
Long-term treatment results and prognostic factors of synchronous and metachronous squamous cell carcinoma of head and neck and esophagus
Introduction
Squamous cell carcinoma of head and neck (HNSCC) is one of the most common malignant tumors. About 7–20% of patients with HNSCC accompany by the second primary tumors of the upper digestive tract and respiratory tract synchronously or metachronously. Similarly, multiple primary cancers are frequently detected in squamous cell carcinoma of esophagus (ESCC). Many studies have reported that about 10–22% of patients with ESCC accompany by the second primary tumors such as stomach cancer, head and neck cancer, lung cancer, and etc. (1-3). In these ESCC patients with multiple primary cancer, HNSCC is the second most common malignancy (4-7). The high incidence of synchronous and metachronous HNSCC and ESCC may attribute to the same epidemiological risk factors such as drinking, smoking, eating habits and so on (8-10). Lim et al. (11) found that tobacco and alcohol were the important pathogenic factors for such patients (P=0.028; P=0.019). In addition, the “regional carcinogenicity” theory also supports the view that the double primary HNSCC and ESCC is caused by exposure to the same carcinogenic substance (9,12). With the application of esophagoscopy supplemented with iodine staining and 18F-FDG PET/CT, the detectable rate of ESCC in HNSCC patients, especially for early-stage disease, has an increasing trend in recent years (10,13,14). In addition, the improved survival after treatment for the first cancer also results in more frequent detection of the second cancer in these patients (15,16).
The treatment options for these patients are faced with pronounced difficulties, and the prognosis is relatively poor (17-21). In addition, the nature of its relative low incidence leads to the lack of high-grade evidence-based treatment consensus for these patients without large series clinical studies focusing on the treatment optimization. The main reason is that most of these diseases are complicated, and the following clinical factors, including the interval time between two kinds of cancer (synchronous or metachronous), the location and clinical stages of two different cancer (22-26), the previous treatment method of the first cancer and so on, need to be taken into consideration before determining the treatment strategy. So, it is very necessary to determine the appropriate treatment strategy through multidisciplinary team to improve the outcomes of these diseases. The currently accepted pattern of care for these patients is combined modality approaches based on surgery, radiotherapy and chemotherapy and/or molecular target therapy (22,27,28). To provide the further information, we retrospectively analyzed the treatment modalities and prognosis of patients with synchronous and metachronous HNSCC and ESCC in our institution. The present study included relative larger numbers of patients than before, and the treatment strategy of all patients was determined by the multidisciplinary team.
Methods
Patients characteristic
All patients with synchronous and metachronous HNSCC and ESCC receiving treatment in Fudan University Shanghai Cancer Center from January 2005 to December 2016 were recruited, except the patients with distant metastasis. Finally, 70 cases met the inclusion criteria. The clinical features, treatment information and outcome data were collected and analyzed through a retrospective review of medical records. According to differences in the interval time between two kinds of cancer, all patients were divided into synchronous group (time interval ≤6 months) and metachronous group (time interval >6 months). In metachronous group, there were 15 patients with ESCC followed by HNSCC and 34 patients with HNSCC followed by ESCC, respectively. The TNM staging systems (AJCC/UICC seventh edition) were used to stage the HNSCC and ESCC separately. The characteristics of all patients were shown in Table 1. There were 11 patients of oropharyngeal cancer, and only 4 patients were tested for p16 status. The expressions of p16 were negative in these 4 patients.
Table 1
| Parameters | Number | Percentage |
|---|---|---|
| Median age (year) | 60 (range, 43–77) | |
| Median time interval between diagnosis of HNSCC and ESCC (months) | 12.7 (range, 0–125.8) | |
| Sex | ||
| Male | 68 | 97.1 |
| Female | 2 | 2.9 |
| Smoking | ||
| Yes | 45 | 64.3 |
| No | 25 | 35.7 |
| Alcohol drinking | ||
| Yes | 40 | 57.1 |
| No | 30 | 42.9 |
| The location of HNSCC | ||
| Oral cavity | 17 | 24.3 |
| Oropharynx | 11 | 15.7 |
| Larynx | 23 | 32.9 |
| Hypopharynx | 15 | 21.4 |
| Multiple sites | 4 | 5.7 |
| The location of ESCC | ||
| Cervical | 8 | 11.4 |
| Upper thoracic | 14 | 20.0 |
| Middle thoracic | 27 | 38.6 |
| Lower thoracic | 13 | 18.6 |
| Multiple sites | 3 | 4.3 |
| Unknown | 5 | 7.1 |
| Clinical stage of HNSCC | ||
| Stage I | 4 | 5.7 |
| Stage II | 8 | 11.4 |
| Stage III | 34 | 48.6 |
| Stage IV | 24 | 34.3 |
| Clinical stage of ESCC | ||
| Stage I | 17 | 24.3 |
| Stage II | 13 | 18.6 |
| Stage III | 26 | 37.1 |
| Stage IV | 14 | 20.0 |
| Occurrence sequence of cancer | ||
| Synchronous disease | 21 | 30.0 |
| ESCC followed by HNSCC | 15 | 21.4 |
| HNSCC followed by ESCC | 34 | 48.6 |
OS, overall survival; HNSCC, squamous cell carcinoma of head and neck; ESCC, squamous cell carcinoma of esophagus.
