
Immunomodulatory features of radiotherapy in lung carcinoma
Introduction
As a standard treatment modality, radiotherapy (RT) has been widely employed for cancer treatment. In addition to its direct inhibition of tumor cells, RT has been long known for its immunomodulatory effects. Therapeutic ionized irradiation includes whole-body and local types according to exposure range; low, intermediate, and high-dose types based on radiation doses; and conventional high-dose and low-fractionated radiotherapies according to treatment regimen; each type was exerting different effects on the immune system. Here, we focused on conventional high-dose and low-fractionated radiation for solid cancers and analyzed their immunomodulatory effects. Studies have shown that (1,2) RT induces immunogenic cell death (3), releasing large amounts of cancer antigens or other dangerous substances (4) for antigen-presenting cell (APC) priming (5) and inducing specific anticancer immune reactions, reflected by DC activation and/or increased CTL cell amounts (6) as well as the “abscopal effect” after RT (7). Such specific events observed upon radiation-associated cancer cell inhibition have promoted the use of RT as a technique of “in situ immunization” (8). It has been demonstrated that even relatively low-dose RT without inducing cell toxicity can also increase the amounts of MHC class I molecules on tumor cells,which can, in turn, augment antitumor immunity (9). Secondly, RT non-specifically stimulates or activates the immune system by the ionizing irradiation itself or via cancer tissue injury after exposure to irradiation, i.e., through non-specific T cell, NK cell, macrophage, and mast cell responses (10). Thirdly, RT drastically decreases cancer burden and subsequently reduces immune tolerance, killing immuno-resistant clones generated through selective pressure during immunoediting, as radiation-associated tumor cell inhibition makes no distinction between cells based on their susceptibility/resistance to immunity, which results in more efficient immune-mediated inhibition of residual cancer clonogens (11). Moreover, ionized irradiation equally kills local immunotolerant immune cells surrounding the lesion, further destroying the immunotolerant cancer fortress and blocking suppressive factors from tumor sojourners, including tumor-associating macrophages, and infiltrating immunosuppressive immune cells (12,13). Despite the above phenomena being well-acknowledged, the effects of ionizing radiation on the immune system remain unclear, and the reported results are controversial. Merrick and colleagues reported that RT induces a “tolerogenic” phenotype by reducing IL-12 production in mature human DCs in vitro, resulting in inhibited priming of naïve CD8+ T cells; meanwhile, adoptive DC transfer into tumors combined with RT and chemotherapy in vivo has been shown to lead to enhanced total cancer regression (14), and macrophages exert anti- or pro-cancer effects based on the immune mediators in the microenvironment (15). In addition, how to augment RT responsiveness by using immune-therapeutics designed to enhance anti-cancer immunity has been studied (16,17). However, no report has discussed the potential negative effects of the treatment on antitumor immunity,
This study performed dynamic monitoring of RT-associated immunomodulation by concomitant assessment of peripheral blood lymphocytes, Th1/Th2 cells, Tc1/Tc2 cells, along with mRNA amounts of multiple immune factors including CD25, CD28, CTLA-4, PD-1, Foxp3, TGF-β, and IL-10. The results provided important insights into the immunomodulatory features of radiation for cancer.
Methods
Blood sample preparation
Thirty patients with lung cancer were enrolled in this study. The patients’ characteristics are listed in Table S1. Blood specimen collection was performed for each patient pre- and post-RT. The study received approval from the Human Ethics Committee of State Key Laboratory of Experimental Hematology, Fifth Medical Center of Chinese PLA General Hospital, Beijing, China, and signed informed consent was provided by every patient. Blood samples (8 mL per patient; 4 mL each placed into heparinized and EDTA anticoagulant tubes) were obtained on days −3 to 0 pretreatment and immediately post-treatment, for flow-cytometry and RT-PCR.
Cellular immune cell analysis
Changes of cellular immune reactions were assessed by evaluating T-and B-lymphocytes, NK cells, Treg, along with other main lymphocyte types with peripheral blood flow cytometry (EPICS XL; Beckman Coulter Inc., USA). Fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-Cy5-, PerCP-, allo-phycocyanin (APC)- and PE-Texas Red (ECD)-conjugated antibodies targeting CD45, CD3, CD4, CD8, CD56, CD19, CD25, and CD28 were purchased from BD Biosciences (USA), and employed for staining as directed by the manufacturer.
Quantitative polymerase chain reaction (RT-qPCR)
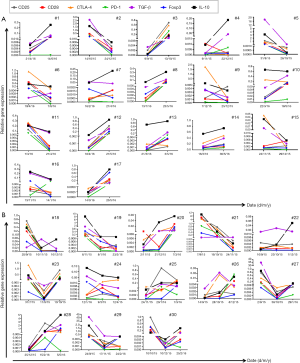
CD25, CD28, CTLA-4, PD-1, Foxp3, TGF-β, and IL-10 mRNA amounts were assessed by RT-qPCR using a specific kit from Beijing Mo Li Tai Bio-Technology (cat. no. 201411; China), as directed by the manufacturer. Briefly, lymphocytes were prepared by centrifugation (400 ×g for 5 minutes) from peripheral blood incubated with red blood cell lysis solution (cat. no. 21510, Dong Fang Hua Hui Biomedical Technology, Beijing, China) as directed by the manufacturer. Total RNA isolation was performed with a kit manufactured by Thermo Fisher Scientific (cat no. 15596-026; China) based on the included protocol. Reverse transcription was carried out with reagents from Promega (cat. no. A3500; USA) as directed by the manufacturer. Amplification was carried out on an AB 7500 Real-Time PCR System (Applied Biosystems, China) for 40 cycles. GraphPad-Prism was employed for analysis (Figure 1).
