Neoadjuvant systemic therapy does not compromise local control after breast-conserving surgery: a single-center, propensity score matching study in China
Introduction
Breast cancer is the most commonly diagnosed cancer and the most common cause of cancer-related death among females (1). The development of multidisciplinary systemic therapy has improved the treatment efficacy of breast cancer. In early-stage breast cancer, breast-conserving surgery (BCS) plus radiotherapy has proved to be equivalent to mastectomy in terms of oncologic safety (2,3), but the application of BCS after neoadjuvant therapy remains controversial. Although unable to improve overall survival (4), neoadjuvant systematic therapy (NST) can degrade the volume of the primary tumor to amplify the possibility of BCS and increase the likelihood of eradicating micrometastatic disease (5,6). BCS after neoadjuvant therapy remains controversial because the improvement in the pathologic complete response (pCR) rate has not been translated into an increase in the BCS rate (7,8). Estimating lesions after NST and surgical decision-making remain challenging for surgeons. A meta-analysis of ten randomized trials investigated long-term outcomes between neoadjuvant and adjuvant therapy in early breast cancer (9). No significant difference was detected for distant metastasis, breast cancer mortality, or overall survival. However, local recurrence was higher in the neoadjuvant group than in the adjuvant group, possibly due to the increase in the BCS rate in the neoadjuvant group. Studies investigating BCS after NST have reported ipsilateral breast tumor recurrence (IBTR) rates ranging from 5% to 11% (10-14). The risk factors associated with IBTR, including clinical stage, pathologic response, a multifocal pattern of residual disease, and lymphovascular space invasion in the specimen, have also been investigated (10-14).
Most studies investigating the outcomes of BCS after NST were one-arm studies or compared BCS after NST with mastectomy (15-18). Two-arm investigations focusing on the IBTR rate are scarce. Our study aimed to explore the IBTR rate of BCS after NST compared to matched initial BCS to estimate the oncologic safety of BCS after tumor downsizing by NST. We also focused on the correlation of IBTR with clinicopathological variables.
Methods
Patients
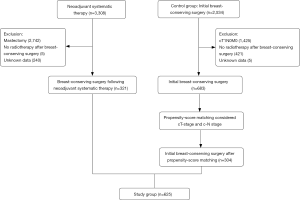
This retrospective study included 321 consecutive patients undergoing BCS following MST during the period June 2008 to June 2017 at Fudan University Shanghai Cancer Center (FUSCC). We also reviewed 683 patients undergoing initial BCS with noninflammatory invasive breast cancer measuring >2 cm and/or axillary lymph node metastasis as a control group. Patients were excluded if they had any one of the following: (I) stage IV disease; (II) no radiotherapy after BCS; (III) without standard trastuzumab therapy when HER2 status was positive; (IV) unknown data (Figure 1). Clinicopathological characteristics and follow-up information were derived from medical records collected by the Department of Surgery of FUSCC. Clinical tumor sizes were assessed on magnetic resonance imaging findings in the baseline assessment.
Surgical approaches
Neoadjuvant chemotherapy was delivered to 252 of the 321 patients with six to eight cycles of anthracycline and/or taxane regimens, while neoadjuvant endocrine therapy was delivered to 69 patients with four to six months of aromatase inhibitors plus ovarian function suppression or aromatase inhibitors alone. All patients with HER2-positive breast cancer received trastuzumab as part of their neoadjuvant regimen. The clinical and radiological response was measured every two cycles. For all patients, the type of breast surgery and axillary surgery were determined by the multidisciplinary team according to NCCN guidelines. When obtaining positive margins in the final pathology, additional excisions were performed. Axillary lymph node dissection (ALND) was the standard treatment for NST patients. For patients presenting clinically lymph node negative, sentinel lymph node dissection (SLND) without ALND was routinely performed. All patients received adjuvant whole-breast radiotherapy with or without the regional nodal area in 25–30 fractions after adjuvant chemotherapy or after surgery if adjuvant chemotherapy was unnecessary. In the control group, adjuvant chemotherapy was delivered to 597 of 683 patients, while adjuvant endocrine therapy only was delivered to 86 patients.
