
HIF2A overexpression reduces cisplatin sensitivity in cervical cancer by inducing excessive autophagy
Introduction
Cervical cancer is the second most common type of cancer among women in developing countries (1,2). Currently, patients with cervical cancer are typically treated by surgery or chemoradiotherapy (3). Cisplatin-based chemotherapy plays a key role in patients with advanced and recurrent cervical cancer. However, with gradual increase in cisplatin resistance in tumor cells, the clinical use of cisplatin is impeded for patients with cervical cancer (4). Furthermore, molecules such as proteins and non-coding RNAs can elevate the cytotoxic effects of cisplatin in cervical cancer (4,5). However, the mechanism of cisplatin resistance is not yet fully elucidated (4). Therefore, further investigation of the mechanisms underlying cisplatin resistance is required to identify additional strategies for enhancing cisplatin cytotoxicity in cervical cancer.
Tumor hypoxia, a common feature among solid tumors (6), affects the efficacy of chemotherapy (7,8). Hypoxia-inducible factors (HIFs) play key roles at the cellular as well as organismal levels in facilitating the adaptation of solid tumors to hypoxia (9). HIF family includes three proteins, HIF1, HIF2, and HIF3. Besides HIF1, accumulating evidence suggests that HIF2 contributes to promoting chemoresistance in solid tumors (10,11). HIF2 is a heterodimer consisting of α (HIF2A) and β subunits. HIF2A, also known as endothelial PAS domain protein-1 (EPAS1) or HIF2α, is a member of the PAS superfamily (MOPs). HIF2A mRNA level is 10-fold higher in cervical cancer tissues than in normal cervical tissue (12). In addition, cytoplasmic HIF2A levels have been shown to correlate with poor response to radiotherapy, and a high ratio of HIF2A-positive tumor-infiltrative macrophages has been observed to increase the risk of local recurrence (13,14). In our previous study, we showed that HIF2A suppression inhibits proliferation, induces G1-phase arrest, and promotes apoptosis in a cervical cancer cell line (CaSki) (15). Thus, we suggested an oncogenic role of HIF2A. However, its role in cisplatin cytotoxicity and the underlying mechanisms in cervical cancer are not yet clear.
Hypoxia-induced autophagy is an important factor that induces chemotherapy resistance in tumor cells (16). Autophagy is a process by which cells digest their own damaged organelles. Recently, autophagy was shown to play a role in the response mechanism of cervical cancer cells to cisplatin (4,7). Inhibition of autophagy using 3-methyladenine (3-MA) or chloroquine (CQ) has been shown to enhance cisplatin cytotoxicity (7). In addition, we showed that HIF2A silencing can suppress cell autophagy under hypoxic conditions (15). Subsequently, we suggested that HIF2A may be involved in promoting sensitivity of cervical cancer to cisplatin by inducing excessive autophagy. In the present study, we established two CaSkis stably overexpressing HIF2A to determine whether HIF2A overexpression decreases the sensitivity of cervical cancer cells to cisplatin and whether HIF2A mediates these effects by inducing excessive autophagy.
Methods
Cell culture
Two cisplatin-sensitive human CaSkis, Hela and CaSki (17,18) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Hela cells were grown in Eagle’s minimum essential medium, and CaSki cells were grown in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS; GIBCO/BRL, Gaithersburg, MD, USA), 100 U/mL penicillin G, and 100 µg/mL streptomycin (Sigma-Aldrich Corp., St. Louis, MO, USA). Cells were cultured at 37 °C in a humidified incubator with 5% CO2.
Construction of HIF2A overexpressing stable cell line
Complete open reading frame (ORF) of HIF2A (NM_001430.5) was amplified by polymerase chain reaction (PCR) using a primer pair, F, TTCGAAATGACAGCTGACAAGGAGAA and R, GGTACCTCAGGTGGCCTGGTCCAGGG, containing BstBI and Kpn1 restriction sites. Further, the amplicon was inserted into pLVX-puro plasmid, which was tagged as pLVX-HIF2A. The plasmids pLVX-HIF2A, psPAX, and pMD2G were transiently transfected into 293T cells. Lentivirus production, quantification, and titration was performed according to standard procedures. Hela and CaSki cells were infected with a negative control lentivirus (LV-NC) or a lentivirus expressing HIF2A (LV-HIF2A).
