
The prognostic value of tumor budding in laryngeal squamous cell carcinoma
Introduction
Laryngeal carcinoma is a commonly diagnosed cancer of head and neck, and more than 90% of which is laryngeal squamous cell carcinoma (LSCC) (1). It has been reported that the 5-year survival of LSCC is decreasing despite improvement of therapy (2). At present, the prognosis and treatment strategies for LSCC is according to the American Joint Committee on Cancer (AJCC) TNM classification. Nevertheless, TNM classification cannot provide accurate prediction value and additional predictor is essential to improve the clinical treatment and the management of patients with LSCC. Histologic subtyping has been reported to classify the risk of patients in several cancers (3-5). However, the value of histologic characteristics in LSCC is unclear. In this study, we aimed to evaluate the histologic features to find new prognostic factor in LSCC.
Since LSCC is accompanied by high local invasion and recurrence, and with different prognosis, the evaluation of histologic features related to local invasion and lymph node metastasis may lead to better stratify patients with LSCC and provide better prognostic outcome.
It has been reported that cancer cells that are located in the invasive tumor front are more aggressive (3). Tumor budding is identified as isolated small tumor clusters composed of less than five non-glandular cancer cells scattered in the invasive tumor front (3). The presence of tumor budding is regarded as characteristic of aggressive cancer and has been previously considered as a new histopathologic marker in several malignancies, including lung squamous cell carcinoma (6), esophageal squamous cell carcinoma (7), colorectal cancer (8), breast cancer (9), and pancreatic ductal adenocarcinoma (10).
Tumor tissues consist of epithelial cells and stroma originated from normal tissues. The stroma may serve as a barrier to constrain cell proliferation and migration in normal tissues while they facilitate tumor progression in tumor tissues. The tumor stroma is composed of inflammatory cells, capillaries, fibroblasts and extracellular matrix (11). Fibroblasts that surround and infiltrate the tumor are also named as cancer associated fibroblasts (CAFs). CAFs can secrete chemokine and growth factor to enhance cells migration and invasion and promote angiogenesis (12). It was reported that tumor-stroma ratio (TSR) has a predictor value in lymph nodes. TSR combined with lymph nodes has been documented to be a significant prognostic parameter in breast cancer (13).
Herein, we evaluated the prognostic significance of tumor budding and TSR on the grounds of slides stained with hematoxylin and eosin (H&E). Our research found that tumor budding and T and N status may serve as reliable independent histologic prognostic markers, and tumor budding should be used in routine histologic diagnosis in LSCC.
Methods
Patients
The patients in this research were diagnosed with LSCC and went surgical resection between 2010 and 2014 at The First Affiliated Hospital of Guangzhou Medical University. The included patients were 51 males, 0 female, without distant metastasis. Clinical characteristics were summarized in Table 1. All patients included might take additional post-operative treatment according to the guideline of NCCN, such as neoadjuvant therapy. And the patients with any adjuvant therapy before surgery were excluded. Tumor stage was categorized on the basis of TNM system by AJCC [2010], and the tumor pathological grade was according to WHO Classification of Head and Neck Tumors [2017]. The research was approved by the ethical committee of The First Affiliated Hospital of Guangzhou Medical University.
Table 1
| Variables | N=51 |
|---|---|
| Sex | |
| Female | 0 |
| Male | 51 |
| Pathologic stage | |
| Stage I–II | 15 |
| Stage III–IV | 36 |
| T-primary tumour | |
| T1–T2 | 33 |
| T3–T4 | 18 |
| Lymphatic invasion | |
| Absent | 45 |
| Present | 6 |
| Differentiation | |
| Well | 13 |
| Moderately | 33 |
| Poorly | 5 |
| Nuclear diameter | |
| Large | 16 |
| Moderate | 23 |
| Small | 12 |
| Age (y) | |
| ≤65 | 30 |
| >65 | 21 |
| Tumor budding, total | |
| High | 19 |
| Low | 32 |
| Tumor budding, max | |
| High | 20 |
| Low | 31 |
| Budding score | |
| 1–4/hpf | 20 |
| 5–9/hpf | 15 |
| >10/hpf | 16 |
| Mitotic count | |
| High | 30 |
| Moderate | 10 |
| Low | 11 |
| TSR | |
| High | 35 |
| Moderate | 8 |
| Low | 8 |
LSCC, laryngeal squamous cell cancer.
