
Histologic transformation of lung adenocarcinoma to squamous cell carcinoma after chemotherapy: two case reports
Introduction
Small-cell lung cancer (SCLC) transformation was reported to be one of the mechanisms of acquired resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) (1). Recently, squamous cell carcinoma (SCC) transformation has been reported to take place after EGFR TKI (2), anaplastic lymphoma kinase (ALK) TKI (3), immunotherapy (4), and even spontaneously (5). Here we present two cases of SCC transformation at resistance to chemotherapy and summarize the cases and potential mechanisms of lung SCC transformation.
Case presentation
Case 1
A 51-year-old female Chinese never-smoker was presented to our hospital with a primary complaint of cough and hemoptysis for 20 days. She had no family history of cancer and was used to be healthy. The physical examination revealed reduced respiratory sound of the right lung. The chest computed tomography (CT) showed a mass in the lower lobe of the right lung (Figure 1A). The bronchoscope revealed adenocarcinoma (ADC), and the immunohistochemical (IHC) staining showed the tumor was positive for thyroid transcription factor 1 (TTF-1), cytokeratin 7 (CK7) and Napsin A, and negative for P63 and P40 (Figure 1B). Gene tests detected no aberrations of EGFR or ALK. She was diagnosed as lung adenocarcinoma, cT2aN2M0 stage IIIA.
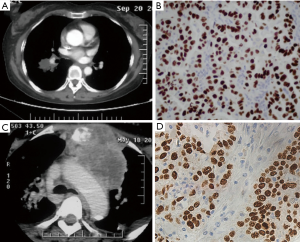
The patient received 4 cycles of cisplatin 75 mg/m2 d1 and pemetrexed 500 mg/m2 d1 Q21d and the sequential radiation of the chest with a dose of 60 Gy/30 f. She showed good adherence, for she completed the whole therapy and follow-up as planned without request for dose reduction, regimen switch, significant delay or interruption. The patient suffered from grade 2 leukopenia during treatment and recovered soon after use of granulocyte colony-stimulating factor (G-CSF). After treatment, the mass attained partial remission (PR). However, 8 months after diagnosis, a mass of the neck appeared and the chest CT found the lymph nodes at the upper mediastinum increased dramatically with a new soft tissue mass in the cervical region (Figure 1C). Then the patient received cervical lymph nodes puncture and found metastatic SCC which was positive for CK5/6, P63 and P40, and negative for TTF-1, Napsin A and synaptophysin (Figure 1D). The gene test found no EGFR, ALK or Kirsten rat sarcoma viral oncogene (KRAS) abnormities. Then she received treatment of docetaxel 75 mg/m2 Q21d for 4 cycles plus cervical radiation with a dose of 36 Gy/20 f and experienced grade 2 myelosuppression during treatment. The disease was stable for 4 months, then the patient lost to follow-up.
Case 2
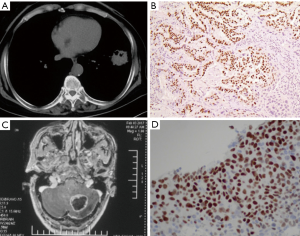
A 61-year-old male Chinese smoker was presented to another hospital with complaint of chest tightness. He had no family history of cancer and had no history of chronic diseases. No positive signs were noticed during physical examination. The chest CT showed a mass in the lower lobe of the left lung (Figure 2A). The patient underwent surgery and the pathology revealed poorly differentiated ADC which was positive for TTF-1 and Napsin A, and negative for P40 (Figure 2B). Gene tests showed no aberrations of EGFR, ALK or KRAS. He was diagnosed as lung adenocarcinoma, pT2aN1M0 stage IIB. Then the patient received adjuvant chemotherapy of paclitaxel 175 mg/m2 d1 and carboplatin AUC 6 Q21d for 4 cycles with grade 1 myelosuppression. The patient suffered from vomiting and headache 9 months later. The brain magnetic resonance imaging (MRI) showed a mass on the left cerebellum with no relapse of the lung (Figure 2C). He received surgery and the pathology revealed metastatic SCC. The tumor was positive of P63 and P40 and focally positive for TTF-1, while negative for CK7 and Napsin A (Figure 2D). Then he received treatment of cisplatin 75 mg/m2 d1 and gemcitabine 1,000 mg/m2 d1,8 Q21d for 6 cycles with good compliance. He suffered from grade 3 neutropenia during treatment and recovered with support of G-CSF. The disease-free survival (DFS) was more than 1 year. The whole diagnosis and treatment processes of the two patients were shown in Figure 3. Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Discussion
Cases of SCC transformation
There have been 26 cases of lung SCC transformation reported. Of which, 22 cases were ADC with the EGFR mutation at first and most of the transformation took place after EGFR TKI resistance; two cases with ALK rearrangement transformed after ALK TKI treatment; one case was after immunotherapy and the other happened spontaneously. Most of these patients were female (19/26) and non- or light smokers (17/19). The most frequent initial gene events were the EGFR Del19 mutation (11/21), EGFR L858R mutation (6/21), L858R + T790M mutation (2/21) and ALK translocation (2/21). Most of them received the corresponding target therapy like gefitinib, erlotinib or crizotinib and the median progression free survival (PFS) was 12 months (range, 3-30 months). The second biopsy revealed SCC with the initial mutation (11/19) or with an extra mutation like T790M or phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PIK3CA) or S768I (7/19) or T790M loss (1/19). Three cases with EGFR mutation continued the first generation TKI and the reaction differed dramatically. Four received carboplatin-based chemotherapy and pemetrexed and vinorelbine seemed effective while gemcitabine not. The two with extra T790M mutation started the third generation EGFR TKI and the efficacy seemed good (one patient received osimertinib and attained PR, the other received rociletinib for more than 10 months).