HIF2A overexpressing stable cell lines were constructed as reported by Guo et al. (19). Briefly, infected cells were passaged twice per week in complete medium (RPMI-1640 medium supplemented with 10% FBS) containing 1 µg/mL puromycin. After 2 weeks, positively screened cell lines were sub-cloned using limiting dilution and then cultured in complete medium containing puromycin for 1 month to generate stable cell lines. The stable cell lines were then bulk cultured for subsequent assays.
Animal model and tumor size measurement
Six-week-old female athymic nude mice (n=70) were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). All mice were housed in a specific pathogen-free animal house and provided free access to food and water. All animal experimental procedures were approved by Institute of Animal Care and Use Committee of First Affiliated Hospital of Gannan Medical University. After adaptive feeding for 3 days, we subcutaneously injected 3×106 cells in 0.2 mL of PBS into the right armpit region. The mice were divided into the following 12 groups: (I) Hela-LV-NC, subcutaneously injected with Hela-LV-NC cells; (II) Hela-LV-HIF2A, subcutaneously injected with Hela-LV-HIF2A cells; (III) Hela-LV-NC+cisplatin, subcutaneously injected with Hela-LV-NC cells and intraperitoneally injected with cisplatin (3 mg/kg in 0.2 mL PBS) once every 3 days; (IV) Hela-LV-HIF2A+cisplatin, subcutaneously injected with Hela-LV-HIF2A cells and intraperitoneally injected with cisplatin (3 mg/kg in 0.2 mL PBS) once every 3 days; (V) Hela-LV-HIF2A+cisplatin+3-MA, subcutaneously injected with Hela-LV-HIF2A cells and intraperitoneally injected with cisplatin and 3-MA (3 mg/kg cisplatin and 2 mg/kg 3-MA in 0.2 mL PBS) once every 3 days; (VI) CaSki-LV-NC, subcutaneously injected with CaSki-LV-NC cells; (VII) CaSki-LV-HIF2A, subcutaneously injected with CaSki-LV-HIF2A cells; (VIII) CaSki-LV-NC+cisplatin, subcutaneously injected with CaSki-LV-NC cells and intraperitoneally injected with cisplatin (3 mg/kg in 0.2 mL PBS) once every 3 days; (IX) CaSki-LV-HIF2A+cisplatin, subcutaneously injected with CaSki-LV-HIF2A cells and intraperitoneally injected with cisplatin (3 mg/kg in 0.2 mL PBS) once every 3 days; (X) CaSki-LV-HIF2A+cisplatin+3-MA, subcutaneously injected with CaSki-LV-HIF2A cells and intraperitoneally injected with cisplatin and 3-MA (3 mg/kg cisplatin and 2 mg/kg 3-MA in 0.2 mL PBS) once every 3 days; (XI) Hela- or CaSki-PBS, subcutaneously injected with Hela or CaSki cells and intraperitoneally injected with PBS (0.2 mL) once every 3 days; and (XII) Hela- or CaSki-cisplatin, subcutaneously injected with HeLa or CaSki cells and intraperitoneally injected with cisplatin (3 mg/kg in 0.2 mL PBS) once every 3 days. Tumor size was measured using calipers, and tumor volume was calculated using the formula, ½ × L × W2, where L is the length and W is the width of the tumor. Four weeks after treatment, mice were killed by cervical dislocation, and tumor tissues were removed and weighed. After washing with PBS, one half of each tissue specimen was fixed with 4% paraformaldehyde while the other half was frozen in liquid nitrogen.
TUNEL assay
TUNEL assay was performed using the In Situ Cell Death Detection Kit (Roche Molecular Biochemicals, Mannheim, Germany) and diaminobenzidine tetrahydrochloride (DAB) chromogen according to the manufacturer’s protocol. Apoptotic cells that stained yellow/brown were counted in 10 random fields per section at 200× magnification using a light microscope (Carl Zeiss, Jena, Germany).
Western blotting
Total protein was extracted from tumor tissues using pre-chilled RIPA buffer (Beyotime, Shanghai, China). Equal amounts of protein per sample were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes. After blocking in 5% non-fat milk at 25 °C for 2 h, the membranes were incubated with anti-human HIF2A polyclonal antibody (1:1,000; Abcam, Cambridge, UK), anti-human autophagy related 5 (ATG5) polyclonal antibody (1:1,500; Abcam), anti-human beclin 1 polyclonal antibody (1:1,000; Abcam), anti-human BCL2-associated X (BAX) polyclonal antibody (1:10,000; Abcam), anti-human B-cell lymphoma 2 (BCL2) polyclonal antibody (1:4,000; Abcam), or anti-human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) polyclonal antibody (1:10,000; Abcam) overnight at 4 °C. After washing with PBS containing 0.1% Tween-20 (PBST), membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:10,000) at 25 °C for 1 h. Afterward, the membranes were rinsed, and protein bands were visualized using an enhanced chemiluminescence (ECL) reagent (Thermo Scientific, Rockford, IL, USA). GAPDH was used as a loading control.
