
Correlation between miR-138-5p expression and efficacy of platinum-based chemotherapy in advanced gastric cancer patients
Introduction
Gastric cancer is one of the most common malignancies worldwide. In China, which has the highest morbidity rate from gastric cancer, the disease is the second most common malignancy in males, the third most common in females, and the second most lethal cancer overall (1). Most early-stage gastric cancer patients experience few symptoms, thus distant metastases are already present in about a third of patients at diagnosis (1). Palliative chemotherapies are the main treatment for advanced gastric cancer, but the prognosis remains dire. The standard first-line platinum-based chemotherapy consists of platinum and 5-fluorouracil (5-FU) (2); however, primary and secondary resistances limit the effective rate of platinum-based therapies to less than 50% (2). Moreover, the drastic differences in patient survival following treatments with the same platinum-based regimen suggest that other factors, such as genetic mutations, may influence the response. Thus, there is an urgent need to find biomarkers that can predict the efficacy of platinum-based therapies and guide individualized treatments. Recent works have shown that microRNAs (miRNAs) are potential biomarkers to predict the efficacy of palliative chemotherapy for advanced gastric cancer.
MiRNAs are a class of single-stranded RNAs that are aberrantly expressed in various cancers. In some cancer types, miRNA expression is closely related to cytotoxic agents’ treatment sensitivity (3). Yang et al. found that overexpression of miR-138-5p in a non-small cell lung cancer (NSCLC) line inhibited DNA damage repair after radiotherapy (4). Wang et al. have suggested that miR-138-5p expression was associated with cisplatin resistance in the NSCLC cell line A549/DDP, which played pivotal roles in nuclear excision repair (NER) and the DNA damage response (5). We previously showed that miR-138-5p regulated the sensitivity of gastric cancer cells to cisplatin, possibly by modulating the expression of DNA repair proteins ERCC1 and ERCC4 (6). However, as most studies have been conducted in cell lines, whether a correlation exists between the efficacy of platinum therapies and the expression of miR-138-5p in tumor tissues and plasma from gastric cancer patients hasn’t been elucidated yet.
To address this, we measured miR-138-5p expression in tissues and plasma from advanced gastric cancer patients and examined its correlation with the efficacy of platinum-based chemotherapies. The findings will contribute to the formulation of individualized regimens for gastric cancer, especially the application of platinum-combined therapies.
Methods
Study participants
The study was approved by the research ethics committee of the First Affiliated Hospital of Anhui Medical University (reference number: Quick-PJ 2016-11-11), and all individuals provided written informed consent. We enrolled a total of 51 patients (32 men, 19 women) treated at the Department of Oncology at the First Affiliated Hospital of Anhui Medical University from December 2014 to December 2015.
The inclusion criteria were: (I) diagnosed with gastric cancer pathologically by gastroscope biopsy or surgery; willing to provide plasma specimens before and after chemotherapies; considered to be stage IV according to the American Joint Committee on Cancer’s Cancer Staging Atlas, 7th edition; (II) not receive palliative radiotherapy or other irrelevant chemotherapies; (III) confirmed to have measurable tumor targets by imaging tests; (IV) Easter Cooperative Oncology Group (ECOG) scores ≤2; (V) no history of other malignancies; (VI) normal hemogram and electrocardiogram and able to tolerate chemotherapy; (VII) willing to receive at least 2 cycles of treatment and cooperate with follow-ups.
Samples of cancerous tissues and paracancerous tissues (2 cm away from the tumor edge) were obtained from 51 and 20 patients respectively, fixed in formalin, and embedded in paraffin. Plasma was prepared from peripheral blood samples collected from the 51 patients (pre-treatment) and 20 healthy volunteers.
Treatment regimens
All patients received the platinum-based chemotherapies. The regimens consisted of cisplatin [75 mg/m2; intravenous (i.v.) on days 1–3 or 1–5] or oxaliplatin (130 mg/m2; i.v. infusion over 2 h on day 1) plus capecitabine (1,000 mg/m2; twice daily on days 1–14) or S-1 (40–60 mg based on body surface area; twice daily on days 1–14. <1.25 m2 =40 mg; 1.25–1.5 m2 =50 mg; >1.5 m2 =60 mg). Paclitaxel (135 mg/m2 on day 1) or docetaxel (75 mg/m2 on day 1) were added based on actual circumstances. Each cycle was separated by a 3-week interval.