Pathological assessment
All specimens were fixed in 10% neutral phosphate-buffered formalin and paraffin-embedded. Slices of typical tumor blocks with a thickness of 4 µm were stained with hematoxylin and eosin (H&E). Estrogen receptor (ER) status, progesterone receptor (PR) status, HER2 expression, and Ki67 proliferative index were tested using immunohistochemical staining in both pretreatment core needle biopsy samples and posttreatment surgical excision samples. Evaluation of TILs in core needle biopsy specimens was accomplished by two pathologists. Stromal TILs were appraised according to the standardization of the international TILs working group (19). Pathological complete response (pCR) was defined as the absence of residual tumor cells in both the breast and axillary lymph nodes (ypT0+ ypN0). Lymphovascular invasion (LVI) was observed in the postoperative slices. Negative margins were defined as “no ink-on-tumor”.
Follow-up protocol
After surgery, patient follow up was scheduled every 3 months in the first 2 years and then every 6 months over the following 3 years. After 5 years, the follow-up frequency was prolonged to once per year. During the follow-up period, patients came to our cancer center to receive a routine examination, comprehensive chest computed tomography (CT), breast MRI, breast ultrasonography, mammography, and abdominal ultrasonography. The follow-up information was compiled by reviewing medical records with a deadline of December 2018. This study was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center (No.050432-4-1212B).
Propensity score matching and statistical analyses
To adjust for significant baseline covariates between the two groups, a propensity score matching procedure was used to decrease latent biases between the two groups. Patients' baseline clinical characteristics, including clinical tumor size and clinical lymph node stage, were considered as covariates for matching. The propensity score matching was carried out by IBM SPSS Statistics version 24.0 software with the number of patients in each group set at 1:1 and Caliper set at 0.05. Baseline standardized mean differences were computed before and after propensity score matching.
Baseline characteristics of the patients were described by frequencies and percentages for categorical variables and by means and standard deviations for normally distributed continuous data, including age, menopausal status, clinical T-stage, clinical N-stage, histological type, ER status, PR status, HER2 expression, pathological T-stage, pathological N-stage and neoadjuvant/adjuvant regimen. Tests of distribution between two groups were conducted by Student’s t-test or Pearson’s x2 test.
IBTR was considered as recurrence in the ipsilateral breast and locoregional recurrence (LRR) as recurrence in the ipsilateral breast, ipsilateral axilla, chest wall, internal mammary, and supraclavicular lymph nodes. Kaplan-Meier survival curves for both groups were used to portray IBTR-free survival. Cox proportional hazards regression was used in univariate and multivariate analyses. All statistical tests were two-sided, and a P value <0.05 was considered statistically significant. SPSS software package version 24.0 (Chicago, IL) was used for all analyses.
Results
Before propensity score matching, 321 patients underwent BCS after NST, and 683 patients underwent initial BCS. Their baseline clinical characteristics are shown in Table 1. Although we selected patients with breast cancer measuring >2 cm and/or axillary lymph node metastasis as controls, there were still significant differences in clinical T-stage (P<0.001), clinical N-stage (P<0.001), ER status (P<0.001), PR status (P<0.001), HER2 status (P<0.001), pathological T-stage (P<0.001), pathological N-stage (P<0.001) and neoadjuvant/adjuvant regimen (P<0.001). Considering the importance of the clinical T-stage and N-stage for BCS in terms of IBTR, we used a propensity score matching process to reduce potential biases.
