT, radiotherapy; RTOG, radiation therapy oncology group; WBI, whole breast irradiation.
The same APBI scheme, but delivered with 3D-CRT technique, was tested by Vinante et al. (25) in a phase II study. Eighty patients (median age: 68 years; range, 60–83) were enrolled from 2008 to 2012. Reported acute toxicities were extraordinarily low: only 8% of patients experienced G1-2 acute skin toxicity. Late toxicities after a median follow-up of 67 months were G1 fibrosis (23.0%), G2 fibrosis (5.0%), fat necrosis (5.0%), and hyperpigmentation or telangiectasia (3.7%).
From the phase III trial published by Meattini et al. (22), patients older than 70 years were 117 with a median age of 74 years (range, 70–85). Patients were randomized in an experimental arm (APBI delivered with IMRT, 30 Gy in 5 non-consecutive daily fractions) and in a control arm (50 Gy in 25 fractions, followed by a boost of 10 Gy in 5 fractions). Toxicity was assessed using RTOG/EORTC scale. Acute toxicity in the experimental arm was as follows: G1: 20.3%, G2: 1.7%, and G3: 1.7%. In the control arm, acute toxicity was scored as G1, G2, and G3 in 32.8%, 25.9%, and 5.1%, respectively. With 5 years median follow-up, only G1 late toxicity was observed in 13.8% and 3.4% of patients in the WBI and APBI arms, respectively.
IORT
Data about IORT studies are summarized in Table 2. Lemanski et al. reported the results of Montpellier phase II trial (RADELEC) (26). From 2004 to 2007, the authors treated 42 patients aged ≥65 years (median age: 72 years; range, 66–80) with EBC using adjuvant IORT after BCS (21 Gy prescribed at 90% isodose). Acute toxicities, scored using the CTCAE v. 3.0 criteria, were as follows: acute wound complication (7.1%), local infection (2.4%), hematoma (11.9). With 72 months median follow-up, 1 patient (2.4%) experienced rib fracture. Regarding cosmetics results based on the physician’s evaluation, a 6.9% incidence of small skin color difference (which probably represents G1 hyperpigmentation) was recorded. Similarly, the authors reported “visible scar that affects the results” defined as “slight” or “significant” in 48.3% and 10.3%, respectively (which may represent a G1-G2 scar retraction/subcutaneous fibrosis).
The study from Tuschy et al. (13) compared 54 patients aged ≥70 years (median age: 76 years; range, 70–95) with 134 younger patients (median 57 years; range: 30–69) treated with IORT from 2002 to 2007 both as sole adjuvant treatment or as a boost before WBI. Delivered doses were not specified and late toxicity was not reported. Acute toxicity was acceptable without significant difference between older and younger patients. In the elderly patients’ group, the main toxicity was breast hematoma (24.1%). Other toxicities were palpable breast seroma (3.7%), erythema (13.0%), mastitis (5.6%), fever (5.6%), tumor bed induration (11.1%), and scar retraction (1.9%). Some toxicity recorded in the axilla was probably related to lymph nodes dissection (50.0% of patients).
Jacobs et al. (18) in their prospective multicenter cohort study compared patients with EBC treated with IORT (23.3 Gy to the 100% isodose) or EB APBI delivered with 3D-CRT or IMRT (38.5 Gy in 10 daily fractions of 3.85 Gy). Enrolled patients were 267 in the IORT arm and 206 in the EB-APBI arm. Median age was 68 years (range, 59–90) and 67 years (range, 59–86) in the IORT and EB-APBI arms, respectively. Only acute toxicity was evaluated using CTCAE v.3.0 criteria and it was acceptable: G2 was 7.0% (IORT) and 3.4% (EBRT) while G3 was 3.3% (IORT) and 1.5% (EBRT). IORT was significantly correlated to increased risk of wound infection (G2: 5.2% and 1.0% in IORT and EBRT group, respectively; G3: 1.9% and 1.5% in IORT and EBRT group, respectively).
Brachytherapy
As shown in Table 2, Khan et al. (21) reported data from an observational prospective registry of patients with EBC treated using a Mammosite device from 2002 to 2004. They compared 537 patients aged ≥70 (median age: 78 years; range, 70–94) with 912 younger patients (median age: 59 years; range 32–70). Acute toxicities were mild and no difference between the two age groups was seen. Seroma was the most frequent side effect (28.9%) in the elderly patient’s group. Other toxicities were fat necrosis (2.4%), infection (8.5%), telangiectasia (7.9%), and scar retraction (6.8%).
Kinj et al. (8) collected data on elderly patients (median age: 77.7 years; range, 65–92) with EBC enrolled in the siFEBI trial (NCT01727011) or treated out of the clinical trial (26 and 22 patients, respectively). BCS was followed by a multicatheter (5–15 vectors, median 11) interstitial HDR-BRT delivering a single fraction of 16 Gy prescribed to the 100% isodose. According to CTCAE v.3.0 criteria, acute G1 toxicity was recorded in 66.7% of patients and was mainly represented by hyperpigmentation (26.7%), dermatitis (6.7%) and breast hematoma (4.4%). G2 acute toxicity was observed in 22.3% of patients (breast hematoma: 4.4%, hyperpigmentation: 4.4%, other: 13.3%) and G3 in 6.7% patients (4.4% patients had G3 breast hematoma and 2.2% had G3 breast infection). With 40 months median follow-up, G1 late toxicity was 86.7%, mainly represented by hypopigmentation of the puncture site (33.3%) and breast fibrosis (26.7%). G1 telangiectasia was recorded in 13.3% of patients while the only reported G2 late toxicity was breast fibrosis (13.3%).
