
Synergistic effect of metformin and EGFR-TKI in the treatment of non-small cell lung cancer
Introduction
Lung cancer is one of the most common malignant tumors and the leading cause of cancer-related death (1). Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancers (2). For patients with advanced NSCLC, comprehensive treatment is the main treatment plan. Platinum-containing chemotherapy is the first-choice treatment plan for advanced patients. The discovery of epidermal growth factor receptor (EGFR) mutation and first-generation application of EGFR-tyrosine kinase inhibitor (TKI) has provided a new method for the treatment of NSCLC (3,4).
First-generation EGFR-TKI mainly includes gefitinib and erlotinib. Its mechanism centers on preventing the activation of EGFR downstream signaling pathway through reversible competition for adenosine-triphosphate (ATP) binding sites, inducing the apoptosis of cancer cells. The IPASS study (5), NEJ002 study (6) and WJTOG3405 study (7) confirmed that progression-free survival (PFS) was significantly prolonged in patients with EGFR mutation after gefitinib treatment. These studies established the role of EGFR-TKI in first-line therapy for advanced NSCLC patients with EGFR mutation. However, after using the first-generation EGFR-TKI, drug resistance is unavoidable.
The second-generation of EGFR-TKI includes afatinib, dacomitinib, and other treatments. Afatinib is characterized by a high selectivity and low molecular weight. It is an irreversible ErbB family inhibitor. It can inhibit the proliferation and metastasis of cancer cells by binding ErbB and blocking signaling pathway (8). In the LUX-Lung 3 study (9), the median PFS in the afatinib group was significantly longer than in the pemetrexed/cisplatin group (11.1 vs. 6.9 months). In the LUX-Lung 6 study (10), the PFS of patients with the exon 19 mutation or L858R mutation treated with afatinib were significantly longer than those treated with gemcitabine/cisplatin (11.0 vs. 5.6 months). Moreover, the afatinib group had a better objective remission rate (ORR) (66.9% vs. 23.0%) and disease control rate (92.6% vs. 76.2%). The LUX-Lung 7 study (11) compared the efficacy of afatinib and gefitinib as the first-line treatment for patients with EGFR mutation. The results showed that the median PFS in the afatinib group was slightly longer (11.0 vs. 10.9 months), and there was no significant difference in safety. Based on these studies, the Food and Drug Administration (FDA) approved afatinib as a first-line treatment for advanced NSCLC patients with the EGFR exon 19 deletion or the exon 21 mutation (L858R mutation) on July 12th, 2013. However, second-generation EGFR-TKI is expensive, and its binding with wild-type EGFR can cause related toxicity. These shortcomings hamper the clinical application of second-generation EGFR-TKI.
Osimertinib is an irreversible third-generation EGFR-TKI, which is sensitive to the EGFR mutation and T790M resistance mutation. The AURA 2 study (12) showed that median PFS was 9.9 months, ORR was 70%, and the 1-year overall survival (OS) rate was 81%. The AURA 3 study (13) showed that the osimertinib group had a longer PFS than the pemetrexed/cisplatin group (10.1 vs. 4.4 months), and the side effects were lower than the chemotherapy group. On November 13th, 2015, the FDA approved osimertinib for the treatment of NSCLC patients with the T790M mutation during or after EGFR-TKI treatment. In the FLAURA study (14), the median PFS was significantly prolonged in the osimertinib group (18.9 vs. 10.2 months), and the level 3 adverse events were less (34% vs. 45%) in the osimertinib group. This established the position of osimertinib as the first-line drug for patients with EGFR mutation. However, the mechanisms of resistance to third-generation EGFR-TKI began to appear, including HER2 amplification, KRAS mutation, and CMET amplification.
Combination therapy is also emerging for new drug resistance mechanisms, for example, combination therapy with EGFR-TKI and pathway inhibitors, including inhibitors of IGF-1R, HER2, PI3K, and mTOR (15-18). Compared with EGFR-TKI alone, these combination therapies can improve the therapeutic effect to some extent. However, these inhibitors also have their disadvantages, such as a higher cost and higher toxicity. The disadvantages limit the clinical application of these treatments. Therefore, in the course of clinical treatment, there is an urgent need for a cheap, low-toxicity, high-efficiency treatment for EGFR-TKI resistance, which will bring better curative effect to patients.