Table 1
| Factor | BCS after NST (n=321) | Initial BCS (n=683) | P |
|---|---|---|---|
| Age, years, median(range) | 48.2±16.3 (21 to 85) | 47.0±12.01 (19 to 87) | 0.638 |
| Menopausal status, n (%) | 0.148 | ||
| Pre-menopause | 195 (60.7) | 447 (65.4) | |
| Post-menopause | 126 (39.3) | 236 (34.5) | |
| Tumor histology, n (%) | 0.085 | ||
| Invasive ductal carcinoma | 210 (65.4) | 644 (94.3) | |
| Others | 6 (1.9) | 39 (5.7) | |
| Unknown (pCR) | 105 (32.7) | ||
| Clinical T-stage, n (%) | <0.001* | ||
| T1 | 51 (15.9) | 74 (10.8) | |
| T2 | 220 (68.5) | 604 (88.4) | |
| T3 | 50 (15.6) | 5 (0.7) | |
| Clinical N-stage, n (%) | <0.001* | ||
| N0 | 109 (34.0) | 391 (57.2) | |
| N1 | 113 (35.2) | 250 (36.6) | |
| N2 | 68 (21.2) | 40 (5.86) | |
| N3 | 28 (8.7) | 1 (0.1) | |
| Nx | 3 (0.9) | 1 (0.1) | |
| ER status, n (%) | <0.001* | ||
| Positive | 166 (51.7) | 490 (71.7) | |
| Negative | 155 (48.3) | 193 (28.3) | |
| PgR status, n (%) | <0.001* | ||
| Positive | 141 (43.9) | 471 (69.0) | |
| Negative | 180 (56.1) | 212 (31.0) | |
| HER2 status, n (%) | <0.001* | ||
| Positive | 102 (31.8) | 121 (17.7) | |
| Negative | 219 (68.2) | 562 (82.3) | |
| Pathological T-stage, n (%) | <0.001* | ||
| Breast pCR | 105 (32.7) | ||
| (yp)Tis | 15 (4.7) | ||
| (yp)T1 | 165 (51.4) | 82 (12.0) | |
| (yp)T2 | 33 (10.3) | 596 (87.3) | |
| (yp)T3 | 3 (0.9) | 5 (0.7) | |
| Pathological N-stage, n (%) | <0.001* | ||
| (yp)N0 | 224 (69.8) | 367 (53.7) | |
| (yp)N1 | 65 (20.2) | 175 (25.6) | |
| (yp)N2 | 29 (9.0) | 105 (15.4) | |
| (yp)N3 | 3 (0.9) | 29 (4.2) | |
| Nx | 7 (1.0) | ||
| Neoadjuvant/adjuvant regimen, n (%) | <0.001* | ||
| Anthracycline, not taxane | 68 (21.2) | 158 (23.1) | |
| Taxane-based | 107 (33.3) | 389 (57.0) | |
| Only endocrine | 69 (21.5) | 86 (12.6) | |
| Other | 77 (24.0) | 50 (7.3) |
*, P<0.05. BCS, breast-conserving surgery; NST, neoadjuvant systematic therapy; pCR, pathologic complete response; ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
After propensity score matching, 304 patients were enrolled in the control group. No significant differences in T-stage and N-stage were observed, as shown in Table 2. With a median follow-up period of 58 months (range, 10–153), 23 patients (7.2%) in the NST group had developed LRR, including 20 with IBTR (6.2%, Table S1), and 26 patients developed recurrences at other sites: eight bone metastases, five brain metastases, four liver metastases, four lung metastases, and five soft tissue metastases.
Table 2
| Factor | BCS after NST (n=321) | Initial BCS (n=304) | P |
|---|---|---|---|
| Age, years, median (range) | 48.2±16.3 (21 to 85) | 45.9±10.96 (23 to 85) | 0.490 |
| Menopausal status, n (%) | 0.068 | ||
| Pre‐menopause | 195 (60.7) | 206 (67.8) | |
| Post‐menopause | 126 (39.3) | 98 (32.2) | |
| Tumor histology, n (%) | 0.124 | ||
| Invasive ductal carcinoma | 210 (65.4) | 287 (94.4) | |
| Others | 6 (1.9) | 17 (5.6) | |
| Unknown (pCR) | 105 (32.7) | ||
| Clinical T-stage, n (%) | 0.144 | ||
| T1 | 51 (15.9) | 36 (11.8) | |
| T2-3 | 270 (84.1) | 268 (88.2) | |
| Clinical N-stage, n (%) | 0.744 | ||
| N0 | 109 (26.7) | 108 (35.5) | |
| N1-3 | 209 (71.7) | 196 (64.5) | |
| Nx | 3 (1.7) | ||
| ER status, n (%) | <0.001* | ||
| Positive | 166 (51.7) | 213 (70.1) | |
| Negative | 155 (48.3) | 91 (29.9) | |