Finally, Smith et al. (11), using the SEER database, retrospectively compared breast cancer patients aged ≥66 (median age 75 years) who were treated from 2002 to 2007 with different treatments: lumpectomy alone, lumpectomy plus adjuvant EB-WBI, and lumpectomy plus adjuvant BRT. They separately reported acute toxicity as post-operative infections or noninfectious post-operative complications. The incidence of post-operative infections was 9.9%, 11.4%, and 16.5% in lumpectomy alone, lumpectomy plus EBRT, and lumpectomy plus BRT, respectively. At multivariable analysis, only BRT was significantly correlated with increased toxicity rates. Regarding noninfectious complications, the incidence was 6.0%, 9.5%, and 18.7% in lumpectomy alone, lumpectomy plus EBRT, and lumpectomy plus BRT groups, respectively. Again, multivariate analysis confirmed the statistical significance of these different rates. The incidence of late toxicity was higher in the BRT group compared to EBRT and lumpectomy alone groups considering fat necrosis (15.3%, 7.7%, and 5.3%, respectively). No significant differences were recorded in terms of rib fracture (4.2%, 4.0%, 5.2% in BRT, EBRT, and lumpectomy alone, respectively) and pneumonitis (<1.0% in all groups).
WBI
Thirteen studies reported data on toxicity in patients treated with WBI. Details on RT schedules and toxicities are summarized in Table 3.
Table 3
| First author, year | Patients (n) | Median age [range], years | Dose to WB/CW | Boost dose | LN dose | RT details | Median FU [range], months | Toxicity evaluation, scale (acute, late) | Acute toxicity (%) | Late toxicity (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ortholan, 2005 | 150 | 78 [42–90] | 32.5 Gy (6.5 Gy/fr)* | 1–2 fr (6.5 Gy/fr)* | 27.5 Gy (5.5 Gy/fr)* | Cobalt or 6 MV photon beam, electron beam (for boost and LN); sequential boost: 33.1%; LNI: 31.8% | 65 | RTOG/EORTC, LENT-SOMA | Erythema: G1: 18.7G2: 9.3G3: 0.0 | Subcutaneous fibrosis: G1: 20.7G2: 14.0G3: 4.7 | |
| Courdi, 2006 | 115 | 83 [64–95] | 32.5 Gy (6.5 Gy/fr)* | 1–3 fr (6.5 Gy/fr)* | 27.5 Gy (5.5 Gy/fr)* | Cobalt or 6 MV photon beam, electron beam (for boost and LN); sequential boost: 80%; LNI: 19% | 41 | RTOG/EORTC, LENT-SOMA | Erythema: G1: 20.9G2: 8.7G3: 0.0 | Subcutaneous fibrosis: G1: 16.5G2: 18.3G3: 5.2 | |
| Fiorica, 2012 | 131 | 78 [75–88] | 50 Gy (2.0 Gy/fr)† | 10–16 Gy (2.0 Gy/fr)† | 0 Gy | 3D-CRT; 6–15 MV photon beam; sequential boost: 45.8% | 56 [6–106] | WHO, RTOG-EORTC | Skin toxicity; G1-2: 58.1; G3: 6.1 | Subcutaneous fibrosis: G1-2: 16.0; G3: 0.0 | |
| Méry, 2014 | 44 | 92 | median 40 Gy, median 10 fr; HF: 77% | NR | NR | Curative intent: 73.0%; palliative intent: 27.0% | 9 [1–65] | CTCAE | G1: 34.0; G2: 23.0; G3: 2.0 | G1: 7.0; G2: 2.0; G3: 0.0 | |
| Cante, 2015 | 83 | 70–80: 93%; >80: 7% | 45 Gy (2.25 Gy/fr)† | up to 50 Gy (2.5 Gy/fr)† | 0 Gy | 3D-CRT; 6 MV photon beams | 60 [36–88] | CTCAE | G1: 40.0; G2: 3.0; G3: 0.0 | G1: 21.0; G2: 6.0; G3: 0.0 | |
| Doré, 2015 | 205 | 81 [52–9]; 94%>70 | 45 Gy (3 Gy/fr)‡ | 9 Gy (3 Gy/fr)‡ | Same as WB | 3D-CRT, 4–10 MV photon beam; and 9–12 mev electron beam (for boost and LN); sequential boost: 47.8%; LNI: 32% | 49 | CTCAE | G1: 65.0; G2: 17.0; G3: 4.0 | Fibrosis: 14.6; Telangiectasia: 8.0; hyperpigmentation: 3.9 | |
| Rovea, 2015 | 298 | 80 [57–89] | 80.9%: 32.5 Gy (6.5 Gy/fr)* 19.1%: 30 Gy (6 Gy/fr)* | 0 Gy | 0 Gy | 3D-CRT or IMRT, 5–6 MV photon beam | 46 [12–84] | RTOG/EORTC, CTCAE | G1: 22.6; G2: 4.8; G3: 1.0; G4: 0.3 | Fibrosis: G1: 31.5; G2: 4.2; G3: 3.5; edema: G1: 7.0; G2: 4.2; G3: 1.4 | Telangiectasia: G1: 1.8 G3: 0.7; hyperpigmentation: G1: 4.6; G2: 2.4; atrophy G1: 2.1; pericarditis G1: 0.4 |