Histologic evaluation
Histologic evaluation was performed on 4 µm hematoxylin and eosin (H&E)-stained slides (1,7). Histologic evaluation was completed by three pathologists who were without any knowledge of the clinical data or the other pathologists’ results. At a later stage, all three pathologists contributed to a consensus assessment for all the variables. Reproducibility was measured using Gwet’s agreement coefficients.
For TSR assessment, the most tumor areas were selected with 4× objective, then, TSR scoring was evaluated using 10× objective. Only fields where stroma and tumor cells are both present were eligible. Stromal cells ratio ≤50% was defined as low stroma ratio (high TSR) and >50% were high stroma (low TSR) (Figure 1A).
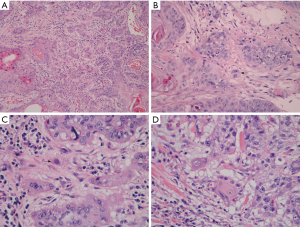
Tumor budding was defined as small tumor nests composed of <5 tumor cells. For evaluating tumor budding, tumor slides were scanned at 10× objective. Subsequently, tumor budding was counted at the most invasion area in 10 fields at ×200 magnification. The tumor budding was analyzed by two ways: the total numbers of tumor budding under 10 HPFs and the maximum numbers per field among 10 HPFs (Figure 1B). >10 tumor budding/10 HPFs or >10 tumor budding/HPF was defined as high total tumor budding or high maximum tumor budding, respectively. <10 tumor budding/10 HPFs or <10 tumor budding/HPF was defined as low total tumor budding or low maximum tumor budding, respectively. Single cell invasion was considered present if any was observed after examination of 50 HPFs at the most invasive area with the maximal number of the smallest tumor nests (Figure 1C).
Nuclear atypia was identified using 40× objective in the area with the highest abnormal nuclear phenotype after scanning the whole slide at 10× objective. For nuclear diameter, we screened 10 HPFs with the largest nuclei and then examined the average nuclear diameter of 100 tumor cells. Small lymphocytes (≈4.0 µm) served as the reference. The nuclear diameter >4 small lymphocytes was classified as large nuclei, while, ≤4 small lymphocytes was classified as small nuclei (Figure 1D). Mitosis evaluation was performed in 50 HPFs areas that contained the highest mitotic activity. Mitotic numbers ≥15/10 HPFs were classified as high mitotic rate, and <15/10 HPFs were classified as low mitotic rate.
Statistical analysis
SPSS 20.0 (IBM, Chicago, IL, USA) was used to perform statistical analysis. Overall survival (OS) was time from the date of surgery to date of death or last follow-up. Recurrence-free survival (RFS) was the time from the date of surgery to the date of disease recurrence or death. OS and RFS assay were evaluated by the Kaplan-Meier method. Multivariate assay was carried out by the Cox proportional hazards regression model and only factors that P value of <0.05 in univariate analysis was included. All statistical tests were used 2-sided and P value less than 0.05 was considered significant.
Results
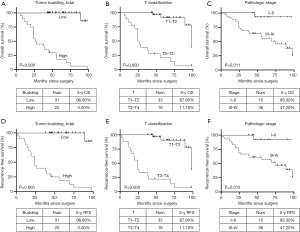
A total of 51 patients were enrolled in the present research. The patients who were lost to follow-up were excluded, and no patients died of other diseases. Clinicopathological features of the patients are demonstrated in Table 1. To evaluate the prognostic value of pathologic characteristics for patients with LSCC, univariate and multivariate analysis were carried out. As shown in Table 2 and Figure 2, univariate analysis demonstrated that tumor budding, T, N, pathologic stage, and most of the pathological factors, which included pathological differentiation, nuclear diameter, nuclear mitotic, tumor budding, tumor nest size, TSR, were significantly correlated with poor 5-year RFS and OS.