| PgR status, n (%) | <0.001* | ||
| Positive | 141 (43.9) | 207 (68.1) | |
| Negative | 180 (56.1) | 97 (31.9) | |
| HER2 status, n (%) | |||
| Positive | 102 (31.8) | 119 (39.1) | 0.054 |
| Negative | 219 (68.2) | 185 (60.9) | |
| Neoadjuvant/adjuvant regimen, n (%) | <0.001* | ||
| Anthracycline, not taxane | 68 (21.2) | 77 (25.3) | |
| Taxane-based | 107 (33.3) | 188 (61.8) | |
| Only endocrine | 69 (21.5) | 27 (8.9) | |
| Other | 77 (24.0) | 12 (3.9) |
*, P<0.05. BCS, breast-conserving surgery; NST, neoadjuvant systematic therapy; pCR, pathologic complete response; ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
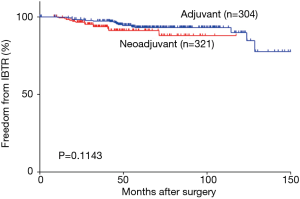
The 3-year IBTR-free survival rates were 93.7% (95% CI, 90.6–96.8%) in the NST group and 96.9% (95% CI, 94.9–98.9%) in the matched initial BCS group. The 3-year disease-free survival (DFS) rate was 87.3% (95% CI, 83.4–91.2%) in the NST group. IBTR events were diagnosed mainly by magnetic resonance imaging (Table S1). The overall pCR rate in the NST group was 31.5% (101/321). LRR after initial BCS occurred in 22 patients (7.2%), 21 of which had IBTR. Figure 2 depicts the IBTR-free survival of the NST group and the initial BCS group. There was no significant difference between the two groups (P=0.154, HR =1.53, 95% CI, 0.82–2.87).
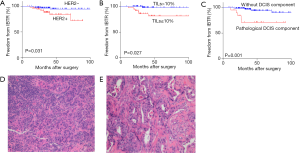
To evaluate independent prognostic factors affecting IBTR in patients undergoing BCS after NST, univariate and multivariate analyses were performed. The results are shown in Table 3 and Figure 3. Pre-NST ER status (P=0.013), pre-NST HER2 status (P=0.022), TILs (P=0.001), and pathologic ductal carcinoma in situ (DCIS) constituent (P=0.001) were associated with a high rate of IBTR in univariate analysis. Next, a multivariate analysis taking these four factors into consideration was performed. Three factors reached statistical significance in the Cox proportional hazard model (Pre-NST HER2 status: HR =3.84, 95% CI, 1.26–11.71, P=0.018; TILs: HR =12.12, 95% CI, 2.62–55.97, P=0.001; pathologic DCIS constituent: HR =8.47, 95% CI, 2.76–26.01, P=0.001).
Table 3
| Factor | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age (<50 vs. ≥50) | 0.71 | 0.30–1.72 | 0.452 | ||||
| Clinical T-stage | |||||||
| cT1 | 1 | ||||||
| cT2 | 0.94 | 0.18–4.67 | 0.937 | ||||
| cT3 | 0.84 | 0.25–3.08 | 0.839 | ||||
| Clinical N-stage | |||||||
| cN0 | 1 | ||||||
| cN1 | 1.02 | 0.34–3.05 | 0.966 | ||||
| cN2 | 1.35 | 0.39–4.68 | 0.635 | ||||
| cN3 | 1.96 | 0.51–7.58 | 0.330 | ||||
| Pre-NST ER (negative vs. positive) | 1.76 | 1.13–2.76 | 0.013* | 3.02 | 0.982–9.28 | 0.054 | |
| Pre-NST PgR (negative vs. positive) | 1.56 | 0.99–2.45 | 0.056 | ||||
| Pre-NST HER2 status (positive vs. negative) | 2.80 | 1.16–6.76 | 0.022* | 3.84 | 1.26–11.71 | 0.018* | |
| Pre-NST Ki67(≤20% vs. >20%) | 1.04 | 0.34–3.17 | 0.951 | ||||
| TIL (≤10% vs. >10%) | 12.11 | 2.71–54.13 | 0.001* | 12.12 | 2.62–55.97 | 0.001* | |
| Neoadjuvant regimen (chemotherapy vs. endocrine therapy) | 2.99 | 0.69–12.91 | 0.142 | ||||
| Margin (negative vs. positive) | 20.29 | 0.00–NA | 0.864 | ||||
| pCR (no vs. yes) | 1.28 | 0.51–3.21 | 0.601 | ||||
| Pathologic ductal carcinoma in situ constituent (yes vs. no) | 5.28 | 2.11–13.25 | 0.001* | 8.47 | 2.76–26.01 | 0.001* | |
| Multifocality (yes vs. no) | 6.83 | 0.91–51.48 | 0.062 | ||||