Statistical analysis
Statistical Package for Social Science (SPSS) 19.0 software package (IBM, Chicago, IL, USA) was used for statistical analyses. Data are presented as mean ± standard deviation (SD) and Student’s t-test or one-way analysis of variance (ANOVA) was used to compare the differences between two or more groups. Differences with P values <0.05 were considered statistically significant.
Results
HIF2A overexpression promotes cell growth in vivo
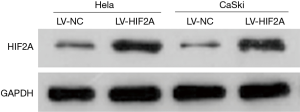
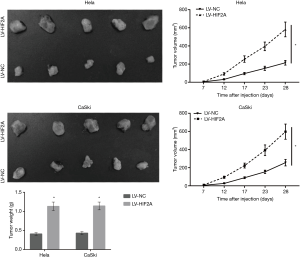
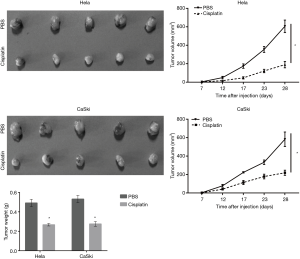
Western blot analysis showed that HIF2A was markedly overexpressed in LV-HIF2A-infected Hela and CaSki cells than in cells infected with LV-NC (Figure 1). This indicated that stable cell lines were successfully constructed with consistent HIF2A expression. Further, we examined the effects of HIF2A overexpression on tumor growth in nude mice. Each of the four stable cell lines were injected subcutaneously into the flanks of nude mice. We found that, from 12 to 28 days after injection, nude mice injected with cells overexpressing HIF2A generated larger tumors than those in mice injected with LV-NC-infected cells (Figure 2). Average tumor volume of the LV-HIF2A group for Hela and CaSki cells at 28 days was significantly larger than that of the LV-NC group (Hela: 582.388±81.534 vs. 214.618±25.754 mm3, P<0.001; CaSki: 597.092±83.592 vs. 255.35±35.749 mm3, P<0.001). Subsequently, tumor tissues obtained from LV-HIF2A-infected mice were found to be heavier than those from LV-NC-infected-mice from 12 to 28 days after injection (Figure 2). Furthermore, average tumor weight in the LV-HIF2A group for Hela and CaSki cells at 28 days was significantly more than that in the LV-NC group (Hela: 1.13±0.11 vs. 0.41±0.03 g, P<0.001; CaSki: 1.14±0.10 vs. 0.43±0.04 g, P<0.001).
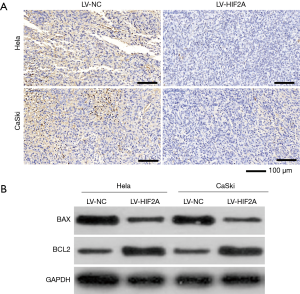
HIF2A overexpression suppresses apoptosis in vivo
TUNEL assay revealed fewer apoptotic cells in tumor tissues from the LV-HIF2A group than from the LV-NC group (Figure 3A). Additionally, expression of the pro-apoptotic protein, BAX, was found to be lower in tumor tissues from the LV-HIF2A group than from the LV-NC group (Figure 3B). Moreover, expression of the anti-apoptotic protein, BCL2, was higher in the LV-HIF2A group than in the LV-NC group (Figure 3B).
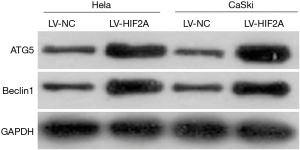
HIF2A overexpression promotes expression of ATG5 and beclin 1 in vivo
Western blot analysis showed that expression of autophagy-related proteins, ATG5 and beclin 1, was higher in tumor tissues from the LV-HIF2A group than from the LV-NC group (Figure 4).