| Giugliano, 2016 | 24 | ≥75 | 42.56 Gy (2.66 Gy/fr)* | 0 Gy | 0 Gy | 3D-CRT, 6 MV photon beam | 15 | RTOG/EORTC, CTCAE and LENT-SOMA | G1: 41.7; G2: 29.2; G3: 4.2 | G1: 31.6; G2: 10.5; G3: 0.0 | |
| 36 | < 75 | G1: 66.7; G2: 11.1; G3: 2.8 | G1: 15.6; G2: 0.0; G3: 0.0 | ||||||||
| Monten, 2017 | 95 | 74 | 28.5 Gy (5.7 Gy/fr)‡ | up to 32.5 Gy (6.5 Gy/fr)‡ or 34.5 Gy (6.9 Gy/fr)‡ if R+ | 27 Gy (5.4 Gy/fr)‡ | IMRT-SIB (prone or supine position) or VMAT-SIB (if LNI) | 6 | CTCAE | G1: 52.6; G2: 10.5; G3: 1.1 | NR | |
| Cao, 2018 | 752 | 75 [70–93] | CF: 35%; HF: 57%; HF schemes: −32.5 Gy (6.25 Gy/fr)*; −40.05 Gy (2.67 Gy/fr)†; −41.6 Gy (3.2 Gy/fr)‡ | 16 Gy (2 Gy/fr)† | same as WB | 3D-CRT: 51.7%LD: 42.7% tomotherapy: 0.1%; LNI: 30%; sequential boost: 22.3% | 88 [5–155] | CTCAE, LENT-SOMA | Dermatitis: G1: 35.7; G2: 15.4; G3: 1.6; breast edema: G1: 1.2; G2: 0.1 dysphagia: G1: 0.4 | Breast deformation; G1: 21.3; G2: 2.8; G3: 0.1; fibrosis: G1: 13.8; G2: 2.9; G3: 0.1; acute coronary syndrome G5: 0.1 | Telangiectasia: G1: 8.9; G2: 3.9; G3: 0.4; arm lymphedema: G1: 4.0; G2: 1.0; G3: 0.1; pulmonary fibrosis G1 0.4pneumonitis G2: 0.1 |
| De Santis, 2018 | 752 | 74 [65–92] | 42.4 Gy (2.65 Gy/fr)† | 10 Gy (2.5 Gy/fr)† if high grade; 16 Gy (2 Gy/fr)† if R+ | 0 Gy | 3D-CRT, photon beam for WB, photon or electron beam for boost sequential boost: 25.3% | 46 [8–102] | RTOG/EORTC | G1: 61.8; G2: 21.3; G3: 1.2 | G1: 14.0; G2: 5.0; G3: 0.0 | |
| Fiorentino, 2018 | 40 | 75 [70–83] | 50 Gy (2.0 Gy/fr)† | Up to 60 Gy (2.4 Gy/fr)† | 0 Gy | SIB-IMRT, 6 MV photon beam | 44 [38–66] | RTOG/EORTC | G1: 62.5; G2: 25.0; G3: 0.0 | Skin toxicity: g1: 32.5; g2: 0.0; g3: 0.0 | Fibrosis: G1: 10.0; G2: 0.0; G3: 0.0 |
| 40 | 72 [65–87] | 40.5 Gy (2.7 Gy/fr)† | Up to 48 Gy (3.2 Gy/fr)† | VMAT-SIB, 6 MV photon beam | 45 [34–70] | G1: 52.2; G2: 2.5; G3: 0.0 | Skin toxicity: G1: 5.0; G2: 0.0; G3: 0.0 | Fibrosis: G1: 5.0; G2: 0.0; G3: 0.0 | |||
| Sanz, 2018 | 486 | 79 [58–97] | 90.7%: 37.5 Gy (6.25 Gy/fr)*; 9.3%: 30 Gy; (5 Gy/fr)* | 1–2 fr added (6.25 or 5 Gy)* | Same as WB | Cobalt, 1.25 MV photon: 13.4%; 3D-CRT, photon or electron: 86.6%sequential boost: 17.5%; LNI: 15.0% | 51 [1–163] | RTOG/EORTC | G1: 38.7; G2: 25.3; G3: 10.5 | Subcutaneous fibrosis: G1: 18.5; G2: 6.0; G3: 2.5; G4: 0.2; telangiectasia: 2.7; edema/mastitis: 1.0 | |
*, weekly fractions; †, daily fractions; ‡, alternate days fractions. 3D-CRT, three-dimensional conformal radiotherapy; BCS, breast conservative surgery; CF, conventional fractionation; CTCAE, common terminology criteria for adverse events; CW, chest wall; fr, fraction; FU, follow-up; HF, hypofractionation; IMRT, intensity modulated radiotherapy; LENT-SOMA, Late Effects Normal Tissue Task Force-Subjective, Objective, Management, Analytic; LD, lateral decubitus; LN, lymph nodes; LNI, lymph nodes irradiation; pts, patients; R+, positive margins after BCS; RT, radiotherapy; RTOG-EORTC, radiation therapy oncology group- European Organisation for Research and Treatment of Cancer; SIB, simultaneous integrated boost; VMAT, Volumetric Modulated Arc Therapy; WB, whole breast; WHO, world trade organization; NR, not reported.
Fiorica et al. (14) reported data on 131 elderly patients (mean age: 78.3 years) treated with EBRT after BCS. Notably, 16.0% of patients were ≥85 years old. The prescribed dose to the whole breast was 50 Gy and 45.8% of patients received a boost on the tumor bed. Acute skin toxicity was scored as G1-2 in 58.1% of patients while only 6.1% of them experienced G3 skin toxicity. Late toxicity was represented by G1-2 fibrosis in 16.0% of patients. The authors found a significant correlation between OS and comorbidities while no correlation was found between toxicity and age or comorbidities.
