
Clinical features and prognosis analysis of 57 patients with primary tracheal tumors
Introduction
Primary tracheal tumors (PTTs) are rare and account for 0.01–0.4% of all malignancies with an annual incidence of 2.6 patients per 1,000,000 people (1,2). The small number of PTTs makes it difficult to investigate the natural history and identify optimal treatment modalities. The clinical manifestations of PTTs are variable, and their symptoms are nonspecific. Bronchoscopy and biopsy are the widely accepted standard for the diagnosis of PTTs, but tumors are typically identified late in their progression. Although complete surgical resection is reported to offer long-term survival in many patients (3), the proportion of operable tumors is relatively low due to the late diagnosis (4). Therefore, treatment strategies for patients with PTTs have not been standardized. In the last decade, radiotherapy, chemotherapy and bronchoscopic intervention in the treatment of PTTs have made significant progress, however few analyses concerning the clinical outcomes have been reported. Our main purpose therefore was to explore the clinical characteristics, evaluate the long-term survival of different treatment approaches, and explore the prognostic factors of PTTs.
Methods
Among patients who underwent bronchoscopy at Xiangya Hospital between January 2009 and May 2019, 90 patients with tracheal neoplasms were recorded. The trachea was defined to extend from cricoid cartilage superiorly to the carina inferiorly. Thirty-three patients (18 males and 15 females) were excluded due to primary malignancy elsewhere (n=31) or inflammatory pseudotumors (n=2). Together, 57 patients met the above criteria and medical histories were evaluated. This study was approved by Xiangya Hospital Institutional Review Board (No. CTXY-110008-2).
Patients’ data, including demographics, presenting symptoms, pathological characteristics, tumor sites, interventions, treatment outcome and recurrence were analyzed. To define the location of tumors, the trachea was equally divided into three parts: the upper, middle and lower third. Ablation, snare, cryotherapy and stent are all included in bronchoscopic interventions.
Student’s t-test was used to compare continuous variables, and chi-squared test was used to compare categorical variables. Survival rates were estimated using the Kaplan-Meier analysis and were compared using a two-sided log-rank test. A P value of 0.05 was considered statistically significant. All analyses were conducted using the SPSS Statistics 18.0 software (SPSS Inc., Chicago, IL, USA).
Results
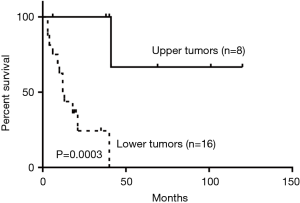
Among the 57 subjects, 36 (63.2%) were male and 21 (36.8%) were female. The mean age for patients with benign tumors was 47.5 [25–70] and 52.3 [15–75] years for patients with malignant tumors. Fourteen (24.6%) tumors were located in the upper third of the trachea, 5 (8.8%) in the middle third, 33 (57.9%) in the lower third. One case (1.8%) infiltrated the whole trachea and 4 (7.0%) were located in both the middle and lower third. Compared to the malignant tumors located in the upper third, those in lower sites showed worse survival outcomes (P=0.0003) (Figure 1).
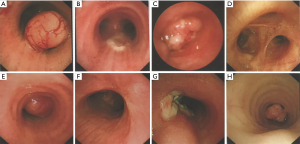
Eight (14.0%) patients had benign tumors, which showed heterogeneous pathologic characterization (Table 1). Dyspnea was the most common symptom in this group. Under bronchoscope, benign tumors were characterized by intraluminal growth with clear boundaries, some of which were pedunculated (Figure 2). Seven (87.5%) patients went through bronchoscopic intervention and 1 (12.5%) underwent complete surgical resection. All benign tumors were successfully removed without recurrence except one case of papilloma.