HIF2A overexpression reduces cisplatin sensitivity but is alleviated by 3-MA
As shown in Figure 5, injecting 3 mg/kg cisplatin once every 3 days could effectively reduce the size and weight of subcutaneous tumors. The data illustrated in Figure 6 suggest that nude mice injected with cells overexpressing HIF2A generated larger tumors than those in mice injected with LV-NC-infected cells with intraperitoneally cisplatin injection. Similarly, tumor tissues from the LV-HIF2A+cisplatin group were heavier than those from the LV-NC+cisplatin group (Figure 6). Furthermore, TUNEL assay showed lower number of apoptotic cells in tumor tissues of the LV-HIF2A group than the LV-NC group (Figure 7A). BAX expression was found to be lower in tumor tissues of the LV-HIF2A+cisplatin group than of the LV-NC+cisplatin group (Figure 7B). Moreover, western blotting data showed that BCL2 expression was higher in the LV-HIF2A+cisplatin group than in the LV-NC+cisplatin group (Figure 7B). Subsequently, the expression of ATG5 and beclin 1 was higher in tumor tissues from LV-HIF2A+cisplatin than LV-NC+cisplatin mice (Figure 7B).
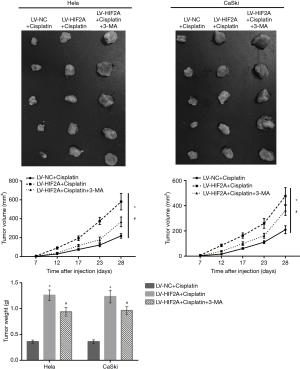
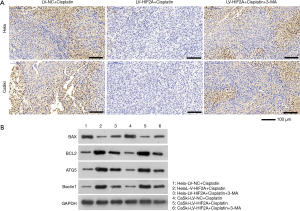
To further investigate whether HIF2A reduces cisplatin sensitivity by inducing autophagy, LV-HIF2A-infected Hela and CaSki cells were treated with 3-MA. Western blot analysis showed that 3-MA evidently decreased the expression of HIF2A overexpression-induced autophagy-related proteins, ATG5 and beclin 1, in mice treated with cisplatin (Figure 7B). This suggested that HIF2A overexpression-induced autophagy was significantly blocked by 3-MA. In addition, tumors of LV-HIF2A+cisplatin+3-MA-treated mice were observed to be smaller than those of LV-HIF2A+cisplatin-treated mice (Figure 6). Tumors from LV-HIF2A+cisplatin+3-MA-treated mice were found to weigh less than those from LV-HIF2A+cisplatin-treated mice (Figure 6). Moreover, the number of apoptotic cells was more in tumor tissues from the LV-HIF2A+cisplatin+3-MA group than from the LV-HIF2A+cisplatin group (Figure 7A). BAX expression was higher while BCL2 expression was lower in tumor tissues from the LV-HIF2A+cisplatin+3-MA group than from the LV-HIF2A+cisplatin group (Figure 7B).
Discussion
Cisplatin is a major chemotherapeutic drug for the treatment of advanced or recurrent cervical cancer. However, resistance to cisplatin significantly compromises the efficacy of the drug in treating the disease. Hypoxia induces cisplatin resistance in solid tumors (7,8,20). HIFs are major regulators for facilitating adaptation to hypoxia, and are also observed to be associated with chemotherapy failure in many types of solid tumors (21). Thus, HIFs may be suitable targets to overcome cisplatin resistance or increase cisplatin sensitivity in cervical cancer. However, such investigations have not been verified in vivo. To our knowledge, this is the first study to investigate the role and underlying mechanisms of HIF2A in mediating cisplatin cytotoxicity in cervical cancer in vivo.
In our previous study, HIF2A expression was observed to increase under hypoxic conditions, while HIF2A suppression inhibited proliferation and autophagy, and promoted apoptosis (15). Our data suggested that HIF2A functions as an oncogene in cervical cancer cells in vitro. Subsequently, in our present study, we investigated the in vivo role of HIF2A using cervical cancer cells stably overexpressing HIF2A. HIF2A overexpression was found to increase the size and weight of subcutaneous tumors indicating that HIF2A overexpression promotes cell growth in vivo. Furthermore, HIF2A overexpression was found to decrease number of apoptotic cells and expression of BAX but increase the expression of BCL2 in subcutaneous tumors. Thus, our data showed that HIF2A overexpression suppresses apoptosis in vivo. The results of the present in vivo study are consistent with the results of our previous in vitro study. Overall, our results suggested that HIF2A functions as an oncogene in cervical cancer cells. Thus, we suggest that suppressing HIF2A expression should be the focus of future research to discover new drugs for treating cervical cancer.