RT
CyberKnife and intensity-modulated radiation therapy (IMRT) were used routinely, based on indications of the patients and their diseases. Total radiation doses varied from 32.5 to 37.5 Gy for CyberKnife; they were delivered in 5 fractions, which were completed within 7 days, with 60–70 Gy for IMRT, delivered in 30–35 fractions of 2 Gy (5 fractions/week).
Results
Effects of radiation on lymphocyte population
Lymphocytes were assessed by cytometry at pretreatment and immediately post-RT in 30 patients. In addition, 13 cases were further evaluated for lymphocyte subpopulations 1 month after IMRT or 2 months after CyberKnife therapy. Lymphocyte subpopulations, including CD3, CD4, CD8, B, NK, Treg, Ts, and CTL cells, were increased or decreased during RT. The most interesting alterations included a prominent reduction of B cells and increases in CD8 and Ts subpopulations. B cells were reduced in 28/30 patients (1.25% to 13.85%, 5.43% in average) with variations >5% in 13 cases. CD8 cells were increased in 22/30 patients (0.2% to 39.2%, 59.22% in average) with variations >5% in 13 cases. Ts cells were increased in 24/30 patients (0.1% to 24.49%, 9.16% in average) with variations >5% in 15 cases. The smallest effect of radiation was observed in the Treg subpopulation in comparison with the other subpopulations. CD3, CD4, NK, and CTL subpopulations were increased or decreased in about half of the cases (Tables 1,S2). As for the associations of the lymphocyte subpopulations, the CD8 increase was highly correlated with Ts cell increase, while B cell reduction was correlated with CD4, CD8, or NK cell changes. Six of the 7 cases with >10% CD8 increase after RT had Ts elevation >10%, whereas the remaining patient had a Ts elevation of 9.37%. Six of the 9 cases with >10% Ts increase after RT had CD8 elevation >10%, whereas 1 case had a CD8 increase of 9.7%, and the remaining two cases had significant CTL elevation (Table S2).
Table 1
| Lymphocyte subpopulations | After radiotherapy | Variation range (%) | Varieties >5% | |
|---|---|---|---|---|
| Up-regulation | Down-regulation | |||
| CD3+ T | 18/30 (average 8.64%) | 11/30 (average 5.09%) | 0–21.5 | 14/30 |
| CD4+ T | 13/30 (average 5.1%) | 17/30 (average 9.92%) | 0.4–35.52 | 16/30 |
| CD8+ T | 22/30 (average 9.22%) | 6/30 (average 2.97%) | 0.2–39.2 | 13/30 |
| B | 2/30 (average 1.12%) | 28/30 (average 5.43%) | 1.25–13.85 | 13/30 |
| NK | 16/30 (average 5.06%) | 13/30 (average 4.94%) | 0–24.4 | 11/30 |
| Treg | 15/30 (average 0.91%) | 15/30 (average 1.14%) | 0.03–3.05 | 0/30 |
| Ts | 24/30 (average 9.16%) | 6/30 (average 3.39%) | 0.1–24.49 | 15/30 |
| CTL | 16/30 (average 2.78%) | 13/30 (average 3.94%) | 0–10.85 | 8/30 |
Effects of radiation on Th1/Th2 balance
Radiation had significant effects on Th1/Th2 and Tc1/Tc2 balances. Among all CD4 cells, Th1 and Th2 cell amounts increased upon RT from −47.84% to +29.28%, and from −3.59% to +15.49%, respectively; meanwhile, in the CD8 population, Tc1 and Tc2 cell levels rose from −64.82% to +36.87%, and from −4.12% to +5.13%, respectively. Variations >5% in CD4 cells were found in 18/30 of the Th1 population and 2/30 of the Th2 population; such variations in the CD8 population were recorded in 25/30 and 1/30 of the Tc1 and Tc2 populations, respectively. The Th1, Th2, Tc1, and Tc2 populations were decreased in 17/30 (11.58% average), 12/30 (1.19% average), 18/30 (30.49% average), and 12/30 (1.19% average) of patients, respectively, and elevated in 13/30 (8.49% average), 18/30 (3.14% average), 12/30 (8.31% in average), and 18/30 (1.87% average) of patients, respectively (Tables 2,S3). These results suggest that radiation exerted greater effects on Th1 and Tc1 cells compared with Th2 and Tc2 cells. However, although fluctuation ranges for Th1 and Tc1 cells were larger, the reduction rates of Th1 and Tc1 cells and the elevated rates of Th2 and Tc2 cells were much higher, suggesting a shift towards Th2 and Tc2 responses after RT. Interestingly, elevation or decrease of Th1, Th2, Tc1, and Tc2 showed no associations with CD3, CD4, CD8, NK, Ts, or CTL fluctuations, suggesting combined effects of the above cell subpopulations.