Evaluation of efficacy
Chemotherapeutic efficacy was evaluated after two treatment cycles according to the Response Evaluation Criteria in Solid Tumors guidelines (RECIST version 1.1) (7) as four outcomes, including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The objective response rate (ORR: CR+PR) and disease control rate (DCR: CR + PR + SD) were also assessed. PFS was defined as the interval from the date of first-line treatment to documented progression or death from any cause. OS was defined as the interval from the first day of treatment to death.
RNA extraction
Total RNAs were isolated from tissue blocks with a mirVana PARIS kit (Thermo Fisher Scientific) according to the manufacturer’s instructions, and its purity was measured using a NanoDrop 1000 spectrophotometer. Only samples with OD260/OD280 ratios between 1.8 and 2.1 were acceptable. After extraction, RNA samples were stored at −80 °C until analyzed. Venous blood was collected with EDTA anticoagulant tubes. Only non-hemolytic specimens were acceptable. Blood samples were centrifuged at 820 ×g for 10 min at 4 °C, and the supernatant was transferred to an RNase-treated tube and re-centrifuged at 1,600 ×g for 10 min. The supernatant was transferred to another RNase-free tube, and total RNA was extracted with a mirVana PARIS kit (Ambion #1556) (Thermo Fisher Scientific) according to the manufacturer’s instructions.
Reverse-transcription quantitative PCR (qRT-PCR)
qRT-PCR was performed using an EzOmics One-Step qPCR Kit (Biomics). U6 mRNA and miR-16 levels were measured as internal references for tissue- and plasma-derived RNA respectively. Reaction mixtures (total 25 µL) consisted of specific primer pairs for miR-138-5p, U6, or miR-16, 2× Master Mix, and RNase-free DEPC water. Reactions were performed in an Mx3000P qRT-PCR system using reverse transcription for 30 min at 42 °C, pre-denaturation at 95 °C for 10 min, annealing for 30 s at 60 °C, and extension for 30 s at 72 °C. After 40 cycles, the fluorescence signal was measured at 72 °C. Relative miR-138-5p levels were calculated using the 2−ΔΔCT method and were normalized by subtracting the Ct values for miR-16 or U6 from that of miR-138-5p.
Statistical analysis
SPSS19.0 software (SPSS Inc., Chicago, IL, USA) was applied to statistical analysis. The Mann-Whitney U test was used for assessing miR-138-5p expression in gastric tissues and plasma. The ability of miR-138-5p expression to predict platinum efficacy was assessed by constructing receiver operator characteristic (ROC) curves. Survival was analyzed by the Kaplan-Meier method and log-rank test. The correlation between miR-138-5p expression in gastric cancer tissues and plasma was evaluated by linear correlation analysis. Univariate and multivariate Cox regression analyses were used to examine whether miR-138-5p expression could predict patients survival. A value of P<0.05 was regarded as statistically significant.
Results
MiR-138-5p expression in advanced gastric cancer
MiR-138-5p expression was measured by qRT-PCR, whose level in gastric cancer tissues was significantly lower than in paracancerous tissues (mean ± SD: 0.087±0.038 and 0.126±0.022, respectively; Z=4.30, P<0.01), as shown in Figure 1A. Similarly, miR-138-5p expression was significantly lower in plasma of patients with advanced gastric cancer than in that of healthy volunteers (0.071±0.037 and 0.097±0.014, respectively; Z=3.0, P<0.01), as shown in Figure 1B.
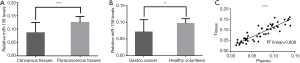
A linear correlation analysis showed that miR-138-5p expression in cancer tissues and plasma were positively correlated (Pearson’s correlation r=0.899, P<0.05; r2=0.808, Figure 1C), suggesting that the miR-138-5p expression in patient’s plasma could represent its counterpart in cancer tissues.
