
Plasma enhance drug sensitivity to bortezomib by inhibition of cyp1a1 in myeloma cells
Introduction
Multiple myeloma (MM), an incurable B cell disease, is characterized by the accumulation of malignant plasma cells in patients’ bone marrow (BM) (1). Patients with MM are at an increased risk of developing infections, anemia, thrombocytopenia, renal failure, and bone disease (2,3). Drug resistance is one of the major problems in MM clinical therapy (4). The interactions between MM cells and the BM microenvironment is considered to play a critical role in MM drug resistance (5). In between, the Notch pathway is important for MM cell growth, migration and drug resistance (6,7). We have demonstrated that Notch activation could induce drug resistance to bortezomib in murine and human MM cells by up-regulating cyp1a1, which was involved in drug metabolism (8). In this study, we used a new technology, called cold atmosphere plasma (CAP), to treat MM cells (9,10). CAP is mainly consisted of free electron and charged ions, which generates reactive oxygen species (ROS) and reactive nitrogen species (RNS) that could have various biological effects (11,12). Thus, gas plasma has been applied in many biomedical fields such as sterilization, dentistry, cosmetology, wound healing, skin disease and cancer therapy (13-15). Combination of different drugs or therapy for a better anti-cancer effect is a common strategy in cancer therapy. Here, we demonstrated for the first time that gas plasma and bortezomib had a synergistic reduction of cell viability in myeloma cells. Gas plasma could enhance the sensitivity to bortezomib in myeloma cells, by inhibiting Notch signaling and cyp1a1, which could be applied as a new combination treatment for a better anti-cancer effect and a lower drug side effect.
Methods
Atmospheric pressure plasma jet (APPJ) generation system.
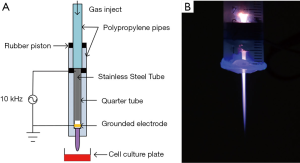
In this study, CAP was produced by a plasma jet device, the schematic diagram of the device structure was shown in Figure 1A. It was modified based on a needle. An inner diameter of 4 mm stainless steel tube was used as a high-voltage electrode and gas inlet. The ground electrode was a 10 mm long copper sheet, which wrapped around the quartz tube at a distance of 10 mm from the nozzle. The plasma generation system is consist of a gas flow controller, a high-voltage power supply, oscilloscope, and the above mentioned APPJ device. It was powered by a 10 kHz sinusoidal power supply at 8 kV peak-to-peak voltage and the He gas flow was maintained at 2 SLM. The plasma plume could directly contact with the cell medium surface.
Optical emission spectroscopy
Optical emission spectra (OES) was detected by an Andor SR-750i grating monochromator (grating grooving 1,200 lines mm−1) within a wavelength range of 300–800 nm. The optical fiber was oriented up the APPJ radial direction at a distance of 1 and 2 cm from the APPJ nozzle.
Cell culture and plasma treatment
LP-1 MM cell line was used in this study and the details could be found in our previous work (16). Cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% foetal calf serum, 100 U/mL penicillin, and 50 µg/mL streptomycin (Corning, Ithaca, NY, USA) in an incubator (Thermo Scientific, Waltham, MA, USA) containing a humidified atmosphere of 5% CO2 at 37 °C. Cells were refreshed 24 h before performing experiments. For plasma treatment, 2×105 cells were cultured in 24 well plates in 300 µL RPMI1640 complete medium and were treated with plasma jet 1.5 cm away from the bottom of the plates. After treatment, cells were continually cultured for further experiments. Ethics approval was not required because no clinical experiments were involved in our paper.
Cell viability assay
In this study, we used CellTiter-Glo Assay kit (Promega, Madison, WI, USA) to detect the cell viability, which based on the quantitative determination of ATP in living cells. 100 µL of cells and 100 µL of Cell-Titer-Glo reagent were mixed into the opaque-walled multi-well plate, then cells were incubated at room temperature for 10 min. The luminescence was determined using the microplate reader (Thermo Scientific, USA) with the protocol of “Luminometric” measurement.
Extracellular and intracellular ROS detection
The ROS levels were monitored using CMH2-DCFDA (Invitrogen, USA) following the manufacturer’s instruction. After plasma treatment for 24 h, 10 µM of ROS dye was incubated with cells for 30 min at 37 °C. Extracellular ROS was measured with a microplate reader (Thermo Scientific) with excitation/emission at 485/530 nm using the protocol for “Fluorometric” measurement. Then, the cells were washed three times with PBS and collected for detection of intracellular ROS fluorescence by a fluorescence microscope (Olympus) with blue light motivating.