Table 2
| Lymphocyte subpopulations | After radiotherapy | Variation range (%) | Varieties >5% | |
|---|---|---|---|---|
| Up-regulation | Down-regulation | |||
| Th1 | 13/30 (average 8.49%) | 17/30 (average 11.58%) | 0.99–47.84 | 18/30 |
| Th2 | 18/30 (average 3.14%) | 12/30 (average 1.19%) | 0.13–15.49 | 2/30 |
| Tc1 | 12/30 (average 8.31%) | 18/30 (average 30.49%) | 0.5–64.82 | 25/30 |
| Tc2 | 18/30 (average 1.87%) | 12/30 (average 1.19%) | 0.01–5.13 | 1/30 |
Effects of radiation on CD25, CD28, PD-1, CTLA-4, Foxp3, TGF-β, and IL-10 gene expression levels
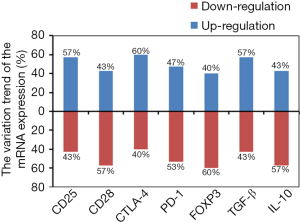
All 30 lung cancer cases were assessed for gene expression in peripheral blood (Figure 1). Of the 30 cases observed, upregulation of the CD25, CD28, CTLA-4, PD-1, Foxp3, TGF-β, and IL-10 genes after RT was found in 17 (57%), 13 (43%), 18 (60%), 14 (47%), 12 (40%), 17 (57%), and 13 (43%) patients, respectively, whereas downregulation was recorded in 13 (43%), 17 (57%), 12 (40%), 16 (53%), 18 (60%), 13 (43%) and 17 (57%), respectively (Figure 2). The up- and down-regulations of genes in various cases were random and individualized, but mainly based on initial expression amounts; i.e., the highly regulated initial expression would result in down-regulation upon RT, and vice versa. In the 13 cases assessed one month after IMRT or two months after CyberKnife, the trends of various parameters right after RT were largely maintained to the third measurement time point, with the lowest signals altering their modulation trends and amplitudes (Figure 1B). Meanwhile, Th1/Th2 and Tc1/Tc2 balance alterations were seemingly associated with Tc1 and Tc1/Tc2 but not Th1/Th2 balance.
Discussion
The present study dynamically assessed cellular immunity during/after RT in 30 patients with lung carcinoma. The major finding was a pronounced reduction of the B lymphocyte population in 28/30 patients (5.43% average), indicating an obvious shift of the immune function from humoral to cellular immunity. Although the exact mechanism remains unclear, it is hard to believe that the reduction of the B cell population was caused by a direct inhibition of local radiation on the B lineage, but rather a passive response to the increased CD8, NK, or CTL populations, as radiation was strongly restricted to the local tumor site. The significant elevations of the CD8 and Ts populations constituted another notable feature of the immune regulation mediated by local RT. CD8 cell increase along with elevated Th2 and Tc2 responses were largely a response of the immune system to direct stimulation by local radiation and the damaged tumor tissue. As there were no significant Th1 and Tc1 increases, in addition to the short observation period, specific anti-tumor immunity could be ruled out. The above results corroborated our previous findings that in comparison with immunomodulation therapy, RT has a direct stimulatory effect on the immune system without profound immunomodulation (18,19). The significant increase of the Ts population could be attributed to increased CD8 cells along with Th2 and Tc2 responses, representing a feedback response to the latter. A stimulatory response of the immune system to local radiation reflected by elevated CD8 population and Th2 and Tc2 responses indicates that such an outcome could be beneficial for initial antitumor immunity but may not last, as Th1 and Tc1 responses are more potent for long-term antitumor immunity. This should be considered carefully and be given due attention when designing subsequent therapeutics.
Therefore, as we cannot expect to cure cancer with a single drug or regimen, combining immunomodulatory therapeutics might be of great significance. RT exhibits a dramatic cancer-cell-killing effect and immunomodulatory potential, but the latter is complex and personalized with both positive and negative effects on long-term antitumor immunity. If the immunomodulatory mechanism mediated by RT is well understood and the immune status is precisely evaluated after RT, jointly applying selective immunomodulatory treatment (immunoediting therapy) or other immune-therapeutics (e.g., immune checkpoint blockers) (20,21) could help selectively maintain positive anticancer responses, correct the imbalance mediated by cancer treatments, and maintain a balanced cellular immunity, for optimal antitumor benefits.
In the simultaneous analysis of immunoregulatory signals, local radiation caused overt regulation of mRNA expression in 7 signals at almost each measurement time point after treatment, and regulation trends greatly depended on initial expression levels. However, associations of mRNA amounts with lymphocyte subpopulation, Th1/Th2, and Tc1/Tc2 responses were not assessed. Nevertheless, the modulation trends of mRNA amounts of these immune factors were largely maintained, along with those of the lymphocyte subpopulations, after 1 month of IMRT or 2 months of CyberKnife with a slight kick-back. These findings suggest that the out-sync of gene expression of the above immune factors and cell responses might be attributed to different time-windows of gene expression and cellular functions; however, the association of mRNA regulation after stimulation by ionized irradiation with pronounced immunoregulation deserves further assessment. Through comparing the effects of RT on the mRNA levels of the above immune factors between CyberKnife and IMRT, and between adenocarcinoma and squamous cell carcinoma groups, it was found that the immunomodulation trends were different between different treatments and between different cancer types. The fact that CyberKnife treatment largely downregulated TGF-β and IL-10 while IMRT-mediated downregulation of these factors implies a differential action of ionizing irradiation on antitumor immunity. This calls for different strategies of immunomodulation therapy to be applied after RT, especially IMRT, as elevated TGF-β and IL-10 amounts in cancer patients generally reflect a poor prognosis. It is believed that peripheral TGF-β and IL-10 expression mainly comes from inhibitory immune cells derived from tumor in cancer patients, and CyberKnife treatment could be more potent in initially killing such cells in the tumor environment than IMRT. Finally, CTLA-4 and PD-1 were downregulated after RT in patients with squamous cell carcinoma and upregulated upon RT in patients with adenocarcinoma; a large cohort is required for further verification of these trends (Figure S1).