Metformin and lung cancer
Metformin is a kind of oral hypoglycemic drug, which has been proven to reduce fasting blood sugar. Since its advent in 1957, it has been used in clinic for more than 60 years (19). Metformin is the preferred oral hypoglycemic agent for patients with type 2 diabetes. It mainly inhibits hepatic gluconeogenesis, reduces hepatic glycogen production, and increases the utilization of glucose by skeletal muscles and fat cells, thereby reducing blood sugar, mainly by activating adenosine 5'-monophosphate-activated protein kinase (AMPK) signaling pathway (20-23).
After analyzing several studies from 2009 to 2013, it was concluded that patients with type 2 diabetes mellitus who use metformin have a lower risk for lung cancer (24). In recent years, experimental studies have shown that metformin can inhibit tumor cell proliferation and improve tumor sensitivity to chemotherapy drugs and small molecule targeted anticancer drugs (25). Therefore, the application of metformin can reduce the incidence of cancer and improve the prognosis of diabetic patients (26). Additionally, studies have further pointed out that in the treatment of type 2 diabetic NSCLC patients with EGFR mutations, a combination of metformin and EGFR-TKI has been shown to have a synergistic effect that delays the onset of resistance and results in longer PFS and OS (27) (PFS: 19.0 vs. 8.0 months, P=0.005; OS: 32.0 vs. 23.0 months, P=0.002) (Figure 1).
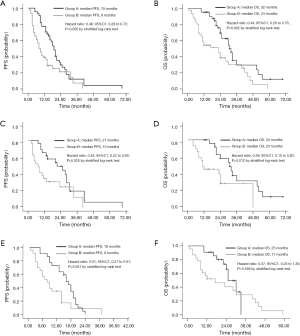
Metformin is associated with reversal of EGFR-TKI resistance
Metformin inhibits the IGF-1R pathway and makes drug-resistant cells re-sensitive to EGFR-TKI
Several studies have reported that the activation of the IGF-1R pathway may lead to a resistance of EGFR-TKI (28-31). IGF-1R is a transmembrane tyrosine-protein kinase receptor expressed on the surface of many types of cells with potential mitogen action. After IGF-1R binds to ligand, phosphorylation of IGF-1R activates RAS/RAF/MAPK and PI3K/AKT/mTOR pathways promote intracellular mitosis, induce cell proliferation and differentiation, while inhibiting apoptosis (32-34).
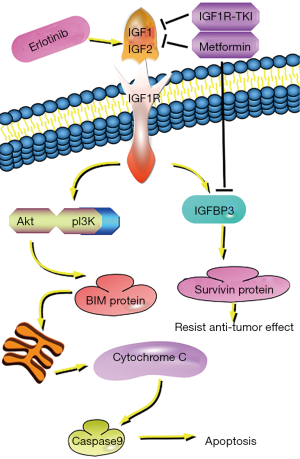
First-generation EGFR-TKI erlotinib increases the level of EGFR/IGF-1R heterodimers in NSCLC cell membranes, activates IGF-IR and its downstream signaling medium IGFBP3 (28,30), stimulates surviving protein synthesis, and counteracts the anti-tumor effect of erlotinib (28). IGF-1R inhibitors can inhibit the expression of IGFBP3 (15,30). While IGF-1R also activates the PI3K/AKT pathway (35,36). The AKT signaling pathway plays an important role in various biological activities such as cell proliferation and apoptosis (33,34), while increasing the kinase activity of AKT and promoting cell transformation can make cells resistant to TKI (37). Inactivation of the AKT signaling pathway leads to a significant increase in BIM protein (38,39). BIM protein promotes mitochondrial release of cytochrome C, whereas cytochrome C activates caspases, which mediate apoptosis via mitochondria (40,41).
