
Bilateral Gamma Knife thalamotomy for treatment of axial tremor
Introduction
Essential tremor (ET) is most frequently marked by tremor of the upper extremities but in some patients either in addition to extremity tremor or in isolation, tremor of the axial portions of the body including the head, voice and lower jaw may be disabling and require intervention. Primidone (mysoline) and beta blockers are the usual pharmacological treatments of ET and other anticonvulsant medications may also be used. In some patients however medical treatment fails, either because it is ineffective after a time or side effects prevent the use of adequate doses. In such circumstances surgical intervention may be considered. Patients with significant head tremor may be embarrassed in public or in more severe cases may be unable to read, use a computer, watch television or movies, drive a motor vehicle safely and may also develop neck pain due to constant head and neck movement. Voice tremor may interfere with communication via telephone or in severe cases even in face to face conversation. The voice handicap associated with ET has multiple dimensions including physical, functional and emotional (1). Hobbies or in some cases an individual’s livelihood may be impaired due to inability to sing or speak. Tremor of the lower jaw, although less common than head or voice tremor, may be extremely embarrassing in public and may interfere with eating, chewing and speaking. In some patients combinations of two or even three of these types of axial tremor may be severely disabling.
A number of reports have described small numbers of patients treated for axial tremor by deep brain stimulation (DBS) usually in the ventral intermediate (VIM) thalamic nucleus (2-8). The reports are rather conflicting in terms of a determination of the effectiveness of unilateral versus bilateral stimulation with some authors having reported excellent relief of axial tremor with unilateral stimulation and others indicating that bilateral stimulation provided the most effective treatment. A recent report suggests that the trajectory of electrode placement may influence the outcome (8). Unfortunately, not all patients are candidates for DBS due to factors such as advanced age, chronic use of anticoagulants, multiple medical conditions (e.g., diabetes, severe coronary artery disease, severe pulmonary disease) or simply a refusal to undergo an open neurosurgical operation or to deal with implanted hardware with its long term problems (e.g., lead fracture, lead displacement, wearing off of effectiveness, and infection) including the need for programming and battery changes at varying intervals.
In such patients, thalamotomy of the VIM nucleus with the Leksell Gamma Unit, the so-called “Gamma Knife” may be considered. In this report we describe our experience with bilateral Gamma Knife thalamotomy (GKT) for treatment of axial tremor.
Materials and methods
Sixty-eight patients with ET either predominantly or only affecting axial structures including head and neck, voice and lower jaw underwent staged bilateral GKT between 2006 and 2012. Tremor severity was evaluated using a 5-step scale [0-4] adapted from the Fahn, Tolosa and Marin, Tremor Rating Scale (9) (Table 1) by a team of experienced medical personnel as in our prior reports. Half point ratings were assigned (e.g., 0.5, 1.5, 2.5, 3.5) for patients in whom the degree of tremor varied over time so that a single ordinal number did not accurately reflect the tremor severity. Median age at the time of the first procedure was 73.6 years, range 54-88 years. All except six patients in whom the usual medications were either ineffective or caused intolerable side effects, had been evaluated and treated prior to the procedures by a neurologist. Patients were not offered GKT unless the tremor score was 2 or greater with maximum tolerable medical treatment. In the six patients who had not undergone prior medical treatment the medications were either refused by the patient or were felt to be contraindicated due to other medical conditions. Median duration of tremor prior to treatment was 8.5 years and ranged from 4-28 years.
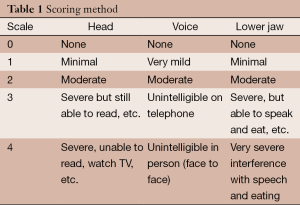
Full table
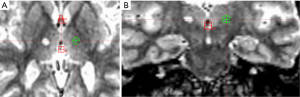
A unilateral GKT was performed in the dominant hemisphere using previously described techniques (10,11). In brief, the Leksell Model G stereotactic frame was attached to the patient’s head using local anesthesia preceded by 1-2 mg of sublingual Lorazepam. A 1.5 T MRI scan was then performed and the target coordinates were determined using the Leksell Gamma Plan. The images were reformatted in the plane of the anterior and posterior commissures and only the Y or anterior-posterior coordinate was determined empirically based on the intercommissural distance, typically 4-5 mm posterior to the mid-commissural point. A coronal MRI image at that A/P coordinate was examined and the center of the intended lesion was placed at the inferior lateral border of the thalamus such that the 50% isodose line of the 4 mm collimator shot coincided with that border (Figure 1A,B). The lesion was made with a single 4 mm isocenter and a maximum radiosurgical dose of 135-140 Gy. Following delivery of the prescribed dose the stereotactic frame was removed and the patient was discharged usually within 30 minutes.