Conclusions
In summary, RT mediated immunomodulation in cancer patients is characterized by a shift from humoral to cellular immunity and significant elevations of CD8 and Ts subpopulations and Th2 and Tc2 responses, indicating an immuno-activating response, which could initially benefit anticancer immunity but may not have an enduring effect. These findings suggest that subsequent immunomodulatory treatment upon RT could help recover the immune balance, maintaining Th1, and Tc1 dominant immune responses for long-term anticancer immunity.
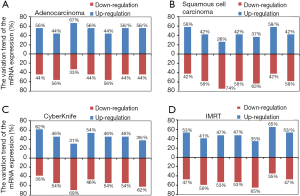
Table S1
| Characteristics | Cases |
|---|---|
| Age (years) | |
| Median | 63 |
| Range | 46–78 |
| Sex | |
| Male | 22 |
| Female | 8 |
| Smoking status | |
| Never-smoker | 10 |
| Smoker | 20 |
| Performance status | |
| 0 | 3 |
| 1or 2 | 27 |
| Pathological type | |
| Adenocarcinoma | 9 |
| Squamous cell carcinoma | 19 |
| No data | 2 |
| c-stage | |
| IIA | 2 |
| IIIA | 7 |
| IB | 3 |
| IIB | 2 |
| IIIB | 7 |
| IV | 9 |
| TNM | |
| T2N0M0 | 3 |
| T2N0M1 | 1 |
| T2N2M0 | 1 |
| T3N0M0 | 1 |
| T3N3M1 | 2 |
| T2N1M0 | 1 |
| T2N3M0 | 1 |
| T2aN2M0 | 2 |
| PT2N2M0 | 1 |
| T3N2M0 | 1 |
| T3N3M0 | 1 |
| T4N2M1 | 1 |
| T4N1M0 | 1 |
| T4N2M0 | 3 |
| T4N3M1 | 1 |
| T3N2M1 | 2 |
| pT3N1M0 | 1 |
| T1N0M1 | 1 |
| T1bN1M0 | 1 |
| T1bN2M0 | 1 |
| T4N3M0 | 2 |
| T4N3M1 | 1 |
| Treatment | |
| Intensity modulated radiation therapy (IMRT) | 17 |
| CyberKnife | 13 |
| Response | |
| Complete response | 1 |
| Partial response | 21 |
| Stable disease | 7 |
| Progressive disease | 0 |
| Not evaluable | 1 |
Table S2
| Patients | Date (d/m/y) | T (%) | CD4+ T (%) | CD8+ T (%) | B (%) | NK (%) | Treg (%) | Ts (%) | CTL (%) |
|---|---|---|---|---|---|---|---|---|---|
| #1 | 2022/4/16 | 62.30 | 41.30 | 21.10 | 9.06 | 27.00 | 3.29 | 9.00 | 11.60 |
| 2014/6/16 | 62.10 | 31.60 | 30.60 | 2.08 | 35.10 | 2.80 | 18.40 | 12.00 | |
| #2 | 2014/10/15 | 58.00 | 32.60 | 25.20 | 12.70 | 16.80 | 2.42 | 2.41 | 23.10 |
| 2024/12/15 | 79.40 | 32.20 | 44.50 | 1.39 | 17.30 | 2.01 | 26.90 | 16.90 | |
| #3 | 2009/9/15 | 72.90 | 36.50 | 34.90 | 5.27 | 20.90 | 3.05 | 26.80 | 8.99 |
| 2013/10/15 | 70.60 | 36.90 | 32.00 | 9.25 | 17.70 | 3.53 | 23.80 | 8.17 | |
| #4 | 2009/11/15 | 42.40 | 17.70 | 22.90 | 2.94 | 53.60 | 1.72 | 10.20 | 12.20 |
| 2022/12/15 | 47.60 | 23.50 | 23.20 | 1.38 | 47.90 | 2.25 | 10.30 | 12.40 | |
| #5 | 1931/8/15 | 62.80 | 44.70 | 16.10 | 19.00 | 17.30 | 4.90 | 5.03 | 11.00 |
| 2026/10/15 | 61.70 | 32.80 | 26.80 | 5.15 | 32.10 | 5.94 | 14.40 | 12.10 | |
| #6 | 2019/4/16 | 52.50 | 26.70 | 22.40 | 6.08 | 41.70 | 2.73 | 11.70 | 10.60 |
| 2007/6/16 | 49.40 | 25.20 | 20.20 | 3.46 | 45.30 | 3.08 | 11.90 | 7.32 | |
| #7 | 16/216 | 46.20 | 32.80 | 13.40 | 13.00 | 31.30 | 2.67 | 4.77 | 7.76 |
| 2027/4/16 | 65.20 | 44.10 | 18.40 | 2.41 | 23.40 | 3.16 | 10.10 | 5.98 | |
| #8 | 2013/1/16 | 83.40 | 44.00 | 39.30 | 8.68 | 7.79 | 3.43 | 15.50 | 21.90 |
| 2023/5/16 | 87.30 | 8.48 | 78.50 | 0.53 | 12.40 | 0.76 | 59.50 | 15.90 | |
| #9 | 2007/12/15 | 77.80 | 49.00 | 30.70 | 5.60 | 16.30 | 3.43 | 20.80 | 11.00 |
| 2025/12/15 | 68.80 | 46.90 | 26.40 | 0.42 | 29.70 | 2.51 | 19.00 | 6.53 | |