Correlation between miR-138-5p expression and clinicopathological features of gastric cancer
As shown in Table 1, the miR-138-5p expression in advanced gastric cancer tissues and plasma did not correlate with patients’ sex, ECOG score, tumor location, tumor tissue differentiation, liver or peritoneal metastasis, number of metastatic organs. However, higher miR-138-5p expression in cancer tissues was more likely to occur in patients aged below 60 (P=0.03).
Table 1
| Characteristics | N | Percentage (%) | Plasma | Tissue | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2−ΔΔCT () | t | P | 2−ΔΔCT () | t | P | ||||
| Gender | 0.10 | 0.92 | 0.20 | 0.84 | |||||
| Male | 32 | 62.7 | 0.072±0.038 | 0.088±0.040 | |||||
| Female | 19 | 37.3 | 0.071±0.036 | 0.086±0.036 | |||||
| Age (years) | 1.65 | 0.11 | 2.24 | 0.03 | |||||
| ≥65 | 25 | 49.0 | 0.063±0.037 | 0.075±0.036 | |||||
| <65 | 26 | 51.0 | 0.080±0.036 | 0.098±0.037 | |||||
| ECOG (scores) | 0.86 | 0.40 | 0.19 | 0.85 | |||||
| 0–1 | 40 | 78.4 | 0.069±0.036 | 0.087±0.036 | |||||
| 2 | 11 | 21.6 | 0.080±0.044 | 0.089±0.048 | |||||
| Differentiation | 0.27 | 0.79 | 0.08 | 0.94 | |||||
| Poor | 35 | 68.6 | 0.072±0.038 | 0.087±0.038 | |||||
| Moderate and well | 16 | 31.4 | 0.069±0.036 | 0.088±0.039 | |||||
| Tumor location | 0.26 | 0.80 | 0.11 | 0.91 | |||||
| Proximal and body | 36 | 70.6 | 0.072±0.038 | 0.087±0.036 | |||||
| Distal | 15 | 29.4 | 0.069±0.037 | 0.088±0.045 | |||||
| Liver metastasis | 0.25 | 0.81 | 0.13 | 0.90 | |||||
| Present | 21 | 41.2 | 0.073±0.036 | 0.086±0.037 | |||||
| Absent | 30 | 58.8 | 0.070±0.039 | 0.088±0.040 | |||||
| Peritoneal metastasis | 1.16 | 0.25 | 0.95 | 0.35 | |||||
| Present | 6 | 11.8 | 0.055±0.034 | 0.073±0.031 | |||||
| Absent | 45 | 88.2 | 0.074±0.037 | 0.089±0.039 | |||||
| Number of metastatic organs | 1.12 | 0.27 | 1.12 | 0.27 | |||||
| 1 | 22 | 43.1 | 0.065±0.039 | 0.080±0.038 | |||||
| ≥2 | 29 | 56.9 | 0.076±0.036 | 0.092±0.038 | |||||
ECOG, Easter Cooperative Oncology Group.
Correlation between miR-138-5p expression in cancer tissues and the efficacy of platinum-based chemotherapies
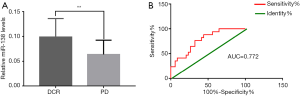
In all 51 advanced gastric cancer patients, among whom the first-line platinum-based chemotherapeutic treatment efficacy could be evaluated, 11 patients showed PR after treatment, whereas 23 patients exhibited SD, and 17 patients presented PD. ORR and DCR were 21.6% and 66.7%, respectively. The miR-138-5p expression in DCR group was higher than PD group (0.099±0.037 and 0.064±0.029, respectively; Z=−3.137, P<0.05, Figure 2A). We constructed ROC curve analysis to identify the optimal miR-138-5p expression cutoff value for DCR prediction. When the Youden index was at its maximum value, the area under the curve (AUC) was 0.772 (95% CI: 0.642–0.901) and the relative expression level of miR-138-5p was 0.081. The sensitivity and specificity for prediction of DCR were 67.65% and 76.47%, respectively (Figure 2B).