Cao et al. (12) reported another retrospective analysis on 752 elderly patients (median: 75 years; range, 70–93) to assess the impact of comorbidities on the outcome of breast cancer patients treated with adjuvant RT after BCS (86.0%) or mastectomy (14.0%). Thirty-five percent of patients were treated with CF and 50 Gy total dose delivered to the whole breast or thoracic wall plus 16 Gy boost when clinically indicated. Different HF schemes were prescribed in 57.0% of patients and RT schedule was not reported in 8% of patients. In this series, 42.7% of patients were treated in lateral decubitus. Seven patients discontinued RT (4 of them definitively) and acute toxicity was mainly represented by dermatitis (G1: 35.7%, G2: 15.4%, G3: 1.6%). Other acute toxicities were breast edema (G1 and G2 in 1.2% and 0.1%, respectively), and G1 dysphagia (0.4%). Late toxicity was assessed using the LENT-SOMA criteria: breast deformation was the most frequent (G1: 21.3%, G2: 2.8%, G3: 0.1%). Fibrosis rates were G1: 13.8%, G2: 2.9%, and G3: 0.1%, while telangiectasia rates were G1: 8.9%, G2: 3.9%, and G3: 0.4%. Other late toxicities were arm lymphedema (G1: 4%, G2: 1%, G3: 0.1%) and pulmonary toxicity (G1 pulmonary fibrosis: 0.4%; G2 pneumonitis: 0.1%). One patient with cardiovascular history died of myocardial ischemia 24 months after the end of RT. In this analysis, the authors found a significant correlation between age ≥80 years and dermatitis and breast pain. Moreover, they found a higher risk of RT discontinuation in patients aged ≥80 years or with comorbidities.
Fiorentino et al. (6) published in 2018 a retrospective analysis on 80 elderly patients. They analysed two groups: 40 patients (median age: 75 years) treated with IMRT and CF and 40 patients (median age: 72 years) treated with VMAT and HF schedule. No G3 toxicity was recorded. Acute G1 and G2 toxicities rates were 62.5% and 25.0% in CF group while in the HF group the corresponding rates were 52.2% and 2.5%, respectively. Incidence of late G1 skin toxicity was 32.5% and 5.0% in CF and HF group, respectively and G1 fibrosis rate was 10.0% and 5.0% in CF and HF, respectively. The authors concluded that RT in elderly patients is well tolerated and VMAT technique provides lower toxicity rates. Regarding the subgroup of patients treated with CF, Fiorentino et al. (27) published in the same year another analysis evaluating the impact of comorbidity on compliance and toxicity. The authors reported a correlation between comorbidity and acute G2 toxicity.
Similarly, Giugliano et al. (5) analysed the comorbidities associated to compliance and the correlation of comorbidities and age with toxicity in 60 patients (mean age: 73.3) treated with a slightly HF regimen. Comorbidities did not significantly impact on toxicity nor on compliance to RT. Furthermore, no difference was registered comparing toxicity rates in patients ≥75 years old and patients <75 years old. Overall, acute toxicity rates were as follow: G1: 56.7%, G2: 18.2%, and G3: 3.2%. Finally, the authors reported 18.3% G1 and 3.3% G2 late toxicity. However, the median follow-up (15 months) was too short to consider these percentages as definitive results.
Other studies testing slightly HF regimens were those from De Santis et al. (20), Cante et al. (17), and Dorè et al. (16).
De Santis et al. (20) reported data on 752 patients with EBC and a median age of 74 years (range, 65–92; 78.9% of patients were >70 years old) treated using the following HF prescription: 42.2 Gy in 16 fractions with a sequential boost of 10 or 16 Gy in 2 Gy/fraction. Acute G1, G2, and G3 toxicity rates were 61.8%, 21.3%, and 1.2%, respectively. G2–G3 acute toxicities were significantly more frequent in patients who received a boost, especially when RT was delivered with photons beams. With 46 months median follow-up, G1 and G2 late toxicity rates were 14.0% and 5.0%, respectively.
Cante et al. (17) reported the results of an observational prospective study on 83 elderly EBC patients (70–80 years: 93%; >80 years: 7%) treated between 2005 and 2012 with HF-WBI (45 Gy to the whole breast and simultaneous boost to the surgical bed up to 50 Gy in 20 daily fractions). G1 and G2 acute toxicity rates were 40.0% and 3.0%, respectively. Late toxicity was G1 in 21.0% of patients (fibrosis, atrophy, telangiectasia, hyperpigmentation, and striae in 9.0%, 3.0%, 2.0%, 5.0%, and 2.0% of patients, respectively) and G2 in 6.0% of patients (fibrosis, telangiectasia, and hyperpigmentation in 3.0%, 1.0%, and 2.0% of patients, respectively).
Doré et al. (16) reported their experience on 205 patients who underwent postoperative RT after BCS and with a median age of 81 years (range, 52–91; 94.0% patients were ≥70 years old). RT was delivered over 5 weeks with 3 fraction/week on alternate days. Total dose was 45 Gy (in 3 Gy/fraction) with a boost on the surgical bed in 47.8% of treated patients. Overall, 32.0% of patients underwent also prophylactic nodal irradiation. G1, G2, and G3 acute toxicity rates were 65.0%, 17.0%, and 4.0%, respectively. Recorded late toxicity was as follows: 14.6% fibrosis, 8.0% telangiectasia, and 3.9% hyperpigmentation.
Several studies analysed HF schemes delivering RT in only 5–6 fraction (5–6.5 Gy/fraction). Some of them (7,15,19) reported on patients series treated before 2000.