Real-time PCR analysis
Total RNA was extracted from cells by RNA kit II (Omega Bio-Tec Inc., USA) following manufacturer’s instructions and quantified with Nano Drop spectrophotometry (BioTek, USA). We used 2 µg total RNA to synthesize first strand cDNA by RevertAid first strand cDNA synthesis kit (Thermo Scientific). Real-time PCR was performed on Bio-Rad CFX Connect™ Real-time System (Bio-Rad, USA) and amplified with an optimized cycling condition: 5 min at 95 °C, then 10 s at 95 °C and 30 s at 60 °C for 38 cycles. The total reaction system was 20 µL: 10 µL 2× QuantiFast SYBR Green PCR MasterMix (Qiagen, Germany), 1 µL of cDNA templates, 0.5 µM primer and 8 µL of DNAase-RNase Free water. The primers were provided by Shenggong Company (Shanghai, China) and the sequences were used in our previous studies (8,17).
Cyp1a1 enzyme activity assay
Twenty four hours after plasma treatment for different time, cells were harvested and washed with PBS for 3 times. Then the cyp1a1 enzyme activity was measured by P450-GloTM cyp1a1 Assay (Promega, USA) according to the manufacturer’s instructions.
Statistical analysis
All values are presented as mean ± SD of three independent experiments. Differences between groups were evaluated using one-way ANOVA and student T test. P<0.05 was considered statistically significant.
Results and discussion
Generation of He plasma by plasma jet
In this study, we generated the plasma through a device modified by a needle. The profile of the plasma jet device is shown in Figure 1A. He plasma was powered by a stainless steel tube with a voltage of 10 kHz/8 kV, which was also used as He gas injection. He gas flow was regulated at a ratio of 2 SLM. Figure 1B shows the discharging image of the He plasma jet.
Detection of emission spectra
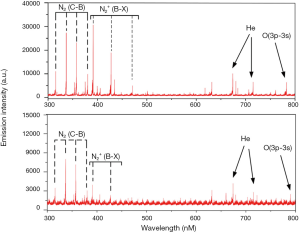
Since there are many particles with high energy states in the plasma, by detecting their characteristic lines in the emission spectroscopy, the distribution of various particles in the plasma could be understood. The optical fiber was placed 1 and 2 cm away from the plasma jet and the emission spectra was shown in Figure 2. Several characteristic lines of N, He and O were marked in the spectra, and the emission intensity is negatively correlated with the distance from the plasma jet.
Plasma enhance the sensitivity to bortezomib
Bortezomib is a first line drug for MM clinical treatment, we investigated whether gas plasma has a synergistic effect with bortezomib in MM cells. The cell cytotoxicity of different concentration of bortezomib was tested by cell viability assay 24 and 48 h after co-incubation (Figure 3A). Meanwhile, the reduction of cell viability by plasma treatment for different durations was investigated 24 and 48 h after treatment (Figure 3B). We chose 1 and 3 nM concentration of bortezomib and 30 s of plasma treatment for synergistic effects analysis. After 24 h co-treated with bortezomib and plasma, we found that 30 s of plasma treatment could both significantly enhance the sensitivity to 1 and 3 nM of bortezomib treatment (Figure 3C).

Plasma increased ROS levels
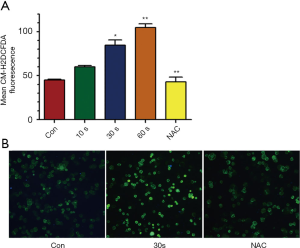
Biological effects induced by plasma treatment were mostly related to ROS generation. We detected the extracellular and intracellular ROS accumulation after plasma treatment by micro-plate reader and fluorescence microscope. It showed that the extracellular ROS level was increased by plasma treatment in a time dependent manner, while the ROS scavenger (NAC) could prevent the ROS accumulation by plasma treatment (Figure 4A). The intracellular ROS was also increased after plasma treatment as the fluorescent intensity was higher than the control and NAC could reverse it (Figure 4B).