IGF-1R inhibitors, such as α-IR3, AG-1024, or R1507 interact with EGFR-TKI to enhance TKI-induced cell growth inhibition and apoptosis (42). Moreover, knockdown of IGF-1R by siRNA can also enhance the sensitivity of EGFR-TKI resistant cells to EGFR-TKI (43). Studies have also shown that metformin can also inhibit IGF-1R signaling in cancer cells (44-46). In short, metformin can restore the sensitivity of EGFR-TKI-resistant cells to EGFR-TKI by inhibiting the IGF-1R pathway, and inhibit the expression of IGFBP3 (30), down-regulate AKT, and enhance BIM-mediated synergistic anti-tumor effects (43) (Figure 2).
Metformin inhibits IL-6 and TGF-β signaling pathways to reverse epithelial-mesenchymal transformation (EMT) and overcome TKI resistance
EMT refers to the biological process of epithelial cells transforming into mesenchymal phenotype cells through specific procedures, which is an important biological process for epithelial-derived malignant tumor cells to acquire migration and invasion ability. It is characterized by the loss of the polarity of epithelial cells and the loss of connection with the basement membrane, which enhances the motility of cancer cells and increases invasion, proliferation and metastasis (47,48). EMT and EGFR-TKI are closely related to lung cancer cell sensitivity (49,50). In vitro studies showed that the resistance of mesenchymal phenotype to EGFR-TKI was higher than that of the epithelial phenotype (51). AXL is a marker of EMT and is up-regulated in NSCLC patients with EGFR-TKI resistance, with AXL’s activation being an important cause of EGFR-TKI resistance (52).
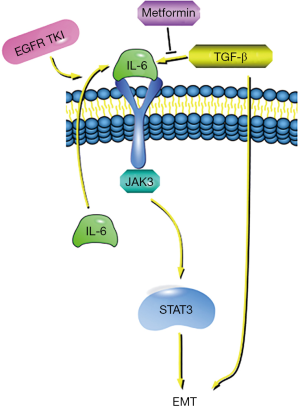
EGFR-TKI treatment causes IL-6 to activate IL-6R in an autocrine manner, thus causing IL-6R/JAK1/STAT3 signaling to activate (53). The IL-6R signaling pathway is more strongly activated in TKI-resistant cells compared to sensitive cells (54). Activation of signaling pathways by IL-6 is a key factor in the development of EMT in tumor cells (55). Also, studies have reported that TGF-β is an important driver of the EMT genetic program (48,56), and can induce activation of the IL-6 axis signaling pathway in lung cancer cells (48,57). Therefore, TGF-β and IL-6 are considered to be important targets for overcoming EGFR-TKI resistance in lung cancer cells (48,57-59).
Studies have shown that metformin can impair the TGF-β-induced mesenchymal state in a variety of pathological processes. It can hinder TGF-β-promoted EMT processes (56), reduce IL-6 secretion, thereby inhibiting signaling in the IL-6R/JAK1/STAT3 pathway (55,60), and restore the sensitivity of drug-resistant cells to EGFR-TKI. Also, some experiments have found that when IL-6 was added to EGFR-TKI drug-resistant cell lines pretreated with metformin, the effect of metformin disappeared. The cell lines resumed their resistance to EGFR-TKI, and re-activated the IL-6 signaling pathway (54). This suggests that metformin can overcome TKI resistance by reversing the EMT process and inhibiting the IL-6 signaling pathway, making cells re-sensitive to EGFR-TKI (61). Therefore, reversing the EMT process and preventing IL-6 signaling pathway transduction may be an effective way to improve the response of EGFR-TKI therapy (Figure 3).
Metformin and EGFR-TKI inhibit tumor cell growth through the LKB1-AMPK-mTOR pathway
Metformin and gefitinib have synergistic effects in LKB1 wild-type NSCLC cells and show significant anti-proliferative and pro-apoptotic activities, which are dependent on the LKB1 mutation status (61). Algire and colleagues reported that (62) cancer cells lacking LKB1 protein expression did not respond to metformin in vitro. LKB1 gene alterations are more often detected in NSCLC compared to small cell lung cancer, and the frequency of LKB1 genetic alterations is higher in cancer cell lines with KRAS mutations (63).