Treatment modalities
The treatment strategy of all patients was determined by the multidisciplinary team. The treatment modalities of all patients were shown in Table 2. For 21 patients with synchronous disease, 10 sites of HNSCC received surgery (3 receiving surgery through the peroral approach, 2 for partial laryngectomy and 5 for total oesopharyngolaryngectomy) and 7 received postoperative radiotherapy with or without chemotherapy. Other 11 sites of HNSCC received radiotherapy, and 7 patients also received chemotherapy and/or anti-EGFR target therapy. Sixteen sites of ESCC received surgery [8 receiving endoscopic submucosal dissection (ESD) or endoscopic mucosal resection (EMR), 3 for thoracotomy, 5 for total oesopharyngolaryngectomy] and 10 received postoperative radiotherapy with or without chemotherapy. Other 5 sites of ESCC received radiotherapy, and 2 patients also received chemotherapy.
Table 2
| The patient groups | Surgery ± radio(chemo) therapy | Radio(chemo) and/or target therapy | χ2 | P |
|---|---|---|---|---|
| Treatment of HNSCC | ||||
| Synchronous cancer | 10 (47.6) | 11 (52.4) | ||
| ESCC followed by HNSCC | 9 (60.0) | 6 (40.0) | 0.538 | 0.463 |
| HNSCC followed by ESCC | 27 (79.4) | 7 (20.6) | 5.960 | 0.015 |
| Treatment of ESCC | ||||
| Synchronous cancer | 16 (76.2) | 5 (23.8) | ||
| ESCC followed by HNSCC | 10 (66.7) | 5 (33.3) | 0.396 | 0.529 |
| HNSCC followed by ESCC | 24 (70.6) | 10 (29.4) | 0.205 | 0.650 |
OS, overall survival; HNSCC, squamous cell carcinoma of head and neck; ESCC, squamous cell carcinoma of esophagus.
There included 15 patients with ESCC followed by HNSCC and 34 patients with HNSCC followed by ESCC in 49 patients with metachronous disease, respectively. For 15 patients with ESCC followed by HNSCC, 10 sites of ESCC received surgery (6 receiving ESD or EMR, 4 for thoracotomy) and 5 received postoperative radiotherapy with or without chemotherapy. Other five sites of ESCC received radiotherapy, and 3 patients also received chemotherapy. Nine sites of HNSCC received surgery (2 receiving surgery through the peroral approach, 3 for partial laryngectomy, 3 for total laryngectomy, 1 for total pharyngolaryngectomy) and 6 patients received postoperative radiotherapy with or without chemotherapy, 5 received neither radiotherapy nor chemotherapy and 6 were unknown. Other six sites of HNSCC received radiotherapy, and 4 patients also received chemotherapy and/or anti-EGFR target therapy.
For 34 patients with HNSCC followed by ESCC, 27 sites of HNSCC received surgery (4 receiving through the peroral approach, 6 for partial laryngectomy, 7 for total laryngectomy, 10 for total pharyngolaryngectomy) and 19 patients received postoperative radiotherapy with or without chemotherapy, 3 received neither radiotherapy nor chemotherapy and 5 were unknown. Other 7 sites of HNSCC received radiotherapy, and 5 patients also received chemotherapy and/or anti-EGFR target therapy. Twenty-four sites of ESCC received surgery (10 receiving ESD or EMR, 14 for thoracotomy) and 19 patients received postoperative radiotherapy with or without chemotherapy, 3 received neither radiotherapy nor chemotherapy and 2 were unknown. Other 10 sites of ESCC received radiotherapy, and 8 patients also received chemotherapy.