Table 2
| Variables | 5-y OS (%) | P | 5-y RFS (%) | P |
|---|---|---|---|---|
| Age | 0.776 | 0.900 | ||
| ≤65 | 60% (18/30) | 60% (18/30) | ||
| >65 | 61.9% (13/21) | 61.9% (13/21) | ||
| T | 0.000 | 0.000 | ||
| T1–T2 | 87.9% (29/33) | 87.9% (29/33) | ||
| T3–T4 | 11.1% (2/18) | 11.1% (2/18) | ||
| N | 0.000 | 0.000 | ||
| N0 | 66.7% (30/45) | 66.7% (30/45) | ||
| N1–N3 | 16.7% (1/6) | 16.7% (1/6) | ||
| Pathologic stage | 0.011 | 0.013 | ||
| Stage I–II | 93.3% (14/15) | 93.3% (14/15) | ||
| Stage III–IV | 47.2% (17/36) | 47.2% (17/36) | ||
| Differentiation | 0.000 | 0.000 | ||
| Well | 53.8% (7/13) | 53.8% (7/13) | ||
| Moderately | 69.7% (23/33) | 69.7% (23/33) | ||
| Poorly | 20.0% (1/5) | 20.0% (1/5) | ||
| Nuclear diameter | 0.005 | 0.003 | ||
| Large | 31.3% (5/16) | 31.3% (5/16) | ||
| Moderate | 78.3% (18/23) | 78.3% (18/23) | ||
| Small | 66.7% (8/12) | 66.7% (8/12) | ||
| Tumour budding, max | 0.000 | 0.000 | ||
| High | 0.0% (0/19) | 0.0% (0/19) | ||
| Low | 96.9% (31/32) | 96.9% (31/32) | ||
| Tumour budding, total | 0.000 | 0.000 | ||
| High | 5.0% (1/20) | 5.0% (1/20) | ||
| Low | 96.8% (30/31) | 96.8% (30/31) | ||
| Budding score | 0.000 | 0.000 | ||
| 1–4/hpf | 80.0% (16/20) | 80.0% (16/20) | ||
| 5–9/hpf | 80.0% (12/15) | 80.0% (12/15) | ||
| >10/hpf | 18.8% (3/16) | 18.8% (3/16) | ||
| Nest size | 0.000 | 0.000 | ||
| Large | 81.2% (13/16) | 81.2% (13/16) | ||
| Intermediate | 64.0% (16/25) | 64.0% (16/25) | ||
| Small | 16.7% (1/6) | 16.7% (1/6) | ||
| Single-cell | 25.0% (1/4) | 25.0% (1/4) | ||
| Mitotic count | 0.010 | 0.011 | ||
| High | 46.7% (14/30) | 46.7% (14/30) | ||
| Mediate | 70.0% (7/10) | 70.0% (7/10) | ||
| Low | 90.9% (10/11) | 90.9% (10/11) | ||
| Histologic subtype | 0.384 | 0.306 | ||
| Keratinization | 62.5% (25/40) | 62.5% (25/40) | ||
| Non-keratinization | 54.5% (6/11) | 54.5% (6/11) | ||
| TSR | 0.027 | 0.031 | ||
| High | 48.6% (17/35) | 48.6% (17/35) | ||
| Moderate | 87.5% (7/8) | 87.5% (7/8) | ||
| Low | 87.5% (7/8) | 87.5% (7/8) |
RFS, recurrence-free survival; OS, overall survival; TSR, tumor-stroma ratio.
As for the multivariate analysis (Table 3), after adjusting for clinical stage, total tumor budding was an independent predictor of the LSCC patients’ prognosis except for T status. Also, the histologic subtype was independently correlated with 5-year RFS (HR =3.381) and the nest size was independently correlated with 5-year OS (HR =1.843). And we found that high-grade tumor budding was associated with higher T stage (P=0.000), smaller nest size (including single-cell) (P=0.005), larger nuclear diameter (P=0.040), advanced clinical stage (P=0.004), worse poorly pathological differentiation (P=0.048) and higher TSR (P=0.005) (shown in Table 4).