Mechanisms
Pre-existing adenosquamous carcinoma
Adenosquamous carcinoma composed 0.4–4% of non-small-cell lung cancer (NSCLC), with each component accounts for at least 10%. Adenosquamous carcinoma could have mutations of EGFR, KRAS, phosphatidylinositol 3 kinase (PI3K) and tumor protein p53 (TP53) (6).
There was a case of adenosquamous carcinoma with single component metastasis reported. A 43-year-old male patient was diagnosed as adenosquamous carcinoma bearing EGFR exon 19 mutation. He received surgery and adjuvant chemotherapy of docetaxel and cisplatin and the DFS was about 6 months. After the occurrence of brain metastasis, the second biopsy revealed ADC only (7).
In case 1, the possibility of pre-existing adenosquamous carcinoma with single component metastasis could not be excluded because of the insufficiency of small biopsy, and pemetrexed might have selectively suppressed the ADC component. But for case 2, since the two biopsies were both surgery samples, it is unlikely to be the adenosquamous carcinoma at the first place.
Interestingly, the mechanism of adenosquamous carcinoma is still under debate. A study found most driver mutations of adenosquamous carcinoma were trunk mutations, indicating a single clone origin (6). In KRAS mouse with ADC, adenosquamous carcinoma happened at liver kinase b1 (LKB1) loss or phosphatase and tensin homologue (PTEN). So even adenosquamous carcinoma could be the result of transformation from ADC to SCC (8).
SCC transformation
Some gene events are related to SCC transformation. Tumor with LKB1 loss showed increasing rate of SCC transformation (9,10). All the human tumors that are double positive of TTF1 and P63 had the LKB1 inactivation, indicating the role of LKB1 (11).
In KRASG12D;LKB1lox/lox (KL) mouse, LKB1 defect causes the imbalance of redox reaction and ROS accumulation, and thus lung tumor plasticity was induced (11). With lineage-tracing experiments, hypoxia deregulation resulted in reduction of lysyl oxidase in LKB1-deficient mice with lung ADC, then collagen disposition decreased, triggered extracellular matrix (ECM) remodeling and P63 upregulation, and ADC gradually transdifferentiated into SCC (9). Reduction of ROS with acetylcysteine decreased the incidence of SCC (11). Besides, Yes-associated protein (YAP) might participate in the process. In mouse and human lung cancer, the activation status of YAP in ADC and SCC was different. The overexpression of YAP inhibits the transformation process while YAP knockdown induces ADC to SCC transformation. In LKB1 defect mice, ECM deprivation might activate the Hippo pathway and inactivate YAP, and then releases the inhibition of DNp63 through zinc finger E-box binding homeobox 2 (ZEB2), and reprograms the SCC differentiation (12).
However, LKB1 deletion alone was not able to cause tumor formation, and various lung cancer histologies generated in KL mouse (8). But 100% of the LKB1;PTEN null tumors expressed SCC markers like P63, and transcriptionally resembled the basal subtype of human SCC, so PTEN might play an essential role in the process of transformation. PTEN (15% of human SCC) negatively regulated PI3K/protein kinase B (AKT) pathway and thus may coordinate with Lkb1 to induce SCC (11). In one case with ALK rearrangement (3), SCC took place after treatment of ALK TKI, the gene test found PTEN loss in addition to ALK alteration, which was in accordance with the mechanism above.
The amplification of Sry related HMG box 2 (SOX2) may also coordinate with LKB1 loss and promote the SCC transformation process (13). The SOX2 expression and LKB1 loss can cooperate to cause a greater incidence of SCC.
Furthermore, inhibitor of nuclear factor kappa-B kinase α (IKKα) knock-in mouse developed spontaneous SCC constantly. It was speculated that IKKα could combine to the promoter of Trim29 and upregulate the transcription of P63, Trim29 and Keratin 5m, which are the markers of SCC (14).
Thus under the stimuli of some gene alterations like EGFR, multi-potent cells may progress to ADC. During the treatment, other genetic mutations such as LKB1 were accumulated and the cells might differentiate into other lineages.