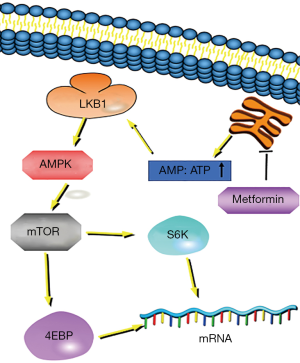
At the cellular level, metformin interferes with mitochondrial respiration and interferes with cellular energy metabolism, resulting in an increased intracellular ratio of AMP:ATP. This can lead to the activation of AMPK by LKB1 (64), a serine/threonine-protein kinase regulator of cellular metabolism (65). Some in vitro and in vivo studies have shown that AMPK activation inhibits mTOR signaling (66-70) and inhibits tumor cell proliferation (71-73). mTOR is an important target protein downstream of AMPK and is involved in the regulation of protein synthesis, cell cycle and apoptosis. When mTOR activity is decreased, phosphorylation of downstream S6K and 4E-BP leads to the inhibition of mRNA translation and the reduction of protein synthesis while playing an anti-tumor role (66-70,74). Thus, metformin and EGFR-TKI inhibit tumor cell growth via the LKB1/AMPK/mTOR pathway (Figure 4).
The use of metformin produces adverse reactions
Domestic reported that the incidence of gastrointestinal reactions of metformin was 15% (Grade I) (75). The symptoms of the reactions are a lack of appetite, nausea, vomiting, abdominal pain, diarrhea, etc., and the incidence rate is 20–30% (76). If a serious gastrointestinal reaction occurs after increasing the dose, the previous lower dose can be tried again, and the dosage subsequently increased according to patient tolerance (77). In addition, sustained release preparations may also be selected to reduce gastrointestinal symptoms in patients. Multiple studies (78-80) have shown that long-term use of metformin can cause a decrease in vitamin B12 levels. Therefore, it is recommended that if patients need long-term use of metformin, vitamin B12 should be appropriately supplemented during the treatment. Treatment of type 2 diabetes includes metformin alone, with little or no hypoglycemia, but a few special patients may have hypoglycemia (6.5%); the most serious and rare adverse reaction is lactic acidosis, which has a low incidence of about 3/100,000, but the mortality rate can be as high as 60% (81).
Conclusions
Drug resistance is a major problem in the treatment of EGFR-mutant NSCLC patients. The second-generation and third-generation EGFR-TKI drugs can bring about certain effects, but they will eventually become resistant. Moreover, combination therapy can have a certain therapeutic effect, but because of its high price and high toxicity, it has been limited in clinical application.
The discovery of the role of metformin in the treatment of lung cancer can provided new ideas in the treatment of EGFR-mutant lung cancer patients. It has been found that metformin can inhibit the IGF-1R pathway and make drug-resistant cells re-sensitive to EGFR-TKI. It can inhibit IL-6 and TGF-β signaling pathways to reverse EMT, overcome TKI resistance, and can also be combined with EGFR-TKI. In application, using the LKB1/AMPK/TOR pathway to inhibit tumor cell growth can bring better PFS and OS to lung cancer patients with the EGFR mutation.