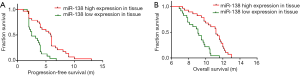
After 18 months of follow-up, all the 51 advanced gastric cancer patients died. The median PFS and OS were 4.0 months (95% CI: 3.76–4.3 months) and 9.9 months (95% CI: 9.2–10.6 months), respectively. Using the cutoff value of 0.081 as the discriminating point between high (n=28) and low (n=23) tissues miR-138-5p expression, the median PFS for the high and low groups were 5.8 months (95% CI: 5.1–6.5 months) and 2.5 months (95% CI: 1.1–3.9 months), respectively (log-rank, χ2=15.94, P<0.05; Figure 3A). Similarly, the median OS of patients with high and low tissues miR-138-5p expression were 11.2 months (95% CI: 10.8–11.6 months) and 9.1 months (95% CI: 8.6–9.6 months) respectively (log-rank, χ2=20.38, P<0.05; Figure 3B).
Correlation between miR-138-5p expression in plasma and the efficacy of platinum-based chemotherapies
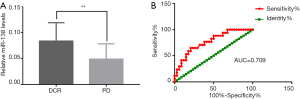
The plasma miR-138-5p expression in DCR group was 0.083±0.036, which was significantly higher than that in PD group (0.048±0.029, Z=−3.107, P<0.01, Figure 4A). ROC curve analysis was also used to identify the optimal miR-138-5p expression cutoff value for DCR prediction here. When the Youden index reached its maximum, the AUC was 0.769 (95% CI: 0.636–0.902) and the plasma expression of miR-138-5p was 0.047. The sensitivity and specificity for prediction of DCR were 79.41% and 64.71% respectively (Figure 4B).
Using the cutoff value of 0.047 to distinguish between high and low plasma miR-138-5p expression, patients in the high (n=33) and low (n=18) expression groups had median PFS of 5.5 months (95% CI: 3.8–7.2 months) and 2.1 months (95% CI: 1.5–2.7 months), respectively (log-rank, χ2=16.48, P<0.05; Figure 5A). The corresponding median OS were 11.1 months (95% CI: 10.4–11.8 months) and 8.9 months (95% CI: 8.1–9.7 months), respectively (log-rank test, χ2=20.2, P<0.05; Figure 5B).

Furthermore, univariate cox and multivariate cox analysis revealed that the miR-138-5p expression in tissues and plasma were both independent predictive factor for PFS and OS in advanced gastric cancer patients (all P<0.05) (Tables 2,3).
Table 2
| Parameters | Categories | HR (95% CI) | P value |
|---|---|---|---|
| PFS | |||
| Age | ≥65 vs. <65 | 1.082 (0.598–1.958) | 0.794 |
| Gender | Male vs. female | 1.093 (0.615–1.941) | 0.763 |
| Grade | G1–2 vs. G3 | 0.784 (0.423–1.453) | 0.444 |
| ECOG | 0–1 vs. 2 | 1.543 (0.775–3.072) | 0.234 |
| Liver metastasis | Present vs. absent | 1.289 (0.729–2.279) | 0.380 |
| Peritoneal metastasis | Present vs. absent | 0.702 (0.295–1.669) | 0.442 |
| Location | Proximal and body vs. distal | 0.772 (0.413–1.443) | 0.409 |
| Number of metastatic organs | 1 vs. ≥2 | 0.764 (0.426–1.369) | 0.366 |
| Chemotherapy regimens | Two drugs vs. three drugs | 1.458 (0.697–3.050) | 0.335 |
| Chemotherapy response | CR + PR vs. PD | 0.011 (0.001–0.093) | <0.001 |
| SD vs. PD | 0.015 (0.002–0.119) | <0.001 | |
| miR–138–5p level (tissue) | Low vs. high | 0.279 (0.146–0.533) | <0.001 |
| miR–138–5p level (plasma) | Low vs. high | 0.190 (0.089–0.405) | <0.001 |
| OS | |||
| Age | ≥65 vs. <65 | 0.875 (0.493–1.554) | 0.649 |
| Gender | Male vs. female | 1.536 (0.855–2.761) | 0.158 |
| Grade | G1–2 vs. G3 | 0.868 (0.472–1.596) | 0.651 |
| ECOG | 0–1 vs. 2 | 1.391 (0.703–2.752) | 0.357 |
| Liver metastasis | Present vs. absent | 1.121 (0.637–1.972) | 0.692 |
| Peritoneal metastasis | Present vs. absent | 0.865 (0.366–2.046) | 0.865 |
| Location | Proximal and body vs. distal | 0.968 (0.525–1.784) | 0.916 |
| Number of metastatic organs | 1 vs. ≥2 | 0.694 (0.384–1.253) | 0.225 |
| Chemotherapy regimens | Two drugs vs. three drugs | 1.739 (0.814–3.714) | 0.153 |
| Chemotherapy response | CR + PR vs. PD | 0.078 (0.031–0.200) | <0.001 |
| SD vs. PD | 0.151 (0.070–0324.) | <0.001 | |
| miR–138–5p level (tissue) | Low vs. high | 0.214 (0.108–0.421) | <0.001 |
| miR–138–5p level (plasma) | Low vs. high | 0.155 (0.071–0.337) | <0.001 |
PFS, progression-free survival; OS, overall survival; ECOG, Easter Cooperative Oncology Group; CR, complete response; PR, partial response; PD, progressive disease; SD, stable disease.