Cisplatin is a widely used platinum-containing chemotherapeutic drug. Cisplatin-induced apoptosis is considered to be major mechanism of cisplatin-based chemotherapy (22). We found that HIF2A overexpression promotes cell growth and suppresses apoptosis in mice intraperitoneally injected with cisplatin. Furthermore, Hela and CaSki are cisplatin-sensitive cells (17,18), and the cisplatin dose used in this study is known to remarkably reduce the size and weight of subcutaneous tumors. All these results suggested that HIF2A overexpression reduces cisplatin sensitivity in cervical cancer cells in vivo. To date, several studies investigating solid tumors for the association between drug resistance and HIFs under hypoxic conditions have mainly focused on HIF-1. Although there is no direct evidence to prove that HIF2A is involved in cisplatin resistance, an increasing number of recent studies have suggested that HIF2A may play an important role in inducing drug resistance in solid tumors. In head-and-neck squamous cell carcinoma, high HIF2A expression is associated with an incomplete response to chemoradiation (23). In hepatocellular carcinoma, HIF2A knockdown by siRNA enhances the efficacy of doxorubicin (24). All these studies support the idea that HIF2A may be involved in inducing drug resistance and may even be a new target to overcome cisplatin resistance.
Beclin 1 and ATG5 are autophagy-related proteins (25). Beclin 1 is a critical component of the class III phosphatidylinositol-3-kinase (PtdIns3K) complex that triggers autophagy in mammalian cells (26,27), while ATG5, an E3-like ligase, is involved in autophagic vesicle formation (27). We found that HIF2A overexpression upregulated both beclin 1 and ATG5 expression in vivo, which suggested that elevated HIF2A levels can trigger autophagy. These results are consistent with the results of our earlier in vitro study, in which HIF2A knockdown suppressed autophagy under hypoxic conditions (15). Additionally, we found that HIF2A overexpression upregulated both beclin 1 and ATG5 expression in mice when intraperitoneally injected with cisplatin. The data suggested that elevated HIF2A levels can also trigger autophagy during cisplatin treatment. Together with its role in tumor growth and apoptosis, we predicted that HIF2A overexpression might trigger excessive autophagy in cervical cancer cells to promote cell growth and thereby suppress apoptosis during cisplatin treatment. Our hypothesis was supported by the following results. The effect of HIF2A overexpression on tumor growth and apoptosis was alleviated in vivo during cisplatin treatment in the presence of 3-MA. Previous studies have also shown that autophagy plays a role in the response mechanism of cervical cancer cells to cisplatin (4,7). Thus, inhibiting excessive autophagy can be one approach to increase cisplatin sensitivity. Likewise, our results suggest that HIF2A may be a new target to inhibit excessive autophagy, which is induced by the hypoxic microenvironment in solid tumors.
Our study had a few limitations. At first, the relationship between HIF2A expression and cisplatin resistance was not investigated in clinical samples of cervical cancer tissues. Secondly, the mechanism underlying HIF2A-induced excessive autophagy was not studied. We plan to focus on these issues in our future investigations.
In conclusion, we reveal that HIF2A overexpression promotes tumor growth and autophagy but suppresses apoptosis in vivo in the presence or absence of cisplatin. The effect of HIF2A overexpression on tumor growth and apoptosis in vivo can be alleviated by 3-MA, with cisplatin treatment. Thus, our results suggest that HIF2A overexpression might reduce cisplatin sensitivity in cervical cancer by inducing excessive autophagy. HIF2A may be considered a new target to increase cisplatin sensitivity by inhibiting hypoxia microenvironment-induced excessive autophagy in solid tumors.