| Pathologic Lymph node metastasis | |||||||
| ypN0 | 1 | ||||||
| ypN1 | 0.62 | 0.34–1.13 | 0.119 | ||||
| ypN2-3 | 1.27 | 0.64–2.51 | 0.500 | ||||
| Lymphovascular invasion (positive vs. negative) | 1.24 | 0.33–4.73 | 0.751 | ||||
| Post-operative chemotherapy (no vs. yes) | 0.84 | 0.24–2.73 | 0.814 | ||||
*, P<0.05. IBTR, ipsilateral breast tumor recurrence; pCR, pathologic complete response; ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2; TILs, tumor infiltrating lymphocytes
Discussion
Our retrospective study discovered that IBTR-free survival after BCS after NST resembled that after initial BCS in patients with invasive breast cancer measuring >2 cm and/or axillary lymph node metastasis after propensity score matching. Based on our single-institution experience, concerns that the application of BCS after NST may increase the risk of IBTR after BCS should be reconsidered.
Most previous studies that investigated the outcomes of BCS after NST were one-arm studies or compared BCS after NST with mastectomy; two-arm studies focusing on the IBTR rate are scarce, possibly because treating patients undergoing mastectomy as the control group would eliminate the applicability of IBTR as an endpoint. Moreover, patients who have a good response to NST are more likely to receive BCS than mastectomy, which leads to a better outcome. This kind of “treatment by indications” bias has influenced previous studies and cannot be completely avoided. Thus, we enrolled patients undergoing initial BCS and used a propensity score matching procedure to adjust for clinical T-stage and N-stage to conduct this nonrandomized study. BCS after NST has rarely been compared with initial BCS (14,20,21), and those studies suggested worse outcomes in patients who underwent BCS after NST compared with initial BCS. However, Mittendorf EA et al. found no differences in LRR-free survival rates when comparing the presenting clinical stage (P=NS) between two groups (14). This observation is in agreement with our result that BCS after NST did not significantly increase the risk for IBTR (BCS after NST vs. initial BCS, P=0.154, HR =1.53, 95% CI, 0.82–2.87), as shown in Figure 2. These results suggest that the characteristics of the tumor and patients’ baseline characteristics underlie the differences in IBTR between BCS and BCS after NST rather than neoadjuvant therapy itself.
Previous studies revealed the IBTR after BCS after NST was associated with clinical T-stage, clinical N-stage, nuclear grade, lymph node metastasis, LVI, and pathologic response (10,13,22). However, information about nuclear grade and LVI is difficult to estimate in surgical specimens after NST and are not reported for pre-NST core needle biopsy specimens. Given this situation, we applied propensity score matching to balance the clinical T-stage and N-stage. Propensity score matching was used recently to compare BCS and mastectomy in several retrospective studies (23,24). After propensity score matching, clinical T-stage (P=0.144) and clinical N-stage (P=0.744) were no longer significantly different between the two groups (Table 2). ER status (P<0.001), PR status (P<0.001) and neoadjuvant/adjuvant regimen (P<0.001) remained significantly different, but these variables are not thought to be related to IBTR.