Table 1
| Patients | Sex/age | Chief complaint | Smoking | Location | Pathology | Treatment | Result |
|---|---|---|---|---|---|---|---|
| 1 | F/38 | Cough | No | Middle | Papilloma | Cryotherapy | Alive |
| 2 | M/25 | Dyspnea | No | Lower | Leiomyoma | Snare | Alive |
| 3 | F/26 | Dyspnea | No | Upper | Hamartoma | Cryotherapy | Alive |
| 4 | F/64 | Dyspnea | No | Middle | Hamartoma | Surgery | Alive |
| 5 | M/49 | Dyspnea | Yes | Lower | Fibroma | Ablation | Alive |
| 6 | M/56 | Dyspnea | Yes | Upper | Fibroma | Snare | Alive |
| 7 | M/70 | Cough | No | Upper | Plasmacytoma | Snare | Alive |
| 8 | F/52 | Cough | No | Lower | Plasmacytoma | Snare | Alive |
Upper, upper third of trachea; middle, middle third of trachea; lower, lower third of trachea.
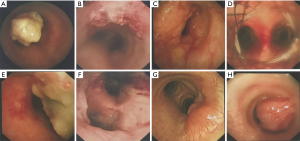
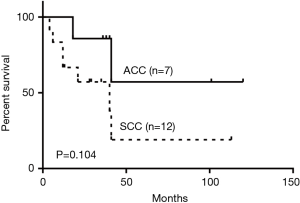
Forty-nine (86.0%) patients had malignant tumors with squamous cell carcinoma (SCC) being the most frequent histologic type (40.8%). Adenoid cystic carcinoma (ACC) was the second most frequent (20.4%). The rest were composed of a variety of malignant pathologies including small cell carcinoma, adenocarcinoma and lymphoma (Table 2). SCC was more likely to occur with increased age and showed male predominance (Table 3). 70% of the SCC subjects were smokers while 40% of ACC patients smoked. Cough (68.6%) and dyspnea (43.1%) were the most common symptoms. Hemoptysis, hoarseness and dysphagia were noted in 35.3%, 7.8% and 3.9% of malignant tumors respectively. The main features of the bronchoscopic descriptions of malignant tumors included exophytic growth or intraluminal growth, which were more likely to invade or infiltrate surrounding tissues (Figure 3). The 5-year survival rate for all malignant PTTs was 13.8%. The 5-year survivals rates for SCC and ACC were 8.3% and 28.6% respectively. No significant difference was observed between the survival of SCC and ACC (P=0.104) (Figure 4).
Table 2
| Characteristics | N (%) | Mean age | Range | P |
|---|---|---|---|---|
| Overall | 49 | 52.3 | 15–75 | |
| Sex | 0.112 | |||
| Male | 55.0 | 32–75 | ||
| Female | 46.4 | 15–74 | ||
| Histology | 0.274 | |||
| SCC | 20 (40.8) | 55.1 | 32–74 | |
| ACC | 10 (20.4) | 45.5 | 15–75 | |
| Small cell carcinoma | 6 (12.2) | 47.0 | 27–73 | |
| Adenocarcinoma | 5 (10.2) | 61.8 | 49–74 | |
| Lymphoma | 3 (6.1) | 51.0 | 34–64 | |
| Cancerization | 5 (10.2) | 55.4 | 38–63 |
ACC, adenoid cystic carcinoma; SCC, squamous cell carcinoma.
Table 3
| Characteristics | SCC | ACC | P |
|---|---|---|---|
| Age, mean [range] | 55.1 [32–74] | 45.5 [15–75] | 0.088 |
| Sex, n [%] | 0.041 | ||
| Male | 17 [85] | 5 [50] | |
| Female | 3 [15] | 5 [50] | |
| Smoking, n [%] | 14 [70] | 4 [40] | 0.114 |
| Sites, n [%] | 0.044 | ||
| Upper | 3 [15] | 6 [60] | |
| Middle | 2 [10] | 1 [10] | |
| Lower | 14 [70] | 3 [30] | |
| Survival rates, % | |||
| 3-year | 33.3 | 57.1 | 0.377 |
| 5-year | 8.3 | 28.6 | 0.243 |
ACC, adenoid cystic carcinoma; SCC, squamous cell carcinoma.