Ortholan et al. (7) reported long-term results of a series including 150 elderly patients treated between 1987 and 1999. Median age was 78 years with only 17 patients (11.3%) <70 years old. Mastectomy was performed in 28.5% of patients and 31.8% subjects were irradiated on the regional lymph nodes (27.5 Gy in 5.5 Gy/fractions). The HF regimen was 32.5 Gy in 5 weekly fractions with a boost on the surgical bed delivered in 33.1% of patients. Acute G1 and G2 erythema was recorded in 18.7% and 9.3% of patients, respectively. Late toxicity was mainly subcutaneous fibrosis: G1 in 20.7%, G2 in 14.0%, and G3 in 4.7% of patients.
Courdi et al. (15) reported on a similar experience from the same institution on 115 patients treated from 1987 to 1999. Median age was 83 years and only subjects not amenable to surgery were included in the analysis. RT, delivered with the same scheme described above (7), was the primary treatment combined with endocrine therapy both in neoadjuvant and/or adjuvant setting. Even if in this analysis only elderly and frail patients were included, toxicity rates were satisfactory: G1 and G2 acute erythema were recorded in 20.9% and 8.7% of cases, respectively, and late G1, G2, and G3 fibrosis was registered in 16.5%, 18.3%, and 5.2% of patients, respectively.
Rovea et al. (10) analysed 298 elderly women (median age: 80 years) with non-metastatic breast cancer treated with adjuvant HF-RT. Most patients (80.9%) were treated with the same scheme described above (32.5 Gy in 5 weekly fractions), while 19.1% were treated with 30 Gy in 5 weekly fractions. Acute toxicity rates were G1: 22.6%, G2: 4.8%, G3: 1.0%, and G4: 0.3%. Treatment interruption occurred in 2 patients because of severe skin reactions. Late toxicity was mainly represented by fibrosis (G1: 31.5%, G2: 4.2%, and G3: 3.5%), oedema (G1: 7.0%, G2: 4.2%, and G3: 3.5%), telangiectasia (G1: 1.8%, and G3: 0.7%), hyperpigmentation (G1: 4.6%, G2: 2.4%), and atrophy (G1: 2.1%). One case of asymptomatic pericarditis was registered 5 months after treatment.
In 2017, Monten et al. (24) reported the results of a phase I-II trial that considered the incidence of clinically relevant dermatitis (grade ≥2) as the primary endpoint and enrolled 95 patients with locally advanced breast cancer. Mean age was 73.6 years, with 34.7% of patients younger than 70 years and 13.7% older than 79 years. Mastectomy was performed in 24.2% of patients. Total dose was 28.5 Gy in 5 fractions over 12 days (at least 1 day between two consecutive fractions) to the whole breast or to the chest wall. When indicated, a boost to the surgical bed was delivered using a simultaneous integrated boost technique and the dose to regional lymph nodes was 27 Gy. Acute toxicity was scored as G1, G2, and G3 in 52.6%, 10.5%, and 1.1% of patients, respectively. Only boost delivery was significantly associated with G2–3 toxicity.
Recently, Sanz et al. (19) reported data on weekly HF in a group of 486 patients with comorbidities or social frailties who could not receive a standard treatment. Median age was 79 years and all tumor stages were included. Mastectomy was previously performed in 20.0% of patients and 15.0% of patients received prophylactic nodal irradiation. Most patients (90.7%) received RT in 6 weekly fractions (6.25 Gy), up to a total dose of 37.5 Gy. The latest enrolled patients (9.3%) were treated with a slightly modified schedule (30 Gy in 6 weekly fractions) and a boost was delivered in 17.5% of patients. Acute G1, G2, and G3 toxicity rates were 38.7%, 25.3%, and 10.5%, respectively. The incidence of late G1, G2, and G3 fibrosis was 18.5%, 6%, and 2.5%, respectively, while 1 patient (0.2%) experienced G4 late fibrosis. Other recorded late toxicities were telangiectasia (2.7%) and oedema/mastitis (1%). A small percentage (1.8%) of patients did not complete RT.
Méry et al. (9) reported a retrospective analysis on 44 nonagenarian patients (mean age: 92 years). RT schedule was not reported and only 59.0% were previously treated with surgery. RT aim was symptoms palliation in 27.3% of patients. Acute G1, G2, and G3 toxicity rates were 34.0%, 23.0%, and 2.0%, respectively. The only patient who presented G3 toxicity did not complete RT. Late toxicity was evaluated in the only 16 patients with follow-up >6 months. G1 and G2 late toxicity rates were 7.0% and 2.0%, respectively.
Discussion
Aim of our review was to collect the fragmentary information available in literature, to analyze the potential correlation between older age and worse RT-related acute and late toxicity in patients with breast cancer.
This analysis has clear limitations. Primarily, not all studies reported both acute and late toxicities. Moreover, in some studies the severity of toxicity was not specified. Furthermore, different toxicity scales were used to specify and grade toxicity. For example, studies using LENT-SOMA scale considered cosmetic related outcomes as toxicity. On the other hand, CTCAE criteria split acute and late toxicity in several categories in the same organ/tissue. Therefore, the percentages often reported using this scale are not representative of the exact number of patients who presented a specific grade of toxicity in a specific tissue (i.e., skin or subcutaneous tissue) because a single patient can experience more than one toxicity. Follow-up duration and percentage of patients lost at follow-up are the other two essential issues which can limit late toxicity evaluation and are quite inhomogeneous in the analysed studies. Patients selection and the definition of “elderly” were also inhomogeneous in the series included in our analysis. We chose to be inclusive considering all studies where patients’ age was at least 60 years because in literature only few studies consider more selective age ranges. Moreover, due to lack of comorbidity description in most studies and correlation between older age and comorbidity incidence, it is difficult to distinguish the impact of age or concomitant diseases on toxicity. Finally, patients’ characteristics were inhomogeneous in terms of tumour stage and molecular subtype, surgical and systemic treatment performed, and RT characteristics.