The most challenging question for the application of BCS after NST is the reduction of IBTR. We analyzed factors that might influence IBTR. Several factors including tumor stage, surgical margins, and residual pathologic tumor size have been reported. Akay et al. established a prognostic index named the MD Anderson prognostic index (MDAPI) to distinguish patients at high risk of LRR who underwent BCS after NAC (25). The MDAPI considered factors including clinical N2/N3 disease, LVI, residual tumor size >2 cm and multifocal residual disease. In our single-institution cohort, HER2 status, TILs and pathologic DCIS constituent were significantly associated with IBTR in univariate and multivariate analyses. The diversity of predictive factors might reflect the enrollment of patients with early-stage breast cancer in our study, whereas previous studies included only local advanced breast cancer. The value of TILs in effectively predicting outcomes in both neoadjuvant and adjuvant settings has been confirmed (26,27), especially in HER2-positive and triple-negative molecular subtypes. TILs were associated with pCR, DFS and overall survival in breast cancers treated with neoadjuvant therapy (28,29). We also observed potential utility of TILs in predicting IBTR after BCS after NST. With respect to molecular subtype, triple-negative and HER2-positive subtypes have been reported to predict higher rates of IBTR (30,31). In our study, this trend was not so obvious, but we found that HER2 positivity was an independent predictive factor for IBTR (Table S2). We previously found that the presence of DCIS was significantly related to positive margins (32). In the present study, we found that DCIS was significantly associated with IBTR, possibly because of the smaller tumor-to-margin distance. Tumor multifocality is always considered a factor in IBTR. The most common shrinkage pattern following NST of this kind of tumor is nest-like (33). Multifocal residual disease was not common in our study because mastectomy was performed only when the tumor magnetic resonance imaging shrinkage pattern resembled a nest.
This study also has limitations. Clinical T-stage and clinical N-stage, which were considered as covariates for propensity score matching, are both categorical data; however, the potential for bias still exists. Large, randomized controlled trials would provide the best evidence. The second limitation is the small sample size and the relatively short follow-up period. There were only a few IBTR events, which could have influenced the results. The third limitation is missing data, especially TILs, which may have affected our results. The comparison of initial BCS and BCS with NST requires additional evidence to achieve a better understanding.
Conclusions
In conclusion, our study demonstrates that BCS after NST and initial BCS have equivalent IBTR-free survival. BCS after NST is a safe and effective surgery for patients. Furthermore, HER2 positivity, TILs ≤10% and pathologic DCIS constituent are factors associated with higher IBTR rates in patients undergoing BCS after NST.
Table S1
| No. | Primary breast cancer | Diagnosis and treatment of IBTR | ||||
|---|---|---|---|---|---|---|
| Pathologic stage | Molecular subtype | Imaging diagnosis | Salvage local therapy | Systemic therapy | ||
| 1 | ypT1N1M0 | HR+/HER2− | MRI | No | Chemotherapy | |
| 2 | ypT0N2M0 | HR+/HER2− | Ultrasonography | Mastectomy | Chemotherapy | |
| 3 | ypT0N0M0 | HR+/HER2+ | MRI | Mastectomy | Chemotherapy | |
| 4 | ypT0N0M0 | HR-/HER2+ | MRI | Mastectomy | Chemotherapy | |
| 5 | ypTisN0M0 | HR-/HER2+ | MRI | No | Chemotherapy | |
| 6 | ypTisN0M0 | HR+/HER2− | Needle core biopsy | Mastectomy | Chemotherapy | |
| 7 | ypT2N0M0 | TNBC | MRI | Mastectomy | Chemotherapy | |
| 8 | ypT1N0M0 | HR+/HER2+ | MRI | Mastectomy | Endocrine therapy | |
| 9 | ypT1N1M0 | TNBC | MRI | No | Chemotherapy | |