Table 3
| Parameters | Categories | Plasma | Tissue | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| PFS | ||||||
| Chemotherapy response | CR + PR vs. PD | 0.018 (0.002–0.162) | <0.001 | 0.017 (0.002–0.146) | <0.001 | |
| SD vs. PD | 0.019 (0.002–0.153) | <0.001 | 0.020 (0.003–0.164) | <0.001 | ||
| miR–138-5p level (tissue) | High vs. low | 0.439 (0.200–0.961) | 0.039 | |||
| miR–138-5p level (plasma) | High vs. low | 0.378 (0.160–0.889) | 0.026 | |||
| OS | ||||||
| Chemotherapy response | CR + PR vs. PD | 0.110 (0.039–0.312) | <0.001 | 0.119 (0.042–0.336) | <0.001 | |
| SD vs. PD | 0.150 (0.067–0.339) | <0.001 | 0.209 (0.092–0.477) | <0.001 | ||
| miR-138-5p level (tissue) | High vs. low | 0.275 (0.130–0.583) | 0.001 | |||
| miR-138-5p level (plasma) | High vs. low | 0.283 (0.119–0.672) | 0.004 | |||
PFS, progression-free survival; OS, overall survival; CR, complete response; PR, partial response; PD, progressive disease; SD, stable disease.
Discussion
Our previous studies demonstrated that the expression of miR-138-5p was significantly lower in the cisplatin-resistant advanced gastric cancer cell line SGC-7901/DDP compared with the sensitive line SGC-7901. We also showed that miR-138-5p overexpression could partially reverse cisplatin resistance by negatively regulating ERCC1 and ERCC4 enzyme activity and inhibiting the NER pathway (6). The results above suggested that miR-138-5p might be a potential therapeutic target, a predictive biomarker for sensitivity to platinum-based therapies, and/or a guide for individualized treatment. However, few studies have examined the miR-138-5p expression in advanced gastric cancer tissues or plasma, or its relationship with the efficacy of platinum-based chemotherapies. Therefore, we collected cancer tissues and plasma from 51 patients with advanced gastric cancer to explore whether the miR-138-5p expression can predict therapeutic efficacy and patients’ prognosis.
MiR-138-5p is considered to be an anti-oncogene in many type of cancer cells include NSCLC cell (8), cervical cancer cell (9), oral squamous cell carcinoma (10), gallbladder cancer cell (11) and pancreatic cancer cell (12) and etc. Most studies on the effects of miR-138-5p upon drug responses have suggested that it has chemosensitizing activity. Wang et al. reported that the miR-138-5p expression was correlated with and played a role in cisplatin sensitivity of A549/DDP by negatively regulating ERCC1 and DNA repair (5). Yang et al. confirmed that miR-138 can diminish DNA repair in tumors by directly acting on H2AX (4). In osteosarcoma cells, upregulation of miR-138 increased the expression of the apoptosis effector protein caspase-3, which targeted EZH2 and enhanced cisplatin sensitivity (13). Studies in NSCLC cells showed that miR-138-5p enhanced doxorubicin sensitivity by targeting E-cadherin and ZEB2, downregulating vimentin, and inhibiting epithelial to mesenchymal transition (14). Han et al. showed that elevated miR-138 expression enhanced the sensitivity of NSCLC cells to cisplatin via CCND3 (15). In the cervical cancer HeLa cell line, miR-138-5p enhanced its sensitivity to 5-FU and adriamycin by downregulating FAK expression (16). Nevertheless, little is known about the involvement of miR-138-5p in gastric cancer.