We considered all available RT techniques (BRT, IORT, EB), RT targets (surgical bed, whole breast, thoracic wall, lymph nodes), radiation sources (cobalt, photon beams, electron beams), and possible fractionation (conventional, daily HF, weekly HF). This choice led to substantially non-comparable results in the selected studies.
Even considering all these limitations, our findings suggest that RT of breast cancer is well tolerated even in elderly patients with toxicity rates comparable to those of the general population. Therefore, toxicity incidence and severity seem not strictly related to age in this setting.
In fact, none of the studies which directly compared age subgroup (13,14,21) found age-related differences. Only Cao et al. reported an increased risk of dermatitis in patients ≥80 years old and a correlation between age ≥80 years and comorbidities with RT interruption. However, it should be noted that toxicity rates were low and toxicity grades were mild in their analysis (12).
In the different analyzed studies, acute toxicity G ≥3 ranged from 0.0% to 10.5% of patients and late toxicity from 0.0% to 13.0% of patients. RT discontinuation/interruption rate varied from 0.0% to 2.0% as shown in Table 4. More specifically, the cumulative acute toxicity G ≥3 rates in all evaluable patients were 2.0%, 6.7%, and 5.2% in patients treated with EB-APBI, BRT, and IORT, respectively. Cumulative late G ≥3 toxicity in evaluable patients treated with EB-APBI was 2.8% while no late G ≥3 toxicity was recorded in studies reporting on BRT and IORT.
Table 4
| RT setting | First author, year | Years of enrollment | Acute toxicity ≥ G3 (%) | Late toxicity ≥ G3 (%) | RT discontinuation/interruption (%) |
|---|---|---|---|---|---|
| APBI | Sayan, 2017 | 2006–2013 | NR | 13.0 | NR |
| Vinante, 2019 | 2008–2012 | 0.0 | 0.0 | 0.0 | |
| Meattini, 2015 | 2005–2013 | 1.7 | 0.0 | NR | |
| Jacobs, 2018 | 2011–2016 | 3.0 | NR | 0.0 | |
| % in evaluable patients | 2.0 | 2.8 | 0.0 | ||
| BRT | Khan, 2013 | 2002–2004 | NR | NR | NR |
| Kinj, 2018 | 2012–2015 | 6.7 | 0.0 | 0.0 | |
| Smith, 2014 | 2002–2007 | NR | NR | NR | |
| % in evaluable patients | 6.7 | 0.0 | 0.0 | ||
| IORT | Lemanski, 2013 | 2004–2007 | NR | 0.0 | 0.0 |
| Tuschy, 2013 | 2002–2007 | NR | NR | 0.0 | |
| Jacobs, 2018 | 2011–2016 | 5.2 | NR | 0.0 | |
| % in evaluable patients | 5.2 | 0.0 | |||
| WBI-CF | Meattini, 2015 | 2005–2013 | 5.1 | 0.0 | NR |
| Fiorica, 2012 | 2000–2007 | 6.1 | 0.0 | 0.0 | |
| Cao, 2018 | 2003–2009 | 1.6 | 0.8 | 0.9 | |
| Fiorentino, 2018 | 2011–2015 | 0.0 | 0.0 | 0.0 | |
| % in evaluable patients | 2.3 | 0.6 | 0.7 | ||
| WBI-HF daily | Fiorentino, 2018 | 2011–2015 | 0.0 | 0.0 | 0.0 |
| Giugliano, 2016 | 2011–2013 | 3.3 | 0.0 | 0.0 | |
| Cante, 2015 | 2005–2012 | 0.0 | 0.0 | 0.0 | |
| De Santis, 2018 | 2009–2017 | 1.2 | 0.0 | 0.0 | |
| Doré, 2015 | 2004–2012 | 4.0 | NR | 1.9* | |
| Mery, 2014 | 2003–2013 | 2.0 | 0.0 | 2.0 | |
| % in evaluable patients | 1.7 | 0.0 | 0.4 | ||
| HF weekly | Rovea, 2015 | 2007–2013 | 1.3 | 7.7 | 0.7 |
| Ortholan, 2005 | 1987–1999 | 0.0 | 4.7 | NR | |
| Courdi, 2006 | 1987–1999 | 0.0 | 5.2 | 0.0 | |
| Sanz, 2018 | 1992–2006 | 10.5 | 2.7 | 1.8† | |
| Monten, 2017 | NR | 1.1 | NR | NR | |
| % in evaluable patients | 4.9 | 4.7 | 1.2 | ||
*, only temporary interruption of RT; †, 57.1% temporary interruption. APBI, accelerated partial breast irradiation; BRT, brachytherapy; CF, conventional fractionation; HF, hypofractionated; IORT, intraoperative radiotherapy; NR, not reported; RT, radiotherapy; WBI, whole breast irradiation.
Between the different APBI techniques, the highest percentage (6.7%) of acute G ≥3 toxicity was reported by Kinj et al. who delivered a single 16 Gy HDR-BRT dose to the tumor bed (8), and the lowest G ≥3 acute toxicity rates were recorded in EB-APBI studies (0.0–3.0%) (18,22,25). The highest late toxicity incidence (13.0%) was registered by Sayan et al. using EB-APBI with HF-accelerated regimen (40 Gy in 10 daily fractions) (23).
In patients treated with WBI, the overall rates of G ≥3 toxicity were 3.0% for acute toxicity and 1.8% for late toxicity. Comparing different RT fractionations, we observed higher toxicity rates in studies on weekly HF as expected from a radiobiological point of view particularly for late effects. In fact, acute G ≥3 toxicity was 2.3%, 1.7%, and 4.9% in CF, daily HF, and weekly HF studies, respectively, while late G ≥3 toxicity was 0.6%, 0.0%, and 4.7% in CF, daily HF, and weekly HF, respectively.