| 10 | ypT1N1M0 | HR+/HER2+ | MRI | Breast-conserving surgery | No | |
| 11 | ypT1N1M0 | HR+/HER2+ | MRI | Mastectomy | Endocrine therapy | |
| 12 | ypTisN0M0 | HR-/HER2+ | Mammography | Mastectomy | Endocrine therapy | |
| 13 | ypT1N0M0 | HR-/HER2+ | MRI | No | Chemotherapy | |
| 14 | ypT1N0M0 | HR+/HER2+ | MRI | Mastectomy | Endocrine therapy | |
| 15 | ypT1N1M0 | HR+/HER2+ | MRI | Mastectomy | Chemotherapy | |
| 16 | ypT1N3M0 | TNBC | MRI | Mastectomy | Chemotherapy | |
| 17 | ypT2N0M0 | TNBC | MRI | Mastectomy | Chemotherapy | |
| 18 | ypTisN0M0 | TNBC | MRI | Mastectomy | Chemotherapy | |
| 19 | ypT1N1M0 | TNBC | MRI | Mastectomy | Chemotherapy | |
| 20 | ypT2N1M0 | HR+/HER2+ | MRI | Mastectomy | Chemotherapy | |
MRI, magnetic resonance imaging; IBTR, ipsilateral breast tumor recurrence; HR, estrogen receptor; HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
Table S2
| Molecular subtype | HR+/HER2−, N=149 | HR+/HER2+, N=58 | HR−/HER2+, N=44 | TNBC, N=70 |
|---|---|---|---|---|
| pCR | 16 (10.7%) | 14 (24.1%) | 29 (65.9%) | 36 (51.4%) |
| IBTR | 3 (2.0%) | 7 (12.1%) | 4 (9.1%) | 6 (8.6%) |
| LRR | 5 (3.4%) | 7 (12.1%) | 5 (11.4%) | 6 (8.6%) |
IBTR, ipsilateral breast tumor recurrence; pCR, pathologic complete response; HR, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2; TILs, tumor infiltrating lymphocytes.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.11.23). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center (No.050432-4-1212B). Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Wang L, Ouyang T, Wang T, et al. Breast-conserving therapy and modified radical mastectomy for primary breast carcinoma: a matched comparative study. Chin J Cancer Res 2015;27:545-52. [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [Crossref] [PubMed]
- Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 1997;15:2483-93. [Crossref] [PubMed]
- Caudle AS, Kuerer HM. Breast conservation therapy after neoadjuvant chemotherapy: optimization of a multimodality approach. J Surg Oncol 2014;110:32-6. [Crossref] [PubMed]
- Mauri D, Pavlidis N, Ioannidis JPA, et al. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 2005;97:188-94. [Crossref] [PubMed]
- de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol 2014;15:1137-46. [Crossref] [PubMed]
- National Cancer Institute. Docetaxel, carboplatin, trastuzumab, and pertuzumab with or without estrogen deprivation in treating patients with hormone receptor-positive, HER2-positive operable or locally advanced breast cancer. Available online: www.clinicaltrials.gov/ct2/show/NCT02003209
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 2018;19:27-39. [Crossref] [PubMed]
- Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol 2004;22:2303-12. [Crossref] [PubMed]
- Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy. Cancer 2005;103:689-95. [Crossref] [PubMed]
- Cance WG, Carey LA, Calvo BF, et al. Long-term outcome of neoadjuvant therapy for locally advanced breast carcinoma: effective clinical downstaging allows breast preservation and predicts outstanding local control and survival. Ann Surg 2002;236:295-302; discussion 302-3. [Crossref] [PubMed]
- Carrara GFA, Scapulatempo-Neto C, Abrahão-Machado LF, et al. Breast-conserving surgery in locally advanced breast cancer submitted to neoadjuvant chemotherapy. Safety and effectiveness based on ipsilateral breast tumor recurrence and long-term follow-up. Clinics (Sao Paulo) 2017;72:134-42. [Crossref] [PubMed]
- Mittendorf EA, Buchholz TA, Tucker SL, et al. Impact of chemotherapy sequencing on local-regional failure risk in breast cancer patients undergoing breast-conserving therapy. Ann Surg 2013;257:173-9. [Crossref] [PubMed]
- Peintinger F, Symmans WF, Gonzalez-Angulo AM, et al. The safety of breast-conserving surgery in patients who achieve a complete pathologic response after neoadjuvant chemotherapy. Cancer 2006;107:1248-54. [Crossref] [PubMed]