However, although metformin has a certain role in the treatment of EGFR-mutant NSCLC patients, we should also be wary of the adverse reactions caused by metformin, including gastrointestinal reactions, hypoglycemia, etc., the dosage of metformin should be carefully controlled, or another drug intervention should be applied.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.11.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet 2000;355:479-85. [Crossref] [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. [Crossref] [PubMed]
- Antonicelli A, Cafarotti S, Indini A, et al. EGFR-targeted therapy for non-small cell lung cancer: focus on EGFR oncogenic mutation. Int J Med Sci 2013;10:320-30. [Crossref] [PubMed]
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [Crossref] [PubMed]
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54-9. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Hirsh V. Afatinib (BIBW 2992) development in non-small-cell lung cancer. Future Oncol 2011;7:817-25. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. [Crossref] [PubMed]
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643-52. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Guix M, Faber AC, Wang SE, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest 2008;118:2609-19. [PubMed]
- Rho JK, Choi YJ, Jeon BS, et al. Combined treatment with silibinin and epidermal growth factor receptor tyrosine kinase inhibitors overcomes drug resistance caused by T790M mutation. Mol Cancer Ther 2010;9:3233-43. [Crossref] [PubMed]
- Ueda S, Basaki Y, Yoshie M, et al. PTEN/Akt signaling through epidermal growth factor receptor is prerequisite for angiogenesis by hepatocellular carcinoma cells that is susceptible to inhibition by gefitinib. Cancer Res 2006;66:5346-53. [Crossref] [PubMed]
- Li D, Shimamura T, Ji H, et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell 2007;12:81-93. [Crossref] [PubMed]
- Kinaan M, Ding H, Triggle CR. Metformin: An Old Drug for the Treatment of Diabetes but a New Drug for the Protection of the Endothelium. Med Princ Pract 2015;24:401-15. [Crossref] [PubMed]
- Hundal RS, Krssak M, Dufour S, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000;49:2063-9. [Crossref] [PubMed]
- Natali A, Ferrannini E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia 2006;49:434-41. [Crossref] [PubMed]
- Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1996;81:4059-67. [PubMed]
- Viollet B, Guigas B, Sanz Garcia N, et al. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012;122:253-70. [Crossref] [PubMed]
- Schmedt N, Azoulay L, Hense S. Re.: "Reduced risk of lung cancer with metformin therapy in diabetic patients: a systematic review and meta-analysis Am J Epidemiol 2014;180:1216-7. [Crossref] [PubMed]
- Rizos CV, Elisaf MS. Metformin and cancer. Eur J Pharmacol 2013;705:96-108. [Crossref] [PubMed]
- Currie CJ, Poole CD, Jenkins-Jones S, et al. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 2012;35:299-304. [Crossref] [PubMed]
- Chen H, Yao W, Chu Q, et al. Synergistic effects of metformin in combination with EGFR-TKI in the treatment of patients with advanced non-small cell lung cancer and type 2 diabetes. Cancer Lett 2015;369:97-102. [Crossref] [PubMed]
- Morgillo F, Woo JK, Kim ES, et al. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res 2006;66:10100-11. [Crossref] [PubMed]
- Morgillo F, Kim WY, Kim ES, et al. Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res 2007;13:2795-803. [Crossref] [PubMed]
- Cortot AB, Repellin CE, Shimamura T, et al. Resistance to irreversible EGF receptor tyrosine kinase inhibitors through a multistep mechanism involving the IGF1R pathway. Cancer Res 2013;73:834-43. [Crossref] [PubMed]
- Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer 2009;10:281-9. [Crossref] [PubMed]
- Martin JL, Baxter RC. Signalling pathways of insulin-like growth factors (IGFs) and IGF binding protein-3. Growth Factors 2011;29:235-44. [Crossref] [PubMed]
- Sheppard K, Kinross KM, Solomon B, et al. Targeting PI3 kinase/AKT/mTOR signaling in cancer. Crit Rev Oncog 2012;17:69-95. [Crossref] [PubMed]