The highest acute toxicity rate was 10.5% in Sanz et al. analysis (19). Due to the high incidence of side effects, from 2012 the authors reduced the prescribed dose from 37.5 Gy in 6 weekly fractions to 30 Gy in 6 weekly fractions. In all other studies, acute G ≥3 toxicity did not exceed 6.1%. The highest late toxicity rate (7.7%) was reported by Rovea et al. who used a regimen of 32.5 or 30 Gy in 5 weekly fractions (10). Instead, it should be emphasized that reported late ≥ G3 toxicity rates were clearly lower in CF and daily HF studies (<1.0%).
In WBI studies, 2 cases of G4 toxicity were reported both in patients treated with weekly HF regimens [1 case of acute G4 toxicity in Rovea’s study (10) and 1 case of late toxicity in Sanz’s study (19)]. These cases represent 0.03% of evaluable patients treated with WBI including both acute and late toxicity. One G5 late toxicity (a death for myocardial infarction in a patient with cardiovascular-disease history) was reported by Cao et al. (12).
In WBI studies, only 23 cases of RT discontinuation/interruption (0.8% of all evaluable patients) were recorded from Méry et al. (9), Doré et al. (16), Sanz et al. (19), Cao et al. (12), and Rovea et al. (10).
Despite the limits mentioned above, our analysis failed to show an excess of RT-related toxicity in elderly patients irrespective of the technique used. Consequently, the large variety of RT options available seems to question the issues leading to RT omission such as logistic difficulties and QoL concerns. In fact, data show that RT can be safely delivered with IORT which can reduce the treatment time as much as possible by combining surgery and RT at the same time (26), or with a single fraction of BRT, or with HF schedules which can reduce the overall treatment duration and/or the number of RT application per week.
The main advantage of BRT and HF-EB over IORT is that at least 15% of patients treated intraoperatively have a definitive histology not suitable for this treatment. Consequently, these patients need also post-operative WBI while IORT comes to be considered as a “boost”. On the contrary, post-operative RT allows a better selection of patients amenable for APBI (8).
Given these considerations, RT omission in elderly patients with breast cancer should be carefully evaluated limiting this option to very selected critical patients, and it should not be driven only by patients’ age evaluation. Even if available data suggests no improvement in OS when adjuvant RT is added to BCS and systemic therapy in older patients, disease free survival is proved to be higher when standard treatment (BCS + RT) is used, regardless of age (3).
Considering that life expectancy of breast cancer healthy women aged 70 and 80 is 15.8 and 8.8 years, respectively (28), comorbidities definition, social-economical frailties evaluation, and geriatric assessment should become routine practice when considering any treatment omission (4). Furthermore, in clinical practice RT is often omitted in this subgroup of patients due to concerns on QoL worsening. However, elderly patients desire to be informed and to share therapeutic decisions with physician (29). Therefore, it is essential to provide exhaustive information on RT pros and cons and to consider that RT is not the only treatment which can affect QoL. For example, endocrine therapy side effects and increased risk of local recurrence with the need of salvage mastectomy after RT omission, should be properly discussed with women because they can seriously affect long term QoL.
In conclusion, the RT role in elderly breast cancer patients need to be further investigated by routinely including this group of patients in prospective clinical trials together with younger patients. Moreover, specific studies on elderly patients are required in order to systematically evaluate comorbidities and geriatric assessment, to report detailed toxicity data, and possibly to analyse QoL using Patients Reported Outcome Measures.
Finally, it is very important to better stratify patients looking at disease stage and comorbidities because when considering de-escalation of breast cancer treatment, it is not obvious that RT is the omissible element. In fact, our analysis suggests that RT tolerability and toxicity are satisfactory also in elderly patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Vincent Vinh-Hung and Nam P Nguyen) for the series “Radiotherapy for Breast Cancer in Advanced Age” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.08.28). The series “Radiotherapy for Breast Cancer in Advanced Age” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Gosain R, Pollock YY, Jain D. Age-related Disparity: Breast Cancer in the Elderly. Curr Oncol Rep 2016;18:69. [Crossref] [PubMed]
- Matuschek C, Bölke E, Haussmann J, et al. The benefit of adjuvant radiotherapy after breast conserving surgery in older patients with low risk breast cancer- a meta-analysis of randomized trials. Radiat Oncol 2017;12:60. [Crossref] [PubMed]
- Popescu T, Karlsson U, Vinh-Hung V, et al. Challenges Facing Radiation Oncologists in The Management of Older Cancer Patients: Consensus of The International Geriatric Radiotherapy Group. Cancers (Basel) 2019;11:371. [Crossref] [PubMed]
- Giugliano FM, Falivene S, Esposito E, et al. Short-course radiotherapy in elderly women with breast cancer: Comparison by age, comorbidity index and toxicity. Int J Surg 2016;33:S92-6. [Crossref] [PubMed]