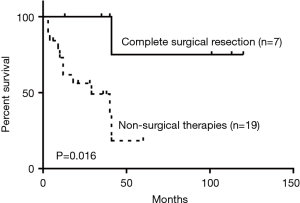
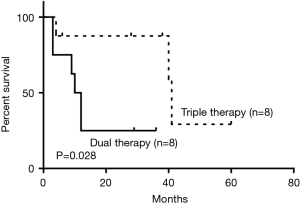
As for the treatment of the malignant PTTs, 7 patients (22.6%) underwent complete surgical resection, 4 (12.9%) went through radiotherapy, 8 (25.8%) received concurrent chemoradiotherapy (dual therapy), 2 (6.5%) were treated with bronchoscopic intervention, 8 (25.8%) received the combination of dual therapy with bronchoscopic intervention (triple therapy), and 2 (6.5%) received symptomatic treatment. Compared to non-surgical intervention group, surgical group showed significant improvement in survival outcomes (P=0.016) (Figure 5). Within the non-surgical intervention group, triple therapy was significantly better than dual therapy (P=0.028) (Figure 6).
Discussion
PTT is a rare disease of the respiratory tract, which could be explained by the decreased surface of the tracheal epithelium, the presence of mucinous secreting cells, and the tracheal laminar air flows unlike those turbulent of the bronchial tree (5,6). In our study, only 57 individuals were diagnosed with PTT out of 58,506 patients who underwent bronchoscopy exam during study period.
Previous studies have reported that 10% PTTs occurring in adults are benign (7). Dyspnea and cough are the most frequent symptoms, but are not universal. Bronchoscopic intervention is reported to be sufficient for tumors with limited extensions, while open tracheal resection and anastomosis have been recommended in patients with widespread lesions. In our study, 7 out of 8 subjects underwent bronchoscopic intervention without recurrence except one case which developed papilloma, which is consistent with previous reports (8).
Malignancy can occur in every parietal layer of the trachea and hence greatly vary histologically. SCC is reported to be the most frequent histologic type, which accounts for one-half to two-thirds of all malignant PTTs; ACC is the second most common, which accounts for 10% to 15% of malignant patients (9). The prevalence of ACC in our study population was similar. However, the frequency of SCC in the cohort described here was less than other reported studies. Environmental difference and a higher percentage of other histologic types in our study might play a role in this divergence. A similar incidence of small cell carcinoma was found in previous reports (10). SCC occurs predominantly in men and smokers in the sixth and seventh decades, while ACC is not smoking related and evenly distributed between genders and peaks in incidence in the fourth and fifth decades. In our study, the mean ages of diagnosis of SCC and ACC were 55.1 and 45.5 years, respectively. The most frequent symptoms of malignant PTTs vary based on the location of the tumor and the histologic type. SCCs often present with hemoptysis, given mucosal ulceration and irritation (11). Hoarseness may develop due to involvement of the recurrent laryngeal nerve by extratracheal extension (12). The location of the tumor, which has rarely been studied in previous reports, seems to have significant prognostic value. In our study, malignant PTTs were mainly located in the upper and lower third of the trachea, which is in agreement with previous reports (13). The location of tumors might play a role in the prognosis. Tumors located in lower third showed worse survival outcomes, which may be ascribed to the predilection of SCC in the lower part of the trachea. The other reason may be that tumors of lower anatomic location are more aggressive (14). Additional studies are warranted to validate this finding.
The prognosis of malignant PTT is dismal and comorbidities are substantial. According to the largest reported study based on surveillance, epidemiology, and end results (SEER), the overall 5-year survival is 23.1–33.3% (15). Different histologic types vary in prognosis. SCCs are more likely to metastasize, and are more resistant to all forms of therapy with a 5-year overall survival rate of 8.4–17.6%. In contrast, 5-year overall survival of ACCs is reported to be 63.1–82.5% (15). The significantly lower 5-year survival rate in our study is likely explained by the low resectability rate.
The optimal treatment for tracheal tumors remains controversial. Complete surgical resection, radiotherapy, chemotherapy and various bronchoscopic intervention all have been used, alone or in combination. Complete surgical resection is reported to be the optimal therapy for PTTs (16). Nouraei reported that 10-year palliation-free survival was 60.8% with curative resection (17). Gaissert reported that Overall 5- and 10-year survival in resected ACC was 52% and 29% (unresectable 33% and 10%) and in resected SCC 39% and 18% (unresectable 7.3% and 4.9%) (18). In our study, patients undergoing complete surgical resection showed significantly better survival compared to those without surgical intervention.