Follow up assessment of tremor severity was obtained 6 months and one year following the procedure. If by one year following completion of the procedure there was at least some improvement in tremor a second contralateral procedure was offered providing that no side effects or complications had occurred and the patient wished to proceed. A second contralateral procedure was performed using an identical technique to the first procedure, between 12 and 21 months (mean 15.5 months) following the first procedure in all patients described in this report. Follow up was then resumed 6 months and one year after the second procedure and then annually thereafter. In addition to assessment of tremor severity, patients were asked to make their own individual estimates of the degree of tremor improvement in follow up. Statistical analysis of the change in tremor scores was assessed after both the first and second procedures compared to the baseline pre-procedure scores, using pairwise comparisons of the pre-operative scores and the scores just prior to the second procedure and again at the time of last follow up, using the Wilcoxon signed rank test. Statistical significance was assumed if the P value was 0.05 or better.
Results
Table 2 following the first GKT head tremor decreased from a pre-operative score of 2.8+/–1.2 to 2.2+/–1.1 an improvement of 21.4%. After the second procedure the score further decreased at the time of last follow up to 1.1+/–0.7 an improvement of 60.7%. Both levels of improvement represent statistically significant values (P<0.001 and P<0.0001 respectively). For voice tremor the pre-operative score of 2.9+/–1.0 decreased to 1.9+/–0.8 after the first procedure an improvement of 34.0% and to 1.2+/–0.6 after the second procedure an overall improvement of 58.6%. Both levels of improvement are statistically significant (P<0.001 and P<0.0001 respectively). For tremor of the lower jaw the pre-operative score of 3.0+/–1.1 decreased to 1.8+/–0.9 after the first procedure, an improvement of 40.0% and to 1.1+/–0.5 after the second procedure an overall improvement of 63.3%. Both levels of improvement are statistically significant (P<0.001 and P<0.0001 respectively).
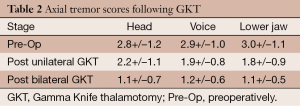
Full table
Two patients sustained permanent complications between 6 and 12 months following the second procedures even though they had shown no complications after the first procedure. This represents a complication rate of 1.5% calculated on a per procedure basis and 2.9% calculated on a per patient basis. In both cases the complications included difficulties with speech and balance.
Discussion
Bilateral GKT provides a significant improvement in axial tremor with a low rate of complications. To our knowledge this is the first description of the use of bilateral GKT to treat axial tremor, although several reports have demonstrated the benefit of GKT on ET of the upper extremities (10-16). A number of reports of the application of DBS to the treatment of axial tremor have appeared in the literature but to quote one set of authors the results have been “inconsistent” (8). An extensive review of the use of DBS to treat ET concluded that in 2 of 17 studies the effect of DBS on head tremor was established with bilateral VIM DBS as being more effective than unilateral VIM DBS (3). In one study voice tremor improved only in patients with severe symptoms and there was no difference between the effect of unilateral versus bilateral stimulation (6). In another study bilateral DBS produced improvement in voice tremor in 83% of patients, much better than with unilateral stimulation (4) and in still another study there was no improvement in voice tremor with either unilateral or bilateral DBS in 19 patients (7).
Bilateral DBS in the VIM often causes difficulty with speech and balance in up to 73% of patients (17) and in a recent large study, 8% of patients who underwent DBS for a variety of indications, including ET, experienced neurological complications of the procedure and 10% experienced hardware related complications including lead fracture and infection which required removal and later replacement of the hardware (18). In addition DBS requires regular programming and replacement of the implanted pulse generator when battery depletion occurs at a maximum of every 5 years and frequently more often in ET patients. Programming and routine battery replacements, in the absence of any complications, add significantly to the cost of DBS and may be relatively easy or difficult. Pedrosa et al. (19) indicated that high frequency DBS produced significant improvement in tremor but simultaneously worsened verbal fluency whereas low frequency stimulation enhanced verbal fluency but did not improve tremor.
Finally, one report suggests that radiofrequency thalamotomy (RFT) may be used as a salvage procedure after ineffective DBS (20), although bilateral RFT is associated with a high complication rate of 20-25% including dysphasia, dysarthria, abulia and even akinetic mutism.
Several prior reports have described the effectiveness of GKT for treatment of extremity tremor in ET. A total of six reports which include over 600 patients in total, indicate that extremity tremor is relieved in a significant number of patients with GKT (10-16). In our own large series there was an average reduction of 51% in tremor severity with 80% of patients experiencing total or near total relief of tremor within 6 months after a GKT procedure (10). Unilateral GKT, in our experience has an 8% rate of temporary complications, a 4% rate of permanent complications and a 1-2% risk of serious permanent complications which may include sensory loss, weakness or paralysis and speech problems (10). Interestingly, in both the current study and our prior published experience with bilateral staged DBS for extremity tremor in ET the complication rate is much lower, about 1-3% and consists almost exclusively of problems with speech and balance (10).
Conclusions
Bilateral GKT appears to offer a safe and effective treatment option for patients with axial ET related tremors. In contrast to DBS in which bilateral procedures may either be performed at the same operative procedure or with a short interim period of a few weeks to a few months, bilateral GKT may only be safely performed with a minimal interval of 1 year in order to insure there are no complications of a unilateral procedure before proceeding to perform the contralateral side. In addition, the results of DBS are immediately apparent, although late tolerance may occur.