- Fiorentino A, Gregucci F, Mazzola R, et al. Intensity-modulated radiotherapy and hypofractionated volumetric modulated arc therapy for elderly patients with breast cancer: comparison of acute and late toxicities. Radiol Med 2019;124:309-14. [Crossref] [PubMed]
- Ortholan C, Hannoun-Lévi JM, Ferrero JM, et al. Long-term results of adjuvant hypofractionated radiotherapy for breast cancer in elderly patients. Int J Radiat Oncol Biol Phys 2005;61:154-62. [Crossref] [PubMed]
- Kinj R, Chand ME, Gal J, et al. Single fraction of accelerated partial breast irradiation in the elderly: Early clinical outcome. Radiat Oncol 2018;13:174. [Crossref] [PubMed]
- Méry B, Assouline A, Rivoirard R, et al. Portrait, treatment choices and management of breast cancer in nonagenarians: An ongoing challenge. Breast 2014;23:221-5. [Crossref] [PubMed]
- Rovea P, Fozza A, Franco P, et al. Once-weekly hypofractionated whole-breast radiotherapy after breast-conserving surgery in older patients: A potential alternative treatment schedule to daily 3-week hypofractionation. Clin Breast Cancer 2015;15:270-6. [Crossref] [PubMed]
- Smith GL, Jiang J, Buchholz TA, et al. Benefit of adjuvant brachytherapy versus external beam radiation for early breast cancer: Impact of patient stratification on breast preservation. Int J Radiat Oncol Biol Phys 2014;88:274-84. [Crossref] [PubMed]
- Cao KI, Salviat F, Laki F, et al. Outcomes of postoperative radiation therapy for breast cancer in older women according to age and comorbidity status: An observational retrospective study in 752 patients. J Geriatr Oncol 2018;9:600-5. [Crossref] [PubMed]
- Tuschy B, Berlit S, Romero S, et al. Clinical aspects of intraoperative radiotherapy in early breast cancer: Short-term complications after IORT in women treated with low energy x-rays. Radiat Oncol 2013;8:95-9. [Crossref] [PubMed]
- Fiorica F, Berretta M, Ursino S, et al. Adjuvant radiotherapy on older and oldest breast cancer patients after conservative surgery: A retrospective analysis. Arch Gerontol Geriatr 2012;55:283-8. [Crossref] [PubMed]
- Courdi A, Ortholan C, Hannoun-Lévi JM, et al. Long-term results of hypofractionated radiotherapy and hormonal therapy without surgery for breast cancer in elderly patients. Radiother Oncol 2006;79:156-61. [Crossref] [PubMed]
- Doré M, Cutuli B, Cellier P, et al. Hypofractionated irradiation in elderly patients with breast cancer after breast conserving surgery and mastectomy: Analysis of 205 cases. Radiat Oncol 2015;10:161. [Crossref] [PubMed]
- Cante D, Franco P, Sciacero P, et al. Hypofractionated whole-breast radiotherapy and concomitant boost after breast conservation in elderly patients. Tumori 2016;102:196-202. [Crossref] [PubMed]
- Jacobs DH, Speijer G, Petoukhova AL, et al. Acute toxicity of intraoperative radiotherapy and external beam-accelerated partial breast irradiation in elderly breast cancer patients. Breast Cancer Res Treat 2018;169:549-59. [Crossref] [PubMed]
- Sanz J, Zhao M, Rodríguez N, et al. Once-Weekly Hypofractionated Radiotherapy for Breast Cancer in Elderly Patients: Efficacy and Tolerance in 486 Patients. Biomed Res Int 2018;2018:8321871. [Crossref] [PubMed]
- De Santis MC, Bonfantini F, Di Salvo F, et al. Hypofractionated Whole-Breast Irradiation With or Without Boost in Elderly Patients: Clinical Evaluation of an Italian Experience. Clin Breast Cancer 2018;18:e1059-66. [Crossref] [PubMed]
- Khan AJ, Vicini FA, Beitsch P, et al. Local control, toxicity, and cosmesis in women >70 years enrolled in the American Society of Breast Surgeons accelerated partial breast irradiation registry trial. Int J Radiat Oncol Biol Phys 2012;84:323-30. [Crossref] [PubMed]
- Meattini I, Saieva C, Marrazzo L, et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy technique compared to whole breast irradiation for patients aged 70 years or older: subgroup analysis from a randomized phase 3 trial. Breast Cancer Res Treat 2015;153:539-47. [Crossref] [PubMed]
- Sayan M, Wilson K, Nelson C, et al. A novel schedule of accelerated partial breast radiation using intensity-modulated radiation therapy in elderly patients: Survival and toxicity analysis of a prospective clinical trial. Radiat Oncol J 2017;35:32-8. [Crossref] [PubMed]
- Monten C, Lievens Y, Olteanu LAM, et al. Highly Accelerated Irradiation in 5 Fractions (HAI-5): Feasibility in Elderly Women With Early or Locally Advanced Breast Cancer. Int J Radiat Oncol Biol Phys 2017;98:922-30. [Crossref] [PubMed]
- Vinante L, Avanzo M, Furlan C, et al. Ten daily fractions for partial breast irradiation. Long-term results of a prospective phase II trial. Breast J 2019;25:243-9. [Crossref] [PubMed]
- Lemanski C, Azria D, Gourgou-Bourgade S, et al. Electrons for intraoperative radiotherapy in selected breast-cancer patients: Late results of the Montpellier phase II trial. Radiat Oncol 2013;8:191. [Crossref] [PubMed]
- Fiorentino A, Mazzola R, Levra NG, et al. Comorbidities and intensity-modulated radiotherapy with simultaneous integrated boost in elderly breast cancer patients. Aging Clin Exp Res 2018;30:533-8. [Crossref] [PubMed]
- Basso U, Brunello A, Pogliani C, et al. Treatment options for early breast cancer in elderly women. Expert Rev Anticancer Ther 2004;4:197-211. [Crossref] [PubMed]
- Wang SY, Kelly G, Gross C, et al. Information Needs of Older Women With Early-Stage Breast Cancer When Making Radiation Therapy Decisions. Int J Radiat Oncol Biol Phys 2017;98:733-40. [Crossref] [PubMed]