| #10 | 2022/3/16 | 69.40 | 46.40 | 23.10 | 6.48 | 24.60 | 4.22 | 9.52 | 13.30 |
| 2018/5/16 | 68.40 | 43.20 | 25.30 | 7.89 | 25.40 | 6.48 | 10.50 | 14.40 | |
| #11 | 2007/3/16 | 82.60 | 53.60 | 32.80 | 9.78 | 9.85 | 2.05 | 17.40 | 15.70 |
| 2027/4/16 | 78.20 | 39.90 | 46.40 | 0.88 | 22.90 | 2.74 | 31.50 | 15.70 | |
| #12 | 2018/4/16 | 69.40 | 34.70 | 32.50 | 6.40 | 22.00 | 4.66 | 22.20 | 9.95 |
| 2027/5/16 | 81.50 | 23.20 | 55.90 | 2.67 | 17.30 | 1.61 | 40.80 | 13.90 | |
| #13 | 2021/3/16 | 74.30 | 41.40 | 32.10 | 11.40 | 15.00 | 3.03 | 15.10 | 15.40 |
| 2003/5/16 | 78.90 | 46.20 | 32.10 | 3.55 | 17.50 | 4.15 | 15.20 | 14.80 | |
| #14 | 2016/3/16 | 80.00 | 42.00 | 33.00 | 4.41 | 15.30 | 3.97 | 15.50 | 14.90 |
| 1931/5/16 | 77.50 | 37.00 | 37.20 | 2.75 | 19.60 | 3.37 | 24.30 | 13.60 | |
| #15 | 2024/11/15 | 84.50 | 20.90 | 48.60 | 2.63 | 12.00 | 1.73 | 38.80 | 6.08 |
| 2029/12/15 | 77.10 | 25.90 | 44.80 | 0.79 | 16.80 | 2.15 | 33.00 | 13.80 | |
| #16 | 2013/11/15 | 76.60 | 57.00 | 24.60 | 4.69 | 17.60 | 2.26 | 11.60 | 12.30 |
| 2014/1/16 | 82.20 | 59.30 | 27.10 | 1.42 | 15.30 | 3.10 | 13.30 | 13.00 | |
| #17 | 2014/3/16 | 71.70 | 51.80 | 19.60 | 11.20 | 16.70 | 5.69 | 4.95 | 15.00 |
| 2020/5/16 | 75.40 | 52.70 | 21.30 | 4.89 | 19.50 | 5.80 | 16.00 | 4.15 | |
| #18 | 2025/9/15 | 66.40 | 43.50 | 22.80 | 11.40 | 26.00 | 1.59 | 9.89 | 12.10 |
| 2010/11/15 | 66.40 | 27.40 | 38.50 | 2.05 | 29.20 | 1.49 | 24.50 | 12.90 | |
| 2010/12/15 | 66.20 | 27.80 | 37.00 | 2.64 | 28.90 | 0.76 | 27.70 | 9.03 | |
| #19 | 2006/11/15 | 76.80 | 33.90 | 31.80 | 4.87 | 17.00 | 2.67 | 10.80 | 19.30 |
| 2008/1/16 | 55.30 | 22.00 | 28.20 | 0.89 | 41.40 | 1.31 | 11.80 | 13.30 | |
| 2023/2/16 | 57.10 | 23.90 | 28.00 | 1.02 | 40.40 | 2.54 | 13.10 | 14.70 | |
| #20 | 2002/11/15 | 76.60 | 48.60 | 34.00 | 2.40 | 22.40 | 2.63 | 21.80 | 9.44 |
| 2021/12/15 | 77.30 | 44.00 | 37.20 | 1.06 | 22.40 | 1.87 | 26.00 | 9.56 | |
| 2007/3/16 | 79.10 | 36.10 | 45.80 | 1.65 | 19.70 | 1.30 | 35.50 | 7.64 | |
| #21 | 2007/9/15 | 84.30 | 48.70 | 33.80 | 8.58 | 6.59 | 6.37 | 13.90 | 19.10 |
| 2016/10/15 | 80.10 | 33.80 | 42.40 | 9.38 | 8.62 | 3.37 | 31.70 | 11.80 | |
| 2024/11/15 | 79.90 | 35.40 | 42.50 | 12.80 | 5.54 | 4.19 | 30.40 | 10.60 | |
| #22 | 2010/9/15 | 86.30 | 48.40 | 36.00 | 4.36 | 9.48 | 4.72 | 12.50 | 21.80 |
| 2023/10/15 | 88.30 | 51.70 | 36.00 | 2.61 | 8.96 | 3.09 | 13.00 | 21.10 | |
| 2001/12/15 | 80.80 | 43.40 | 36.10 | 2.51 | 16.00 | 3.35 | 17.50 | 18.10 | |
| #23 | 1931/7/15 | 68.40 | 30.80 | 39.60 | 7.32 | 25.80 | 2.40 | 26.80 | 12.50 |
| 2011/9/15 | 72.10 | 34.20 | 38.60 | 2.66 | 24.70 | 2.21 | 28.20 | 13.30 | |
| 2013/10/15 | 70.70 | 33.40 | 37.60 | 4.58 | 24.40 | 2.78 | 25.30 | 13.20 | |
| #24 | 2012/1/16 | 74.80 | 44.80 | 27.80 | 6.84 | 18.50 | 3.91 | 10.20 | 16.70 |
| 2009/3/16 | 73.20 | 42.50 | 28.00 | 5.59 | 21.00 | 3.88 | 12.10 | 15.50 | |
| 2012/4/16 | 72.00 | 45.00 | 25.20 | 2.54 | 24.70 | 4.16 | 11.60 | 14.20 | |
| #25 | 2024/11/15 | 76.30 | 47.30 | 28.70 | 9.35 | 12.50 | 3.01 | 16.10 | 14.30 |
| 2020/1/16 | 85.40 | 55.10 | 29.50 | 5.23 | 7.89 | 3.41 | 14.60 | 13.50 | |