- Beriwal S, Schwartz GF, Komarnicky L, et al. Breast-conserving therapy after neoadjuvant chemotherapy: long-term results. Breast J 2006;12:159-64. [Crossref] [PubMed]
- Shin HC, Han W, Moon HG, et al. Breast-conserving surgery after tumor downstaging by neoadjuvant chemotherapy is oncologically safe for stage III breast cancer patients. Ann Surg Oncol 2013;20:2582-9. [Crossref] [PubMed]
- Zhou X, Li Y. Local Recurrence after Breast-Conserving Surgery and Mastectomy Following Neoadjuvant Chemotherapy for Locally Advanced Breast Cancer - a Meta-Analysis. Breast Care (Basel) 2016;11:345-51. [Crossref] [PubMed]
- Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015;26:259-71. [Crossref] [PubMed]
- Cho JH, Park JM, Park HS, et al. Oncologic safety of breast-conserving surgery compared to mastectomy in patients receiving neoadjuvant chemotherapy for locally advanced breast cancer. J Surg Oncol 2013;108:531-6. [Crossref] [PubMed]
- Agarwal G, Sonthineni C, Mayilvaganan S, et al. Surgical Outcomes of Primary Versus Post-Neoadjuvant Chemotherapy Breast Conservation Surgery: A Comparative Study from a Developing Country. World J Surg 2018;42:1364-74. [Crossref] [PubMed]
- Matsuda N, Hayashi N, Ohde S, et al. A nomogram for predicting locoregional recurrence in primary breast cancer patients who received breast-conserving surgery after neoadjuvant chemotherapy. J Surg Oncol 2014;109:764-9. [Crossref] [PubMed]
- Yoo GS, Park W, Yu JI, et al. Comparison of Breast Conserving Surgery Followed by Radiation Therapy with Mastectomy Alone for Pathologic N1 Breast Cancer Patients in the Era of Anthracycline Plus Taxane-Based Chemotherapy: A Multicenter Retrospective Study (KROG 1418). Cancer Res Treat 2019;51:1041-51. [Crossref] [PubMed]
- Chen K, Pan Z, Zhu L, et al. Comparison of breast-conserving surgery and mastectomy in early breast cancer using observational data revisited: a propensity score-matched analysis. Sci China Life Sci 2018;61:1528-36. [Crossref] [PubMed]
- Akay CL, Meric-Bernstam F, Hunt KK, et al. Evaluation of the MD Anderson Prognostic Index for local-regional recurrence after breast conserving therapy in patients receiving neoadjuvant chemotherapy. Ann Surg Oncol 2012;19:901-7. [Crossref] [PubMed]
- Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010;28:105-13. [Crossref] [PubMed]
- Yu X, Zhang Z, Wang Z, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. Clin Transl Oncol 2016;18:497-506. [Crossref] [PubMed]
- Yang X, Rao J, Yang W, et al. Evaluation of the Predictive and Prognostic Values of Stromal Tumor-Infiltrating Lymphocytes in HER2-Positive Breast Cancers treated with neoadjuvant chemotherapy. Target Oncol 2018;13:757-67. [Crossref] [PubMed]
- Ruan M, Tian T, Rao J, et al. Predictive value of tumor-infiltrating lymphocytes to pathological complete response in neoadjuvant treated triple-negative breast cancers. Diagn Pathol 2018;13:66. [Crossref] [PubMed]
- Swisher SK, Vila J, Tucker SL, et al. Locoregional Control According to Breast Cancer Subtype and Response to Neoadjuvant Chemotherapy in Breast Cancer Patients Undergoing Breast-conserving Therapy. Ann Surg Oncol 2016;23:749-56. [Crossref] [PubMed]
- Jwa E, Shin KH, Kim JY, et al. Locoregional Recurrence by Tumor Biology in Breast Cancer Patients after Preoperative Chemotherapy and Breast Conservation Treatment. Cancer Res Treat 2016;48:1363-72. [Crossref] [PubMed]
- Wu S, Zhu Y, Yang Z, et al. Low rate of positive margins and re-excision after partial mastectomy in highly selected breast cancer patients: A Chinese single-institution experience. Oncotarget 2017;8:12225-33. [PubMed]
- Tomida K, Ishida M, Umeda T, et al. Magnetic resonance imaging shrinkage patterns following neoadjuvant chemotherapy for breast carcinomas with an emphasis on the radiopathological correlations. Mol Clin Oncol 2014;2:783-8. [Crossref] [PubMed]