Table 3
| Variables | OS | RFS | |||||
|---|---|---|---|---|---|---|---|
| HR | CI | P | HR | CI | P | ||
| T | |||||||
| T3-4vs. T1-2 | 5.217 | 1.328–20.492 | 0.018 | 5.854 | 1.410–24.306 | 0.015 | |
| Histologic subtype | |||||||
| Keratinization vs. non-keratinization | – | – | – | 3.381 | 1.043–10.960 | 0.042 | |
| Nest size | |||||||
| Large vs. small | 1.843 | 1.043–3.259 | 0.035 | – | – | – | |
| Tumor budding | |||||||
| High vs. low | 30.911 | 3.816–250.353 | 0.001 | 43.561 | 5.124–337.073 | 0.001 | |
RFS, recurrence-free survival; OS, overall survival.
Table 4
| Variables | Tumor budding | P | |
|---|---|---|---|
| Low | High | ||
| Age | 0.771 | ||
| ≤65 | 18 | 12 | |
| >65 | 14 | 7 | |
| T | 0.000 | ||
| T1–T2 | 29 | 4 | |
| T3–T4 | 3 | 15 | |
| N | 0.179 | ||
| N0 | 30 | 15 | |
| N1–3 | 2 | 4 | |
| Nest | 0.005 | ||
| Large | 14 | 2 | |
| Intermediate | 16 | 9 | |
| Small | 1 | 5 | |
| Single-cell | 1 | 3 | |
| Nuclear diameter | 0.040 | ||
| Small | 8 | 4 | |
| Intermediate | 18 | 5 | |
| Large | 6 | 10 | |
| Pathologic stage | 0.004 | ||
| I–II | 14 | 1 | |
| III–IV | 18 | 18 | |
| Differentiation | 0.048 | ||
| Well | 7 | 6 | |
| Moderately | 24 | 9 | |
| Poorly | 1 | 4 | |
| Mitotic | 0.052 | ||
| Low | 10 | 1 | |
| Mediate | 17 | 3 | |
| High | 5 | 15 | |
| TSR | 0.005 | ||
| Low | 7 | 1 | |
| Moderate | 8 | 0 | |
| High | 17 | 18 | |
| Histologic subtype | 0.726 | ||
| Keratinization | 26 | 14 | |
| Non-keratinization | 6 | 5 | |
Discussion
In previous literatures, unfavorable prognostic factors for laryngeal squamous cell carcinoma are listed as advanced tumor stage, subglottic localization, high microscopic grade, increased number and size of metastasizing lymph nodes, presence of extracapsular extension, epidermal growth factor receptor expression, and tumor budding, etc. (14-16). In the present study, we evaluated the prognostic value of histologic factors on the basis of H&E analysis.
Stromal cells take a main role in the cancer invasion and metastasis (17). The TSR has been documented as a significantly independent prognostic factor in several epithelial cancers (18). Also, TSR scoring can be completed based on H&E-stained slides without additional costs and taken less than a minute. TSR evaluation is highly reproducible due to its simplicity and reliability. Therefore, TSR scoring can be as a part of the routine histopathologic report. The patients with higher TSR showed a worse prognosis. But it was not as we expected that TSR was an independent predictor for the LSCC patients’ outcome in Cox-regression analysis. The limited sample may attribute to the result.