| 2001/3/16 | 80.90 | 49.90 | 30.10 | 6.64 | 12.20 | 3.19 | 17.30 | 12.60 | |
| #26 | 2014/9/15 | 78.50 | 38.50 | 33.30 | 9.64 | 12.30 | 2.12 | 14.20 | 20.40 |
| 2028/10/15 | 89.80 | 46.70 | 35.20 | 2.44 | 5.56 | 4.02 | 10.00 | 23.40 | |
| 2008/12/15 | 84.00 | 35.30 | 38.30 | 2.37 | 9.81 | 2.48 | 22.60 | 17.30 | |
| #27 | 2002/9/15 | 77.90 | 48.70 | 30.00 | 6.08 | 16.00 | 5.68 | 9.89 | 19.60 |
| 1930/10/15 | 84.70 | 36.70 | 47.20 | 2.22 | 11.70 | 4.04 | 23.90 | 21.00 | |
| 2029/1/16 | 77.80 | 39.80 | 38.00 | 3.49 | 19.40 | 4.47 | 28.80 | 8.18 | |
| #28 | 2025/12/15 | 87.00 | 43.40 | 38.70 | 7.00 | 5.89 | 3.05 | 25.60 | 12.20 |
| 2015/2/16 | 91.90 | 31.10 | 48.60 | 2.17 | 5.53 | 2.84 | 36.10 | 13.80 | |
| 2005/5/16 | 85.90 | 34.80 | 43.60 | 4.56 | 12.40 | 3.57 | 29.00 | 14.30 | |
| #29 | 2024/9/15 | 65.40 | 29.30 | 29.50 | 16.50 | 17.40 | 3.26 | 12.70 | 16.40 |
| 2017/11/15 | 80.50 | 38.20 | 36.80 | 9.21 | 8.97 | 4.61 | 9.34 | 27.10 | |
| 2024/2/16 | 71.50 | 26.40 | 34.90 | 9.47 | 17.70 | 1.86 | 13.90 | 22.30 | |
| #30 | 2010/10/15 | 74.30 | 34.30 | 37.10 | 3.01 | 23.00 | 2.86 | 21.60 | 16.20 |
| 2010/12/15 | 85.70 | 38.50 | 43.60 | 1.41 | 13.90 | 3.62 | 27.40 | 16.40 | |
| 2025/2/16 | 78.60 | 34.80 | 40.50 | 2.03 | 19.40 | 3.33 | 26.80 | 12.50 |
T cells: CD3+CD19−; CD4+ T cells: CD3+CD4+CD8-; CD8+T cells: CD3+ CD8+CD4-; B cells: CD3-CD19+; NK cells: CD3-CD16+56+; Treg cells: CD4+CD25+CD127low; suppressor T cells (Ts): CD3+CD8+CD28-; cytotoxic T cells (CTL): CD3+CD8+CD28+.
Table S3
| Patients | Date (d/m/y) | Th1 (%) | Th2 (%) | Th1/Th2 | Tc1 (%) | Tc2 (%) | Tc1/Tc2 |
|---|---|---|---|---|---|---|---|
| #1 | 2022/4/16 | 4.38 | 3.25 | 1.35 | 14.10 | 3.43 | 4.11 |
| 2014/6/16 | 5.37 | 3.68 | 1.46 | 21.20 | 3.31 | 6.4 | |
| #2 | 2014/10/15 | 20.60 | 5.41 | 3.81 | 49.40 | 2.63 | 18.8 |
| 2024/12/15 | 17.50 | 20.90 | 0.84 | 66.20 | 1.12 | 59.1 | |
| #3 | 2009/9/15 | 4.98 | 3.92 | 1.27 | 8.57 | 1.46 | 5.87 |
| 2013/10/15 | 12.30 | 2.76 | 4.46 | 40.60 | 1.45 | 28 | |
| #4 | 2009/11/15 | 33.90 | 0.51 | 67.1 | 58.00 | 1.65 | 35.2 |
| 2022/12/15 | 35.80 | 2.77 | 12.9 | 61.00 | 3.21 | 19 | |
| #5 | 1931/8/15 | 19.20 | 0.12 | 160 | 54.60 | 0.75 | 72.8 |
| 2026/10/15 | 15.50 | 3.74 | 4.14 | 61.20 | 0.87 | 70.3 | |
| #6 | 2019/4/16 | 14.10 | 1.67 | 8.44 | 67.90 | 0.29 | 235.8 |
| 2007/6/16 | 0.67 | 0.33 | 2 | 20.10 | 1.15 | 17.5 | |
| #7 | 16/216 | 26.60 | 2.16 | 12.3 | 69.40 | 2.64 | 26.3 |
| 2027/4/16 | 21.40 | 1.51 | 14.2 | 23.70 | 3.97 | 5.97 | |
| #8 | 2013/1/16 | 23.40 | 0.66 | 35.5 | 63.80 | 1.54 | 41.4 |
| 2023/5/16 | 8.54 | 3.55 | 2.41 | 13.50 | 0.62 | 21.8 | |
| #9 | 2007/12/15 | 49.20 | 1.08 | 45.6 | 77.20 | 0.16 | 498.1 |
| 2025/12/15 | 52.20 | 1.69 | 30.9 | 74.80 | 2.78 | 26.9 | |
| #10 | 2022/3/16 | 13.50 | 0.47 | 28.6 | 31.80 | 0.48 | 66.7 |
| 2018/5/16 | 7.79 | 0.71 | 11 | 15.70 | 3.08 | 5.1 | |
| #11 | 2007/3/16 | 20.40 | 0.68 | 30.1 | 61.00 | 0.92 | 66.7 |
| 2027/4/16 | 6.10 | 2.43 | 2.51 | 11.00 | 1.16 | 9.48 | |
| #12 | 2018/4/16 | 24.80 | 4.57 | 5.43 | 50.50 | 1.07 | 47.2 |
| 2027/5/16 | 3.33 | 3.57 | 0.93 | 4.88 | 5.05 | 0.97 | |
| #13 | 2021/3/16 | 8.51 | 3.50 | 2.43 | 38.70 | 1.28 | 30.2 |