- Vázquez de la Torre A, Junyent F, Folch J, et al. PI3 k/akt inhibition induces apoptosis through p38 activation in neurons. Pharmacol Res 2013;70:116-25. [Crossref] [PubMed]
- Gallagher EJ, LeRoith D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol Metab 2010;21:610-8. [Crossref] [PubMed]
- van der Veeken J, Oliveira S, Schiffelers RM, et al. Crosstalk between epidermal growth factor receptor- and insulin-like growth factor-1 receptor signaling: implications for cancer therapy. Curr Cancer Drug Targets 2009;9:748-60. [Crossref] [PubMed]
- Lim SM, Kim HR, Cho EK, et al. Targeted sequencing identifies genetic alterations that confer primary resistance to EGFR tyrosine kinase inhibitor (Korean Lung Cancer Consortium). Oncotarget 2016;7:36311-20. [Crossref] [PubMed]
- Rosich L, Saborit-Villarroya I, Lopez-Guerra M, et al. The phosphatidylinositol-3-kinase inhibitor NVP-BKM120 overcomes resistance signals derived from microenvironment by regulating the Akt/FoxO3a/Bim axis in chronic lymphocytic leukemia cells. Haematologica 2013;98:1739-47. [Crossref] [PubMed]
- Aroui S, Dardevet L, Najlaoui F, et al. PTEN-regulated AKT/FoxO3a/Bim signaling contributes to Human cell glioblastoma apoptosis by platinum-maurocalcin conjugate. Int J Biochem Cell Biol 2016;77:15-22. [Crossref] [PubMed]
- Prenek L, Boldizsar F, Kugyelka R, et al. The regulation of the mitochondrial apoptotic pathway by glucocorticoid receptor in collaboration with Bcl-2 family proteins in developing T cells. Apoptosis 2017;22:239-53. [Crossref] [PubMed]
- Ishii Y, Nhiayi MK, Tse E, et al. Knockout Serum Replacement Promotes Cell Survival by Preventing BIM from Inducing Mitochondrial Cytochrome C Release. PLoS One 2015;10:e0140585. [Crossref] [PubMed]
- Choi YJ, Rho JK, Jeon BS, et al. Combined inhibition of IGFR enhances the effects of gefitinib in H1650: a lung cancer cell line with EGFR mutation and primary resistance to EGFR-TK inhibitors. Cancer Chemother Pharmacol 2010;66:381-8. [Crossref] [PubMed]
- Pan YH, Jiao L, Lin CY, et al. Combined treatment with metformin and gefitinib overcomes primary resistance to EGFR-TKIs with EGFR mutation via targeting IGF-1R signaling pathway. Biologics 2018;12:75-86. [PubMed]
- Abo-Elmatty DM, Ahmed EA, Tawfik MK, et al. Metformin enhancing the antitumor efficacy of carboplatin against Ehrlich solid carcinoma grown in diabetic mice: Effect on IGF-1 and tumoral expression of IGF-1 receptors. Int Immunopharmacol 2017;44:72-86. [Crossref] [PubMed]
- Tandon M, Chen Z, Othman AH, et al. Role of Runx2 in IGF-1Rbeta/Akt- and AMPK/Erk-dependent growth, survival and sensitivity towards metformin in breast cancer bone metastasis. Oncogene 2016;35:4730-40. [Crossref] [PubMed]
- Karnevi E, Said K, Andersson R, et al. Metformin-mediated growth inhibition involves suppression of the IGF-I receptor signalling pathway in human pancreatic cancer cells. BMC Cancer 2013;13:235. [Crossref] [PubMed]
- Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871-90. [Crossref] [PubMed]
- Yadav A, Kumar B, Datta J, et al. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res 2011;9:1658-67. [Crossref] [PubMed]
- Uramoto H, Iwata T, Onitsuka T, et al. Epithelial-mesenchymal transition in EGFR-TKI acquired resistant lung adenocarcinoma. Anticancer Res 2010;30:2513-7. [PubMed]
- Chung JH, Rho JK, Xu X, et al. Clinical and molecular evidences of epithelial to mesenchymal transition in acquired resistance to EGFR-TKIs. Lung Cancer 2011;73:176-82. [Crossref] [PubMed]
- Thomson S, Buck E, Petti F, et al. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res 2005;65:9455-62. [Crossref] [PubMed]
- Zhang Z, Lee JC, Lin L, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet 2012;44:852-60. [Crossref] [PubMed]
- Kim SM, Kwon OJ, Hong YK, et al. Activation of IL-6R/JAK1/STAT3 signaling induces de novo resistance to irreversible EGFR inhibitors in non-small cell lung cancer with T790M resistance mutation. Mol Cancer Ther 2012;11:2254-64. [Crossref] [PubMed]
- Li L, Han R, Xiao H, et al. Metformin sensitizes EGFR-TKI-resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin Cancer Res 2014;20:2714-26. [Crossref] [PubMed]