Inhibition of Notch and cyp1a1 by plasma treatment
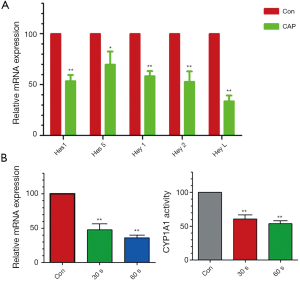
In our previous study, we have demonstrated that cyp1a1, a Cytochrome P450 enzyme for drug metabolism, is involved in bortezomib resistance in myeloma cells (8). Furthermore, Notch pathway is a critical signaling for regulating cyp1a1 activity. Therefore, we investigated whether plasma treatment had effects on these signaling. By real-time PCR we found that plasma treatment could significantly decrease the expression of Notch downstream target genes such as Hes1, Hes5, Hey1, Hey2 and HeyL (Figure 5A). Meanwhile, plasma treatment could down-regulate cyp1a1 expression in a time depend manner (Figure 5B). Besides, the enzyme activity assay showed that cyp1a1 activity was suppressed by gas plasma treatment (Figure 5C).
Conclusions
Drug resistance is one of the major problems encountered in clinical therapy of multiple myeloma treatment. Bortezomib is a first line drug used in the standard treatment and has improved clinical outcome. However, some patients do not respond to bortezomib or they eventually relapse after response (18,19). Besides, the 5 years overall survival is still not satisfied and the combination of several drugs to overcome the multiple drug resistance is applied. In this study, we first tried to couple two different kinds of treatment: the chemotherapy and the plasma treatment, to enhance the anti-tumor effects. Gas plasma is a newly developed technology that has been widely applied in biological and medical applications. We found that gas plasma treatment and bortezomib had a synergistic effect on the induction of myeloma cell death, which may lower the drug concentration to avoid the drug side effects in the future. In previous study we found that cyp1a1 up-regulated by Notch activation could induce drug resistance to bortezomib in myeloma cells (8), so we detected the Notch signaling and cyp1a1 expression after gas plasma treatment. Interesting, gas plasma treatment did suppress the Notch pathway and down-regulate the cyp1a1 expression and enzyme activity. But how the plasma interact with the Notch pathway remains unknown and it is an interesting work to be further investigated. As we know, plasma treatment induces various biological effects mostly through the generation of many ROS (11). It has been reported that Notch signaling could modulate ROS accumulation especially for H2O2 (20,21). To the contrary, ROS could also regulate Notch pathway and affected cell metabolism and cell death (22). In our results, ROS produced by gas plasma discharging could suppress the Notch downstream target genes. Kim et al. reported that ROS could down-regulate Notch signaling and induce cell death in breast cancer cells (23). Cao et al. demonstrated that inhibition of ROS production could up-regulate intracellular Notch and its downstream effectors (24). These results indicated that Notch might be a downstream target regulated by ROS. Furthermore, we found that cyp1a1, an enzyme involved in drug metabolism, was suppressed by plasma treatment induced Notch inhibition. By promoter analysis we found that there were several classical Notch CSL/RBP-J DNA binding sites (25) in the cyp1a1 promoter region (Supplementary) of either from human species or Mus musculus database, indicating that Notch could directly regulate cyp1a1 expression by modulating cpy1a1 mRNA transcription. Our results reported a new strategy to enhance the drug sensitivity by gas plasma treatment, which might be applied in clinical therapy to overcome drug resistance and to reduce side effects during chemotherapy.
In a whole, we used a new technology, the gas plasma, which could generate various ROS, to enhance the sensitivity of myeloma cells to bortezomib treatment. We further pointed out that plasma could inhibit cyp1a1 via Notch signaling and contribute to the synergistic effect with bortezomib.
Supplementary
Acknowledgments
Funding: This work is supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.10.43). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethics approval was not required because no clinical experiments were involved in our paper.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kumar SK. Management of Multiple Myeloma. J Natl Compr Canc Netw 2018;16:624-7. [Crossref] [PubMed]
- Krishnan SR, Jaiswal R, Brown RD, Luk F, Bebawy M. Multiple myeloma and persistence of drug resistance in the age of novel drugs. Int J Oncol 2016;49:33-50. [Crossref] [PubMed]
- Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia 2009;23:10-24. [Crossref] [PubMed]
- Iida S. Mechanisms of action and resistance for multiple myeloma novel drug treatments. Int J Hematol 2016;104:271-2. [Crossref] [PubMed]
- Nikesitch N, Ling SCW. Molecular mechanisms in multiple myeloma drug resistance. J Clin Pathol 2016;69:97-101. [Crossref] [PubMed]
- Garavelli S, Lazzari E, Colombo M, et al. Multiple Myeloma-Associated Drug Resistance: Targeting the Notch Pathway. Haematologica 2015;100:159.