| 2003/5/16 | 3.86 | 3.13 | 1.23 | 23.80 | 2.05 | 11.61 | |
| #14 | 2016/3/16 | 18.50 | 1.12 | 16.5 | 45.70 | 1.87 | 24.4 |
| 1931/5/16 | 6.60 | 3.50 | 1.89 | 19.60 | 3.03 | 6.47 | |
| #15 | 2024/11/15 | 49.60 | 1.96 | 25.3 | 87.00 | 0.30 | 287.1 |
| 2029/12/15 | 30.70 | 0.70 | 43.7 | 76.00 | 0.31 | 249 | |
| #16 | 2013/11/15 | 23.00 | 2.68 | 8.58 | 57.30 | 1.66 | 34.5 |
| 2014/1/16 | 31.50 | 0.86 | 36.6 | 56.70 | 0.91 | 62.2 | |
| #17 | 2014/3/16 | 6.15 | 4.66 | 1.32 | 23.50 | 3.59 | 6.55 |
| 2020/5/16 | 3.61 | 1.07 | 3.37 | 7.07 | 1.63 | 4.34 | |
| #18 | 2025/9/15 | 31.20 | 2.12 | 14.7 | 62.60 | 2.31 | 27.1 |
| 2010/11/15 | 26.50 | 3.78 | 7.01 | 53.20 | 2.95 | 18 | |
| 2010/12/15 | 45.80 | 3.33 | 13.8 | 84.30 | 0.47 | 179 | |
| #19 | 2006/11/15 | 24.00 | 1.62 | 14.8 | 51.10 | 2.15 | 23.8 |
| 2008/1/16 | 19.60 | 0.97 | 20.3 | 68.90 | 0.10 | 710 | |
| 2023/2/16 | 24.30 | 2.65 | 9.17 | 61.00 | 1.23 | 49.6 | |
| #20 | 2002/11/15 | 21.20 | 1.77 | 12 | 72.40 | 0.93 | 77.8 |
| 2021/12/15 | 42.40 | 3.13 | 13.5 | 61.80 | 1.84 | 33.6 | |
| 2007/3/16 | 52.00 | 1.11 | 46.8 | 86.40 | 0.15 | 572 | |
| #21 | 2007/9/15 | 2.30 | 1.12 | 2.05 | 5.79 | 1.61 | 3.6 |
| 2016/10/15 | 4.81 | 10.20 | 0.47 | 40.40 | 5.42 | 7.45 | |
| 2024/11/15 | 28.70 | 3.19 | 9 | 71.90 | 1.74 | 41.3 | |
| #22 | 2010/9/15 | 4.60 | 1.58 | 2.91 | 26.90 | 2.58 | 10.4 |
| 2023/10/15 | 13.40 | 2.46 | 5.45 | 51.30 | 2.33 | 22 | |
| 2001/12/15 | 17.40 | 1.66 | 10.5 | 58.40 | 1.31 | 44.6 | |
| #23 | 1931/7/15 | 52.10 | 1.43 | 36.4 | 74.50 | 1.42 | 52.5 |
| 2011/9/15 | 4.26 | 4.47 | 1 | 9.68 | 0.60 | 16.1 | |
| 2013/10/15 | 47.20 | 0.91 | 51.9 | 81.50 | 0.76 | 107.2 | |
| #24 | 2012/1/16 | 21.50 | 1.33 | 16.2 | 56.90 | 4.61 | 12.3 |
| 2009/3/16 | 6.47 | 1.20 | 5.39 | 30.50 | 0.49 | 62.63 | |
| 2012/4/16 | 4.71 | 0.80 | 5.92 | 23.10 | 2.17 | 10.6 | |
| #25 | 2024/11/15 | 16.20 | 3.10 | 5.23 | 66.20 | 1.04 | 63.7 |
| 2020/1/16 | 17.50 | 1.43 | 12.2 | 65.70 | 0.41 | 162 | |
| 2001/3/16 | 22.50 | 1.72 | 13.1 | 70.80 | 0.72 | 98.3 | |
| #26 | 2014/9/15 | 3.52 | 1.14 | 3.09 | 4.56 | 2.47 | 1.85 |
| 2028/10/15 | 32.80 | 2.36 | 13.9 | 32.30 | 7.60 | 4.25 | |
| 2008/12/15 | 36.50 | 1.43 | 25.5 | 53.90 | 1.26 | 42.8 | |
| #27 | 2002/9/15 | 0.31 | 0.81 | 0.38 | 2.03 | 0.53 | 3.83 |
| 1930/10/15 | 9.71 | 5.64 | 1.72 | 38.90 | 3.11 | 12.5 | |
| 2029/1/16 | 12.50 | 0.67 | 18.5 | 47.60 | 2.39 | 19.9 | |
| #28 | 2025/12/15 | 9.24 | 1.17 | 7.9 | 46.90 | 0.25 | 186 |
| 2015/2/16 | 12.00 | 4.58 | 2.62 | 50.80 | 2.61 | 19.5 | |
| 2005/5/16 | 9.46 | 3.87 | 2.44 | 20.50 | 2.48 | 8.27 | |
| #29 | 2024/9/15 | 31.00 | 3.10 | 10 | 53.90 | 4.97 | 10.8 |
| 2017/11/15 | 25.80 | 4.50 | 5.73 | 39.20 | 8.03 | 4.88 | |
| 2024/2/16 | 46.60 | 1.25 | 37.3 | 62.10 | 0.67 | 93.1 | |
| #30 | 2010/10/15 | 28.40 | 2.43 | 11.7 | 65.30 | 1.70 | 38.4 |
| 2010/12/15 | 41.80 | 1.74 | 24 | 74.20 | 0.52 | 142.7 | |
| 2025/2/16 | 35.10 | 1.15 | 30.5 | 60.60 | 0.57 | 106.1 |
Th1 cells: CD3+ CD4+ IFN-r+ IL-4-; Th2 cells: CD3+ CD4+ IL-4+ IFN-r-; Tc1cells: CD3+ CD8+ IFN-r+ IL-4-; Tc2cells: CD3+ CD8+ IL-4+ IFN-r-.
Acknowledgments