But not all the transforming cases harbored initial gene alterations or took place after specific treatment. There were also researches argued that specific cells may induce specific histology and the initial genetic events may not contribute to ADC-SCC transformation. It was reported that targeting PTEN deletion to basal cells did not increase SCC formation, at least in a keratin promoter driven model. So the function of cancer stem cells (CSCs) should be taken into consideration (15). Maybe under some selection pressure, specific precursor cells undergo a series of complex genetic events, then transformation takes place and the tumor can become resistant to the current treatment.
Most of the reported transformed cases harbored driven gene alterations like EGFR/ALK. It is still unclear whether these tumors with molecular alterations have a higher propensity to transform. Our cases transformed without EGFR mutation or EGFR TKI treatment, just like the previously reported case (5). This research compared the brain metastasis and lung samples of 24 NSCLC patients and found 3 pairs had different histologic types in lung and brain, one of which was lung ADC with brain metastatic SCC. But gene tests were not done in this study. In fact our report was the first of SCC transformation after cytotoxic drugs without any driver genetic abnormalities or target drug selection, which suggests this phenomenon may not be specific to some particular genetic events. But our gene test just including EGFR, ALK and KRAS was too limited to draw this conclusion. So more genetic alterations and signal pathways and CSC markers are warranted to understand the specific mechanism.
In conclusion, we clarified in addition to target therapy, the SCC transformation could also happen after chemotherapy, which necessitates the second biopsy and multiple gene detection after disease progression. Generally, the prognosis of SCC transformation seemed to be fair. For patients with targetable gene aberrations, target therapy could be effective; and chemotherapy could stabilize the disease for months. However, because of the scarcity of SCC transformation, the optimal treatment is still unclear and the new treatment targeting LKB1 or CSC may be promising.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.11.34). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Scher KS, Saldivar JS, Fishbein M, et al. EGFR-mutated lung cancer with T790M-acquired resistance in the brain and histologic transformation in the lung. J Natl Compr Canc Netw 2013;11:1040-4. [Crossref] [PubMed]
- Park S, Han J, Sun JM. Histologic transformation of ALK-rearranged adenocarcinoma to squamous cell carcinoma after treatment with ALK inhibitor. Lung Cancer 2019;127:66-8. [Crossref] [PubMed]
- Hsu CL, Chen KY, Kuo SW, et al. Histologic Transformation in a Patient with Lung Cancer Treated with Chemotherapy and Pembrolizumab. J Thorac Oncol 2017;12:e75-6. [Crossref] [PubMed]
- Jiang M, Zhu X, Han X, et al. Histologic transformation of non-small-cell lung cancer in brain metastases. Int J Clin Oncol 2019;24:375-84. [Crossref] [PubMed]
- Vassella E, Langsch S, Dettmer MS, et al. Molecular profiling of lung adenosquamous carcinoma: hybrid or genuine type? Oncotarget 2015;6:23905-16. [Crossref] [PubMed]
- Burkart J, Shilo K, Zhao W, et al. Metastatic Squamous Cell Carcinoma Component from an Adenosquamous Carcinoma of the Lung with Identical Epidermal Growth Factor Receptor Mutations. Case Rep Pulmonol 2015;2015:283875. [Crossref] [PubMed]
- Ji H, Ramsey MR, Hayes DN, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature 2007;448:807-10. [Crossref] [PubMed]
- Han X, Li F, Fang Z, et al. Transdifferentiation of lung adenocarcinoma in mice with Lkb1 deficiency to squamous cell carcinoma. Nat Commun 2014;5:3261. [Crossref] [PubMed]
- Xu C, Fillmore CM, Koyama S, et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell 2014;25:590-604. [Crossref] [PubMed]
- Li F, Han X, Li F, et al. LKB1 Inactivation Elicits a Redox Imbalance to Modulate Non-small Cell Lung Cancer Plasticity and Therapeutic Response. Cancer Cell 2015;27:698-711. [Crossref] [PubMed]
- Gao Y, Zhang W, Han X, et al. YAP inhibits squamous transdifferentiation of Lkb1-deficient lung adenocarcinoma through ZEB2-dependent DNp63 repression. Nat Commun 2014;5:4629. [Crossref] [PubMed]
- Mukhopadhyay A, Berrett KC, Kc U, et al. Sox2 cooperates with Lkb1 loss in a mouse model of squamous cell lung cancer. Cell Rep 2014;8:40-9. [Crossref] [PubMed]
- Xiao Z, Jiang Q, Willette-Brown J, et al. The pivotal role of IKKα in the development of spontaneous lung squamous cell carcinomas. Cancer Cell 2013;23:527-40. [Crossref] [PubMed]
- Malkoski SP, Cleaver TG, Thompson JJ, et al. Role of PTEN in basal cell derived lung carcinogenesis. Mol Carcinog 2014;53:841-6. [Crossref] [PubMed]