With bilateral GKT, due to the latency period after the first thalamotomy before effectiveness can be judged (1 year) and the necessary waiting period following the second thalamotomy procedure (1 year) the final results of bilateral GKT both in terms of safety and efficacy cannot be determined for 2 years. Nevertheless, for some patients, bilateral GKT may offer their only option for treatment of axial tremors and many patients are unwilling to undergo DBS but are willing to endure the latency period associated with bilateral GKT to avoid the problems associated with DBS. Our study confirms the viability of GKT for the treatment of axial tremors.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.11.01). SV serves as an unpaid editorial board member of Translational Cancer Research. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board. Written informed consent was obtained from the patients for treatment but not needed for the chart review and/ publication.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Louis ED, Gerbin M. Voice handicap in essential tremor: a comparison with normal controls and Parkinson’s disease. Tremor Other Hyperkinet Mov (NY) 2013;3.
- Taha JM, Janszen MA, Favre J. Thalamic deep brain stimulation for the treatment of head, voice, and bilateral limb tremor. J Neurosurg 1999;91:68-72. [PubMed]
- Chopra A, Klassen BT, Stead M. Current clinical application of deep-brain stimulation for essential tremor. Neuropsychiatr Dis Treat 2013;9:1859-65. [PubMed]
- Carpenter MA, Pahwa R, Miyawaki KL, et al. Reduction in voice tremor under thalamic stimulation. Neurology 1998;50:796-8. [PubMed]
- Koller WC, Lyons KE, Wilkinson SB, et al. Efficacy of unilateral deep brain stimulation of the VIM nucleus of the thalamus for essential head tremor. Mov Disord 1999;14:847-50. [PubMed]
- Obwegeser AA, Uitti RJ, Turk MF, et al. Thalamic stimulation for the treatment of midline tremors in essential tremor patients. Neurology 2000;54:2342-4. [PubMed]
- Sydow O, Thobois S, Alesch F, et al. Multicentre European study of thalamic stimulation in essential tremor: a six year follow up. J Neurol Neurosurg Psychiatry 2003;74:1387-91. [PubMed]
- Moscovich M, Morishita T, Foote KD, et al. Effect of lead trajectory on the response of essential head tremor to deep brain stimulation. Parkinsonism Relat Disord 2013;19:789-94. [PubMed]
- Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E. eds. Parkinson’s disease and movement disorders. Baltimore: William & Wilkins, 1993:271-80.
- Young RF, Li F, Vermeulen S, et al. Gamma Knife thalamotomy for treatment of essential tremor: long-term results. J Neurosurg 2010;112:1311-7. [PubMed]
- Young RF. Stereotactic Radiosurgery for Movement Disorders. In: Starr PA, Barbaro NM, Larson PS. eds. Neurosurgical Operative Atlas, 2nd ed: Functional Neurosurgery. New York: Thieme, 2009:165-8.
- Kondziolka D, Ong JG, Lee JY, et al. Gamma Knife thalamotomy for essential tremor. J Neurosurg 2008;108:111-7. [PubMed]
- Young RF, Jacques S, Mark R, et al. Gamma knife thalamotomy for treatment of tremor: long-term results. J Neurosurg 2000;93:128-35. [PubMed]
- Ohye C, Higuchi Y, Shibazaki T, et al. Gamma knife thalamotomy for Parkinson disease and essential tremor: a prospective multicenter study. Neurosurgery 2012;70:526-35; discussion 535-6. [PubMed]
- Kooshkabadi A, Lunsford LD, Tonetti D, et al. Gamma Knife thalamotomy for tremor in the magnetic resonance imaging era. J Neurosurg 2013;118:713-8. [PubMed]
- Régis J, Carron R, Azulay JP, et al. Gamma knife radiosurgery thalamotomy for intractable tremors: a blinded assessment. Abstract: 291. Journal of Radiosurgery and SBRT Vol. 2 Suppl. 1 2013.
- Shih LC, LaFaver K, Lim C, et al. Loss of benefit in VIM thalamic deep brain stimulation (DBS) for essential tremor (ET): how prevalent is it? Parkinsonism Relat Disord 2013;19:676-9. [PubMed]
- Fenoy AJ, Simpson RK Jr. Risks of common complications in deep brain stimulation surgery: management and avoidance. J Neurosurg 2014;120:132-9. [PubMed]
- Pedrosa DJ, Auth M, Pauls KA, et al. Verbal fluency in essential tremor patients: the effects of deep brain stimulation. Brain Stimul 2014;7:359-64. [PubMed]
- Bahgat D, Magill ST, Berk C, et al. Thalamotomy as a treatment option for tremor after ineffective deep brain stimulation. Stereotact Funct Neurosurg 2013;91:18-23. [PubMed]