Funding: The present study was funded by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.11.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study received approval from the Human Ethics Committee of State Key Laboratory of Experimental Hematology, Fifth Medical Center of Chinese PLA General Hospital, Beijing, China, and signed informed consent was provided by every patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Campian JL, Ye X, Brock M, et al. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest 2013;31:183-8. [Crossref] [PubMed]
- Shiao SL, Coussens LM. The tumor-immune microenvironment and response to radiation therapy. J Mammary Gland Biol Neoplasia 2010;15:411-21. [Crossref] [PubMed]
- Lauber K, Munoz LE, Berens C, et al. Apoptosis induction and tumor cell repopulation: the yin and yang of radiotherapy. Radiat Oncol 2011;6:176. [Crossref] [PubMed]
- Beyer C, Stearns NA, Giessl A, et al. The extracellular release of DNA and HMGB1 from Jurkat T cells during in vitro necrotic cell death. Innate Immun 2012;18:727-37. [Crossref] [PubMed]
- Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009;114:589-95. [Crossref] [PubMed]
- Takeshima T, Chamoto K, Wakita D, et al. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res 2010;70:2697-706. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys 2012;84:879-80. [Crossref] [PubMed]
- Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203:1259-71. [Crossref] [PubMed]
- Stangl S, Gross C, Pockley AG, et al. Influence of Hsp70 and HLA-E on the killing of leukemic blasts by cytokine/Hsp70 peptide-activated human natural killer (NK) cells. Cell Stress Chaperones 2008;13:221-30. [Crossref] [PubMed]
- Liang H, Deng L, Chmura S, et al. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J Immunol 2013;190:5874-81. [Crossref] [PubMed]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39-51. [Crossref] [PubMed]
- Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol 2010;22:231-7. [Crossref] [PubMed]
- Moyer JS, Li J, Wei S, et al. Intratumoral dendritic cells and chemoradiation for the treatment of murine squamous cell carcinoma. J Immunother 2008;31:885-95. [Crossref] [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [Crossref] [PubMed]
- Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol 2009;9:480-90. [Crossref] [PubMed]
- Pilones KA, Kawashima N, Yang AM, et al. Invariant natural killer T cells regulate breast cancer response to radiation and CTLA-4 blockade. Clin Cancer Res 2009;15:597-606. [Crossref] [PubMed]
- Yang W, Wang W, Liu B, et al. Immuno-modulation Characteristics by Thermal Ablation Therapy in Cancer Patients. Asia Pac J Clin Oncol 2018;14:e490-e497. [Crossref] [PubMed]
- Wang W, Liu B, Liu G, et al. Fundamental effects of PD-1 antibody on the body: a brief report. Onco Targets Ther 2016;9:4137-41. [Crossref] [PubMed]
- Liu G, Yang W, Guo M, et al. Effective modulation of CD4(+)CD25 (+high) regulatory T and NK cells in malignant patients by combination of interferon-alpha and interleukin-2. Cancer Immunol Immunother 2012;61:2357-66. [Crossref] [PubMed]
- Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 2005;11:728-34. [PubMed]