In this study, we found that miR-138-5p expression was significantly lower in cancer tissues and plasma than in paracancerous tissues and normal plasma. This result herein was consistent with the researches aforementioned, suggesting that miR-138-5p might act as an anti-oncogene and a reduction in its expression contributed to the development of gastric cancer. The miR-138-5p expression was positively linked with the efficacy, PFS, and OS of first-line platinum-based regimens, which was in parallel with our previous studies on the effects of miR-138-5p on SGC7901/DDP and SGC7901 cells (6). Meanwhile, a similar chemosensitizing activity, which was unveiled in previous studies on miR-138-5p, was also observed here.
Recent studies have revealed that miRNAs can be readily detected in plasma, and the level often corresponded with that in tumor tissues (17). Microarray and PCR analysis of plasma samples have identified six miRNAs (miR-1, miR-20a, miR-27a, miR-34, miR-378, and miR-423-5p) that could serve as diagnostic biomarkers for gastric cancer, with miR-378 being specific for early stages. Other studies have demonstrated that circulating miR-214, miR-122, and miR-192 are novel biomarkers for gastric cancer that indicated severer metastases (18,19). Also, circulating miR-200c could predict the sensitivity of esophageal cancer to neoadjuvant chemotherapies and act as a prognostic biomarker (20). Our study identified a positive relationship between the miR-138-5p levels in tumor and plasma (Pearson’s correlation r=0.899, P<0.05), which was consistent with the study of Tsujiura et al. (17), confirming that the plasma miR-138-5p expression could perform as a surrogate for tumor tissue biopsy to predict platinum-based chemotherapies efficacy and avoid difficulties in clinical collection of tumor tissues.
Previous studies have indicated that in advanced gastric cancer, those who responded well to first-line chemotherapies survive longer. Therefore, it would be of great benefit to screen patients for platinum sensitivity before drafting the first-line treatment regimen. Here, we found that the DCR, PFS and OS of platinum-based chemotherapies were significantly better in patients with a tissue miR-138-5p expression higher than 0.081 and a plasma level above 0.047. Univariate and multivariate analysis confirmed the higher miR-138-5p expression was independently correlated with longer PFS and OS.
The ORR and DCR of our cohort of 51 patients were 21.6% and 66.7%, respectively, and the corresponding PFS and OS were 4.0 months (95% CI: 3.7–4.3 months) and 9.9 months (95% CI: 9.2–10.6 months) respectively. Overall, these findings agreed with the results of previous studies. In advanced gastric cancer patients, due to the short post-diagnosis lifetime, ineffective chemotherapy may increase suffering and compromising life quality. Thus, miR-138-5p expression may be used to predict the efficacy of postoperative adjuvant chemotherapy to avoid ineffective attempts and prolong disease free survival.
Conclusions
In conclusion, miR-138-5p expression in both cancer tissues and plasma could be potential biomarkers for predicting platinum-based treatment efficacy and prognosis of advanced gastric cancer, therefore guiding individualized treatments in clinical practice. Study limitations included the small sample size, chemotherapy regimens included cisplatin and oxaliplatin, and the focus on a single miRNA. Future studies should investigate the potential interactions among different miRNAs. Validation of our findings in a large prospective randomized clinical trial and stratified analysis of cisplatin and oxaliplatin will help to elaborate individualized treatments for gastric cancer in the clinics.