- Sullivan NJ, Sasser AK, Axel AE, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene 2009;28:2940-7. [Crossref] [PubMed]
- Cufí S, Vazquez-Martin A, Oliveras-Ferraros C, et al. Metformin against TGFbeta-induced epithelial-to-mesenchymal transition (EMT): from cancer stem cells to aging-associated fibrosis. Cell Cycle 2010;9:4461-8. [Crossref] [PubMed]
- Yao Z, Fenoglio S, Gao DC, et al. TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci U S A 2010;107:15535-40. [Crossref] [PubMed]
- Gao SP, Mark KG, Leslie K, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest 2007;117:3846-56. [Crossref] [PubMed]
- Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell 2009;15:79-80. [Crossref] [PubMed]
- Witta SE, Gemmill RM, Hirsch FR, et al. Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res 2006;66:944-50. [Crossref] [PubMed]
- Morgillo F, Sasso FC, Della Corte CM, et al. Synergistic effects of metformin treatment in combination with gefitinib, a selective EGFR tyrosine kinase inhibitor, in LKB1 wild-type NSCLC cell lines. Clin Cancer Res 2013;19:3508-19. [Crossref] [PubMed]
- Algire C, Amrein L, Bazile M, et al. Diet and tumor LKB1 expression interact to determine sensitivity to anti-neoplastic effects of metformin in vivo. Oncogene 2011;30:1174-82. [Crossref] [PubMed]
- Matsumoto S, Iwakawa R, Takahashi K, et al. Prevalence and specificity of LKB1 genetic alterations in lung cancers. Oncogene 2007;26:5911-8. [Crossref] [PubMed]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 2011;13:1016-23. [Crossref] [PubMed]
- Karbowniczek M, Robertson GP, Henske EP. Rheb inhibits C-raf activity and B-raf/C-raf heterodimerization. J Biol Chem 2006;281:25447-56. [Crossref] [PubMed]
- Gotlieb WH, Saumet J, Beauchamp MC, et al. In vitro metformin anti-neoplastic activity in epithelial ovarian cancer. Gynecol Oncol 2008;110:246-50. [Crossref] [PubMed]
- Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 2009;8:909-15. [Crossref] [PubMed]
- Liu B, Fan Z, Edgerton SM, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle 2009;8:2031-40. [Crossref] [PubMed]
- Wang LW, Li ZS, Zou DW, et al. Metformin induces apoptosis of pancreatic cancer cells. World J Gastroenterol 2008;14:7192-8. [Crossref] [PubMed]
- Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res 2011;71:3196-201. [Crossref] [PubMed]
- Green AS, Chapuis N, Lacombe C, et al. LKB1/AMPK/mTOR signaling pathway in hematological malignancies: from metabolism to cancer cell biology. Cell Cycle 2011;10:2115-20. [Crossref] [PubMed]
- Han D, Li SJ, Zhu YT, et al. LKB1/AMPK/mTOR signaling pathway in non-small-cell lung cancer. Asian Pac J Cancer Prev 2013;14:4033-9. [Crossref] [PubMed]
- Lin CC, Yeh HH, Huang WL, et al. Metformin enhances cisplatin cytotoxicity by suppressing signal transducer and activator of transcription-3 activity independently of the liver kinase B1-AMP-activated protein kinase pathway. Am J Respir Cell Mol Biol 2013;49:241-50. [Crossref] [PubMed]
- Belda-Iniesta C, Pernia O, Simo R. Metformin: a new option in cancer treatment. Clin Transl Oncol 2011;13:363-7. [Crossref] [PubMed]
- Blonde L, Rosenstock J, Mooradian AD, et al. Glyburide/metformin combination product is safe and efficacious in patients with type 2 diabetes failing sulphonylurea therapy. Diabetes Obes Metab 2002;4:368-75. [Crossref] [PubMed]
- Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403. [Crossref] [PubMed]
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193-203. [Crossref] [PubMed]
- Pflipsen MC, Oh RC, Saguil A, et al. The prevalence of vitamin B(12) deficiency in patients with type 2 diabetes: a cross-sectional study. J Am Board Fam Med 2009;22:528-34. [Crossref] [PubMed]
- de Jager J, Kooy A, Lehert P, et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ 2010;340:c2181. [Crossref] [PubMed]
- Kos E, Liszek MJ, Emanuele MA, et al. Effect of metformin therapy on vitamin D and vitamin B(1)(2) levels in patients with type 2 diabetes mellitus. Endocr Pract 2012;18:179-84. [Crossref] [PubMed]
- Bailey CJ, Turner RC. Metformin. N Engl J Med 1996;334:574-9. [Crossref] [PubMed]