Diagnosis of PTT is often delayed for months or years due to its nonspecific symptoms. Honings reported that 2.3 times more patients could have been suitable candidates for curative resection if the malignancy had been identified earlier (17,19). Advanced disease is often complicated by locoregional spread which is not amenable to surgical intervention and requires combined therapy in order to improve long-term survival (20). Studies have been reported that 5-year survival rates of 50% can be acquired with adequately dosed radiotherapy for patients with ACC (21). Systemic chemotherapy has also been reported for PTTs with more extensive lesions (22).
Advances in interventional pulmonology techniques allow for new treatment of patients with PTTs. Bronchoscopic intervention could work in part as surgery, minimize the risk of the migration of the resected tumor, rapidly alleviate airway obstruction, improve patients’ quality of life and prolong their survival (23-26). Still, few studies have explored the effect of the combination of radiotherapy, chemotherapy and bronchoscopic intervention. In our study, we found that the triple therapy showed significantly better survival in comparison with dual therapy. In some patients, to ensure the delivery of radiation to the target region, it is advisable to perform bronchoscopic intervention first, with recanalization of the airway, followed by radiotherapy and chemotherapy, which could increase the median survival up to 15–20 months (27). However, complications from bronchoscopic intervention have been reported and should be considered when deciding the most appropriate intervention (28,29).
Conclusions
PTTs are an understudied group of malignancies that carry significant health burden to the patients. The lack of research on this group of patients is likely due to the infrequency of their occurrence. Histologic type, tumor location, and clinical intervention have important impacts on patient prognosis. There appears to be a consensus that complete surgical resection is the optimal therapy for long-term survival, however, is frequently not an option due to tumor location or local metastasis. Triple therapy is an effective modality for unresectable malignant PTTs. However, our results should be viewed with caution given the retrospective nature and size of our clinical cohort. Additional studies are warranted to identify optimal screening and treatment options for this difficult disease.
Acknowledgments
We are grateful to Dr. Jonathan Alder who helped to check the language and wordings of the manuscript.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.11.55). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Xiangya Hospital Institutional Review Board (No. CTXY-110008-2). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xie L, Fan M, Sheets NC, et al. The use of radiation therapy appears to improve outcome in patients with malignant primary tracheal tumors: a SEER-based analysis. Int J Radiat Oncol Biol Phys 2012;84:464-70. [Crossref] [PubMed]
- Agrawal S, Jackson C, Celie KB, et al. Survival trends in patients with tracheal carcinoma from 1973 to 2011. Am J Otolaryngol 2017;38:673-7. [Crossref] [PubMed]
- Rea F, Zuin A. Tracheal resection and reconstruction for malignant disease. J Thorac Dis 2016;8:S148-52. [PubMed]
- Madariaga MLL, Gaissert HA. Overview of malignant tracheal tumors. Ann Cardiothorac Surg 2018;7:244-54. [Crossref] [PubMed]
- Palma AD, Sollitto F, Loizzi D, et al. Acute care and long-term results in treatment of tracheal tumors: monocentric experience of the last seven years. J Rare Disord 2016;2:1.