- Milner LA. Notch signaling: a key to the pathogenesis of multiple myeloma? Blood 2004;103:3253-4. [Crossref]
- Xu D, Hu J, De Bruyne E, et al. Dll1/Notch activation contributes to bortezomib resistance by upregulating CYP1A1 in multiple myeloma. Biochem Biophys Res Commun 2012;428:518-24. [Crossref] [PubMed]
- Kong MG, Kroesen G, Morfill G, et al. Plasma medicine: an introductory review. New J Phys 2009;11:115012. [Crossref]
- Fridman G, Friedman G, Gutsol A, et al. Applied plasma medicine. Plasma Process Polym 2008;5:503-33. [Crossref]
- Graves DB. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J Phys D Appl Phys 2012;45:263001. [Crossref]
- Xu D, Liu D, Wang B, et al. In Situ OH Generation from O2- and H2O2 Plays a Critical Role in Plasma-Induced Cell Death. PloS One 2015;10:e0128205. [Crossref] [PubMed]
- Weltmann KD, Metelmann HR, von Woedtke T. Low temperature plasma applications in medicine. Europhysics News 2016;47:39-42. [Crossref]
- Bekeschus S, Schmidt A, Weltmann KD, et al. The plasma jet kINPen–A powerful tool for wound healing. Clin Plasma Med 2016;4:19-28. [Crossref]
- von Woedtke T, Reuter S, Masur K, et al. Plasmas for medicine. Phys Rep 2013;530:291-320. [Crossref]
- Xu D, Wang B, Xu Y, et al. Intracellular ROS mediates gas plasma-facilitated cellular transfection in 2D and 3D cultures. Sci Rep 2016;6:27872. [Crossref] [PubMed]
- Xu D, Hu J, Xu S, et al. Dll1/Notch activation accelerates multiple myeloma disease development by promoting CD138+MM-cell proliferation. Leukemia 2012;26:1402-5. [Crossref] [PubMed]
- Ludwig H, Beksac M, Blade J, et al. Current multiple myeloma treatment strategies with novel agents: a European perspective. Oncologist 2010;15:6-25. [Crossref] [PubMed]
- Palumbo A, Rajkumar SV. Treatment of newly diagnosed myeloma. Leukemia. 2009;23:449-56. [Crossref] [PubMed]
- Kamarehei M, Yazdanparast R. Modulation of notch signaling pathway to prevent H2O2/menadione-induced SK-N-MC cells death by EUK134. Cell Mol Neurobiol 2014;34:1037-45. [Crossref] [PubMed]
- Yu HC, Bai L, Yue SQ, et al. Notch signal protects non-parenchymal cells from ischemia/reperfusion injury in vitro by repressing ROS. Ann Hepatol 2013;12:815-21. [Crossref] [PubMed]
- Caliceti C, Nigro P, Rizzo P, et al. ROS, Notch, and Wnt Signaling Pathways: Crosstalk between Three Major Regulators of Cardiovascular Biology. Biomed Res Int 2014;2014:318714. [Crossref] [PubMed]
- Kim TH, Woo JS, Kim YK, et al. Silibinin Induces Cell Death through Reactive Oxygen Species-Dependent Downregulation of Notch-1/ERK/Akt Signaling in Human Breast Cancer Cells. J Pharmacol Exp Ther 2014;349:268-78. [Crossref] [PubMed]
- Cao Y, Fang YX, Cai JY, et al. ROS functions as an upstream trigger for autophagy to drive hematopoietic stem cell differentiation. Hematology 2016;21:613-8. [Crossref] [PubMed]
- Persson LM, Wilson AC. Wide-Scale Use of Notch Signaling Factor CSL/RBP-J kappa in RTA-Mediated Activation of Kaposi's Sarcoma-Associated Herpesvirus Lytic Genes. J Virol 2010;84:1334-47. [Crossref] [PubMed]