Acknowledgments
We thank Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding: This project was funded by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.11.25). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the research ethics committee of the First Affiliated Hospital of Anhui Medical University (reference number: Quick-PJ 2016-11-11), and all individuals provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schlansky B, Sonnenberg A. Epidemiology of noncardia gastric adenocarcinoma in the United States. Am J Gastroenterol 2011;106:1978-85. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286-312. [Crossref] [PubMed]
- Huang D, Wang H, Liu R, et al. miRNA27a is a biomarker for predicting chemosensitivity and prognosis in metastatic or recurrent gastric cancer. J Cell Biochem 2014;115:549-56. [Crossref] [PubMed]
- Yang H, Luo J, Liu Z, et al. MicroRNA-138 Regulates DNA Damage Response in Small Cell Lung Cancer Cells by Directly Targeting H2AX. Cancer Invest 2015;33:126-36. [Crossref] [PubMed]
- Wang Q, Zhong M, Liu W, et al. Alterations of microRNAs in cisplatin-resistant human non-small cell lung cancer cells (A549/DDP). Exp Lung Res 2011;37:427-34. [Crossref] [PubMed]
- Ning J, Jiao Y, Xie X, et al. miR1385p modulates the expression of excision repair crosscomplementing proteins ERCC1 and ERCC4, and regulates the sensitivity of gastric cancer cells to cisplatin. Oncol Rep 2019;41:1131-9. [PubMed]
- Watanabe H, Okada M, Kaji Y, et al. New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1). Gan To Kagaku Ryoho 2009;36:2495-501. [PubMed]
- Li J, Wang Q, Wen R, et al. MiR-138 inhibits cell proliferation and reverses epithelial-mesenchymal transition in non-small cell lung cancer cells by targeting GIT1 and SEMA4C. J Cell Mol Med 2015;19:2793-805. [Crossref] [PubMed]
- Ou L, Wang D, Zhang H, et al. Decreased Expression of miR-138-5p by lncRNA H19 in Cervical Cancer Promotes Tumor Proliferation. Oncol Res 2018;26:401-10. [Crossref] [PubMed]
- Zhuang Z, Xie N, Hu J, et al. Interplay between DeltaNp63 and miR-138-5p regulates growth, metastasis and stemness of oral squamous cell carcinoma. Oncotarget 2017;8:21954-73. [Crossref] [PubMed]
- Ma F, Zhang M, Gong W, et al. MiR-138 Suppresses Cell Proliferation by Targeting Bag-1 in Gallbladder Carcinoma. PLoS One 2015;10:e0126499. [Crossref] [PubMed]
- Yu C, Wang M, Li Z, et al. MicroRNA-138-5p regulates pancreatic cancer cell growth through targeting FOXC1. Cell Oncol (Dordr) 2015;38:173-81. [Crossref] [PubMed]
- Zhu Z, Tang J, Wang J, et al. MiR-138 Acts as a Tumor Suppressor by Targeting EZH2 and Enhances Cisplatin-Induced Apoptosis in Osteosarcoma Cells. PLoS One 2016;11:e0150026. [Crossref] [PubMed]
- Jin Z, Guan L, Song Y, et al. MicroRNA-138 regulates chemoresistance in human non-small cell lung cancer via epithelial mesenchymal transition. Eur Rev Med Pharmacol Sci 2016;20:1080-6. [PubMed]
- Han LP, Fu T, Lin Y, et al. MicroRNA-138 negatively regulates non-small cell lung cancer cells through the interaction with cyclin D3. Tumour Biol 2016;37:291-8. [Crossref] [PubMed]
- Golubovskaya VM, Sumbler B, Ho B, et al. MiR-138 and MiR-135 directly target focal adhesion kinase, inhibit cell invasion, and increase sensitivity to chemotherapy in cancer cells. Anticancer Agents Med Chem 2014;14:18-28. [Crossref] [PubMed]
- Tsujiura M, Ichikawa D, Komatsu S, et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer 2010;102:1174-9. [Crossref] [PubMed]
- Zhang KC, Xi HQ, Cui JX, et al. Hemolysis-free plasma miR-214 as novel biomarker of gastric cancer and is correlated with distant metastasis. Am J Cancer Res 2015;5:821-9. [PubMed]
- Chen Q, Ge X, Zhang Y, et al. Plasma miR-122 and miR-192 as potential novel biomarkers for the early detection of distant metastasis of gastric cancer. Oncol Rep 2014;31:1863-70. [Crossref] [PubMed]
- Tanaka K, Miyata H, Yamasaki M, et al. Circulating miR-200c levels significantly predict response to chemotherapy and prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. Ann Surg Oncol 2013;20:S607-15. [Crossref] [PubMed]