- Honings J, Gaissert HA, van der Heijden HF, et al. Clinical aspects and treatment of primary tracheal malignancies. Acta Otolaryngol 2010;130:763-72. [Crossref] [PubMed]
- Diaz-Mendoza J, Debiane L, Peralta AR, et al. Tracheal tumors. Curr Opin Pulm Med 2019;25:336-43. [Crossref] [PubMed]
- Ahn Y, Chang H, Lim YS, et al. Primary tracheal tumors: review of 37 cases. J Thorac Oncol 2009;4:635-8. [Crossref] [PubMed]
- Macchiarini P. Primary tracheal tumours. Lancet Oncol 2006;7:83-91. [Crossref] [PubMed]
- Gelder CM, Hetzel MR. Primary tracheal tumours: a national survey. Thorax 1993;48:688-92. [Crossref] [PubMed]
- Sherani K, Vakil A, Dodhia C, et al. Malignant tracheal tumors: a review of current diagnostic and management strategies. Curr Opin Pulm Med 2015;21:322-6. [Crossref] [PubMed]
- Thotathil ZS, Agarwal JP, Shrivastava SK, et al. Primary malignant tumors of the trachea - the Tata Memorial Hospital experience. Med Princ Pract 2004;13:69-73. [Crossref] [PubMed]
- Zhengjaiang L, Pingzhang T, Dechao Z, et al. Primary tracheal tumours: 21 years of experience at Peking Union Medical College, Beijing, China. J Laryngol Otol 2008;122:1235-40. [Crossref] [PubMed]
- Zhao Y, Zhao H, Fan L, et al. Adenoid cystic carcinoma in the bronchus behaves more aggressively than its tracheal counterpart. Ann Thorac Surg 2013;96:1998-2004. [Crossref] [PubMed]
- Urdaneta AI, Yu JB, Wilson LD. Population based cancer registry analysis of primary tracheal carcinoma. Am J Clin Oncol 2011;34:32-7. [Crossref] [PubMed]
- Högerle BA, Lasitschka F, Muley T, et al. Primary adenoid cystic carcinoma of the trachea: clinical outcome of 38 patients after interdisciplinary treatment in a single institution. Radiat Oncol 2019;14:117. [Crossref] [PubMed]
- Nouraei SM, Middleton SE, Nouraei SA, et al. Management and prognosis of primary tracheal cancer: a national analysis. Laryngoscope 2014;124:145-50. [Crossref] [PubMed]
- Gaissert HA, Grillo HC, Shadmehr MB, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg 2004;78:1889-96; discussion 1896-7.
- Honings J, Gaissert HA, Verhagen AF, et al. Undertreatment of tracheal carcinoma: multidisciplinary audit of epidemiologic data. Ann Surg Oncol 2009;16:246-53. [Crossref] [PubMed]
- Gaafar AH, Shaaban AY, Elhadidi MS. The use of metallic expandable tracheal stents in the management of inoperable malignant tracheal obstruction. Eur Arch Otorhinolaryngol 2012;269:247-53. [Crossref] [PubMed]
- Je HU, Song SY, Kim DK, et al. A 10-year clinical outcome of radiotherapy as an adjuvant or definitive treatment for primary tracheal adenoid cystic carcinoma. Radiat Oncol 2017;12:196. [Crossref] [PubMed]
- Behringer D, Könemann S, Hecker E. Treatment approaches to primary tracheal cancer. Thorac Surg Clin 2014;24:73-6. [Crossref] [PubMed]
- An J, Yang HP, Hu CP, et al. Multinodule abnormalities of the tracheobronchus: bronchoscopy findings and clinical diagnosis. Clin Respir J 2017;11:440-7. [Crossref] [PubMed]
- Scarlata S, Graziano P, Lucantoni G, et al. Endoscopic treatment of primary benign central airway tumors: results from a large consecutive case series and decision making flow chart to address bronchoscopic excision. Eur J Surg Oncol 2015;41:1437-42. [Crossref] [PubMed]
- Lin CY, Chung FT. Central airway tumors: interventional bronchoscopy in diagnosis and management. J Thorac Dis 2016;8:E1168-76. [Crossref] [PubMed]
- Luo T, Zhou H, Meng J. Clinical characteristics of tracheobronchopathia osteochondroplastica. Respir Care 2019;64:196-200. [Crossref] [PubMed]
- Kanaev SV, Arseniev AI, Barchuk AS, et al. Treatment of neoplastic lesions of the central bronchi and trachea using endotracheobronchial surgery, intraluminal brachytherapy, combined radiotherapy and chemoradiotherapy. Vopr Onkol 2015;61:62-70. [PubMed]
- Vishwanath G, Madan K, Bal A, et al. Rigid bronchoscopy and mechanical debulking in the management of central airway tumors: an Indian experience. J Bronchology Interv Pulmonol 2013;20:127-33. [Crossref] [PubMed]
- Hamouri S, Novotny NM. Primary tracheal schwannoma a review of a rare entity: current understanding of management and followup. J Cardiothorac Surg 2017;12:105. [Crossref] [PubMed]