Tumor budding has been documented as a morphologic phenomenon of tumor invasion and to be a poor prognostic predictor in several cancers (19-22). In our research, we counted the total numbers of tumor buds using 10 HPFs and the maximum numbers in 1 HPF. Only total budding number is independently associated with 5-year RFS and OS. It has been uncovered that tumor budding is related with epithelial mesenchymal transition (EMT) to enhance tumor cell migration and invasion (23-26). Tumor budding maximum number does not show independent effect, which may be due to being difficult to agree between different pathologists when counting the maximal number of tumor budding in 1 HPF. Budding seems to be associated with the nuclear location of b-catenin, which is related to E-cadherin aberrations, along with loss of the expression of epithelial cell adhesion molecule (Ep-CAM). These changes are caused by the loss of intercellular adhesions (27). In the process of EMT, cells lose epithelial features and gain mesenchymal characteristics. Dysregulation of EMT can enhance cancer invasion and metastasis formation. However, the detailed molecular mechanism by which tumor budding enhances tumor invasion and metastasis is unclear.
The degree of keratinization was found to be independently related to the occurrence and survival of OSCC patients (28,29). Our results supported this conclusion, so as a possible risk factor for recurrence, keratinization should be considered in decisions regarding adjuvant therapy. In several kinds of squamous cell carcinoma, tumor cell nest size was highly prognostic on these malignancies (7), and tumor nest size showed independent prognosis on the LSCC patients’ 5-year OS. Further studies are necessary.
In conclusion, similar with previous researches about other malignancies, tumor budding based on the H&E-stained specimens, which can be carried out easily, can be a part of routine histopathologic reporting for LSCC. We think tumor budding will be better stratify patients with LSCC and provide better prognostic outcome. As it is a limited sample research, it needs an enlarged and multi-center LSCC samples to certify the conclusion.
Acknowledgments
Funding: This research work was funded by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.11.28). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The research was approved by the ethical committee of The First Affiliated Hospital of Guangzhou Medical University. The present work was performed after taking informed consent from the patient and a sincere effort has been made to uphold patient confidentiality.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Han L, Tang M, Xu X, et al. MiR-143-3p suppresses cell proliferation, migration, and invasion by targeting Melanoma-Associated Antigen A9 in laryngeal squamous cell carcinoma. J Cell Biochem 2018; [Epub ahead of print]. [PubMed]
- Chen L, Zeng H, Yang J, et al. Survival and prognostic analysis of preoperative inflammatory markers in patients undergoing surgical resection for laryngeal squamous cell carcinoma. BMC Cancer 2018;18:816. [Crossref] [PubMed]
- Bànkfalvi A, Piffko J. Prognostic and predictive factors in oral cancer: the role of the invasive tumour front. J Oral Pathol Med 2000;29:291-8. [Crossref] [PubMed]
- Bryne M. Is the invasive front of an oral carcinoma the most important area for prognostication? Oral Dis 1998;4:70-7. [Crossref] [PubMed]
- Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol 2005;29:167-78. [Crossref] [PubMed]
- Masuda R, Kijima H, Imamura N, et al. Tumor budding is a significant indicator of a poor prognosis in lung squamous cell carcinoma patients. Mol Med Rep 2012;6:937-43. [Crossref] [PubMed]
- Jesinghaus M, Boxberg M, Konukiewitz B, et al. A Novel Grading System Based on Tumor Budding and Cell Nest Size Is a Strong Predictor of Patient Outcome in Esophageal Squamous Cell Carcinoma. Am J Surg Pathol 2017;41:1112-20. [Crossref] [PubMed]