Acknowledgments
Funding: This study is supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.11.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived. All animal experimental procedures were approved by Institute of Animal Care and Use Committee of the Army Medical University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Islami F, Siegel RL, et al. Global Cancer in Women: Burden and Trends. Cancer Epidemiol Biomarkers Prev 2017;26:444-57. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Small W Jr, Bacon MA, Bajaj A, et al. A global health crisis. Cancer 2017;123:2404-12. [Crossref] [PubMed]
- Zhu H, Luo H, Zhang W, et al. Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Des Devel Ther 2016;10:1885-95. [Crossref] [PubMed]
- Abu N, Hon KW, Jeyaraman S, et al. Long noncoding rnas as biotargets in cisplatin-based drug resistance. Future oncology (London, England) 2018;14:3085-95. [Crossref] [PubMed]
- Gray LH, Conger AD, Ebert M, et al. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 1953;26:638-48. [Crossref] [PubMed]
- Xu Y, Yu H, Qin H, et al. Inhibition of autophagy enhances cisplatin cytotoxicity through endoplasmic reticulum stress in human cervical cancer cells. Cancer Lett 2012;314:232-43. [Crossref] [PubMed]
- Teicher BA. Hypoxia and drug resistance. Cancer Metastasis Rev 1994;13:139-68. [Crossref] [PubMed]
- Welsh SJ, Powis G. Hypoxia inducible factor as a cancer drug target. Curr Cancer Drug Targets 2003;3:391-405. [Crossref] [PubMed]
- Zhao J, Du F, Luo Y, et al. The emerging role of hypoxia-inducible factor-2 involved in chemo/radioresistance in solid tumors. Cancer Treat Rev 2015;41:623-33. [Crossref] [PubMed]
- Ghattass K, Assah R, El-Sabban M, et al. Targeting hypoxia for sensitization of tumors to radio- and chemotherapy. Curr Cancer Drug Targets 2013;13:670-85. [Crossref] [PubMed]
- Zhang L, Chen Q, Hu J, et al. Expression of HIF-2alpha and VEGF in Cervical Squamous Cell Carcinoma and Its Clinical Significance. Biomed Res Int 2016;2016:5631935. [PubMed]
- Kawanaka T, Kubo A, Ikushima H, et al. Prognostic significance of HIF-2alpha expression on tumor infiltrating macrophages in patients with uterine cervical cancer undergoing radiotherapy. J Med Invest 2008;55:78-86. [Crossref] [PubMed]
- Kim MK, Kim TJ, Sung CO, et al. Clinical significance of HIF-2alpha immunostaining area in radioresistant cervical cancer. J Gynecol Oncol 2011;22:44-8. [Crossref] [PubMed]
- Jiang L, Shi S, Shi Q, et al. Similarity in the functions of HIF-1alpha and HIF-2alpha proteins in cervical cancer cells. Oncol Lett 2017;14:5643-51. [PubMed]
- Wu HM, Jiang ZF, Ding PS, et al. Hypoxia-induced autophagy mediates cisplatin resistance in lung cancer cells. Sci Rep 2015;5:12291. [Crossref] [PubMed]
- Zhang X, Pan C, Zhou L, et al. Knockdown of st6gal-i increases cisplatin sensitivity in cervical cancer cells. BMC Cancer 2016;16:949. [Crossref] [PubMed]
- Subramanian PD, An Z, Yu JR, et al. Silencing of fused toes homolog enhances cisplatin sensitivity in cervical cancer cells by inhibiting epidermal growth factor receptor-mediated repair of DNA damage. Cancer Chemother Pharmacol 2016;78:753-62. [Crossref] [PubMed]
- Guo H, Xia B. Collapsin response mediator protein 4 isoforms (CRMP4a and CRMP4b) have opposite effects on cell proliferation, migration, and invasion in gastric cancer. BMC Cancer 2016;16:565. [Crossref] [PubMed]
- Zhao W, Xia SQ, Zhuang JP, et al. Hypoxia-induced resistance to cisplatin-mediated apoptosis in osteosarcoma cells is reversed by gambogic acid independently of HIF-1alpha. Mol Cell Biochem 2016;420:1-8. [Crossref] [PubMed]
- Rohwer N, Cramer T. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat 2011;14:191-201. [Crossref] [PubMed]
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 2014;740:364-78. [Crossref] [PubMed]
- Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int J Radiat Oncol Biol Phys 2002;53:1192-202. [Crossref] [PubMed]
- He C, Sun XP, Qiao H, et al. Downregulating hypoxia-inducible factor-2alpha improves the efficacy of doxorubicin in the treatment of hepatocellular carcinoma. Cancer Sci 2012;103:528-34. [Crossref] [PubMed]
- Ashkenazi A, Bento CF, Ricketts T, et al. Polyglutamine tracts regulate beclin 1-dependent autophagy. Nature 2017;545:108-11. [Crossref] [PubMed]
- He R, Peng J, Yuan P, et al. Divergent roles of BECN1 in LC3 lipidation and autophagosomal function. Autophagy 2015;11:740-7. [Crossref] [PubMed]
- Zhong Y, Wang QJ, Li X, et al. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol 2009;11:468-76. [Crossref] [PubMed]