- Mehta A, Goswami M, Sinha R, Dogra A. Histopathological Significance and Prognostic Impact of Tumor Budding in Colorectal Cancer. Asian Pac J Cancer Prev 2018;19:2447-53. [PubMed]
- Voutsadakis IA. Prognostic role of tumor budding in breast cancer. World J Exp Med 2018;8:12-7. [Crossref] [PubMed]
- Lohneis P, Sinn M, Klein F, et al. Tumour buds determine prognosis in resected pancreatic ductal adenocarcinoma. Br J Cancer 2018;118:1485-91. [Crossref] [PubMed]
- Acs G, Esposito NN, Kiluk J, et al. A mitotically active, cellular tumor stroma and/or inflammatory cells associated with tumor cells may contribute to intermediate or high Oncotype DX Recurrence Scores in low-grade invasive breast carcinomas. Mod Pathol 2012;25:556-66. [Crossref] [PubMed]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006;6:392-401. [Crossref] [PubMed]
- Vangangelt KMH, Tollenaar LSA, van Pelt GW, et al. The prognostic value of tumor-stroma ratio in tumor-positive axillary lymph nodes of breast cancer patients. Int J Cancer 2018;143:3194-200. [Crossref] [PubMed]
- Sarioglu S, Acara C, Akman FC, et al. Tumor budding as a prognostic marker in laryngeal carcinoma. Pathol Res Pract 2010;206:88-92. [Crossref] [PubMed]
- Ünlü M, Cetinayak HO, Onder D, et al. The prognostic value of tumor-stroma proportion in laryngeal squamous cell carcinoma. Turk Patoloji Derg 2013;29:27-35. [Crossref] [PubMed]
- Akman FC, Dag N, Ataman OU, et al. The impact of treatment center on the outcome of patients with laryngeal cancer treated with surgery and radiotherapy. Eur Arch Otorhinolaryngol 2008;265:1245-55. [Crossref] [PubMed]
- Eriksen AC, Sorensen FB, Lindebjerg J, et al. The prognostic value of tumour stroma ratio and tumour budding in stage II colon cancer. A nationwide population-based study. Int J Colorectal Dis 2018;33:1115-24. [Crossref] [PubMed]
- van Pelt GW, Kjaer-Frifeldt S, van Krieken J, et al. Scoring the tumor-stroma ratio in colon cancer: procedure and recommendations. Virchows Arch 2018;473:405-12. [Crossref] [PubMed]
- Küçük Ü, Ekmekci S, Cakir E, et al. Prognostic significance of tumor budding in muscle invasive urothelial carcinomas of the bladder. Turk J Urol 2018;45:273-8. [Crossref] [PubMed]
- Lino-Silva LS, Salcedo-Hernández RA, Gamboa-Domínguez A. Tumour budding in rectal cancer. A comprehensive review. Contemp Oncol (Pozn) 2018;22:61-74. [Crossref] [PubMed]
- Weis CA, Kather JN, Melchers S, et al. Automatic evaluation of tumor budding in immunohistochemically stained colorectal carcinomas and correlation to clinical outcome. Diagn Pathol 2018;13:64. [Crossref] [PubMed]
- Kanitakis J, Karayannopoulou G. Prognostic significance of tumor budding in cutaneous squamous cell carcinoma. J Am Acad Dermatol 2018;79:e5. [Crossref] [PubMed]
- Taira T, Ishii G, Nagai K, et al. Characterization of the immunophenotype of the tumor budding and its prognostic implications in squamous cell carcinoma of the lung. Lung Cancer 2012;76:423-30. [Crossref] [PubMed]
- Yamaguchi Y, Ishii G, Kojima M, et al. Histopathologic features of the tumor budding in adenocarcinoma of the lung: tumor budding as an index to predict the potential aggressiveness. J Thorac Oncol 2010;5:1361-8. [Crossref] [PubMed]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009;119:1420-8. [Crossref] [PubMed]
- Hong KO, Oh KY, Shin WJ, et al. Tumor budding is associated with poor prognosis of oral squamous cell carcinoma and histologically represents an epithelial-mesenchymal transition process. Hum Pathol 2018;80:123-9. [Crossref] [PubMed]
- Gosens MJ, van Kempen LC, van de Velde CJ, et al. Loss of membranous Ep-CAM in budding colorectal carcinoma cells. Mod Pathol 2007;20:221-32. [Crossref] [PubMed]
- Wolfer S, Elstner S, Schultze-Mosgau S. Degree of Keratinization Is an Independent Prognostic Factor in Oral Squamous Cell Carcinoma. J Oral Maxillofac Surg 2018;76:444-54. [Crossref] [PubMed]
- Park HJ, Cha YJ, Kim SH, et al. Keratinization of Lung Squamous Cell Carcinoma Is Associated with Poor Clinical Outcome. Tuberc Respir Dis (Seoul) 2017;80:179-86. [Crossref] [PubMed]