The statistical results showed that the ratio of receiving surgery for the sites of HNSCC in patients with HNSCC followed by ESCC was significantly higher than in patients with synchronous disease (Table 2).
Statistical analysis
All statistical analyses were performed by using SPSS version 19.0. Overall survival (OS) rate for these patients was calculated. The OS was defined from the diagnosis of second primary tumor to the last follow-up or the death from any cause. Kaplan-Meier method was used to evaluate OS, and log-rank test was used to analyze the difference in different groups. The univariate and multivariate analysis by Cox proportional hazard model were performed to determine the factors associated with survival outcomes. For all tests, a two-sided P<0.05 was considered to be significant.
Results
The treatment outcomes
The median follow-up time of all patients was 33.7 (0.5–130.0) months. Fifty-five patients had been confirmed dead until last follow-up. Among 55 deaths, 14 (25.5%) died of distant metastasis, 22 (40.0%) died of loco-regional recurrence (6 for esophagus and 16 for head and neck), 9 (16.4%) died of loco-regional recurrence with distant metastasis 4 (7.3%) died of other disease and 6 (10.9%) died of unknown reason. The 1-, 2- and 3-year OS rates were 77.1%, 57.1% and 37.1%, respectively (Figure 1). The median survival time of all patients was 33.5 months.
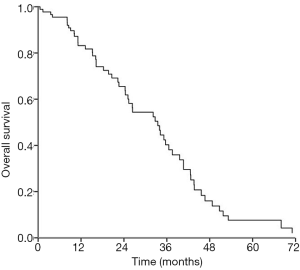
Prognostic factors
The Kaplan-Meier plots showed that the 3-year OS was significantly correlated with stage of ESCC (P=0.003), synchronous or metachronous cancer (P=0.035) and with or without receiving surgery for both cancer (P=0.002) (Figure 2; Table 3). The occurrence sequence of cancer and the clinical stages of both cancer were the variables suggesting a significant trend for OS (P=0.088; P=0.080). The patient age and clinical stage of HNSCC were not the significant variables (P=0.850; P=0.426) (Table 3). The multivariate analysis showed that the clinical stage of ESCC (P=0.011) and receiving surgery for both cancer or not were the independent prognostic factors for OS (Table 4).
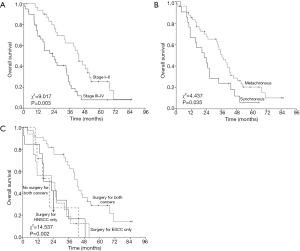
Table 3
| Variables | 3-year OS | HR (95% CI) | χ2 | P |
|---|---|---|---|---|
| Age (year) | ||||
| ≤60 | 41.3 | 0.950 (0.559–1.616) | 0.036 | 0.850 |
| >60 | 43.0 | |||
| Clinical stage of ESCC | ||||
| I–II | 62.3 | 0.426 (0.244–0.743) | 9.017 | 0.003 |
| III–IV | 25.1 | |||
| Clinical stage of HNSCC | ||||
| I–II | 47.6 | 0.747(0.365–1.532) | 0.633 | 0.426 |
| III–IV | 40.7 | |||
| Clinical stages of HNSCC and ESCC | 5.044 | 0.080 | ||
| Stage I–II for both cancer | 66.7 | 0.345 (0.119–0.999) | 3.850 | 0.050 |
| Stage III–IV for one of cancer | 53.5 | 0.640 (0.367–1.118) | 2.459 | 0.117 |
| Stage III–IV for both cancer | 27.1 | |||
| Synchronous or metachronous cancer | ||||
| Synchronous | 23.8 | 1.839 (1.043–3.241) | 4.437 | 0.035 |
| Metachronous | 50.7 | |||
| Occurrence sequence of cancer | 4.871 | 0.088 | ||
| ESCC followed by HNSCC | 70.0 | 0.450 (0.208–0.972) | 4.131 | 0.052 |
| HNSCC followed by ESCC | 42.0 | 0.596 (0.325–1.094) | 2.787 | 0.095 |
| Synchronous disease | 23.8 | |||
| Receiving surgery | 14.537 | 0.002 | ||
| Surgery for both cancer | 65.7 | 0.320 (0.117–0.872) | 4.956 | 0.026 |
| Surgery for ESCC only | 18.8 | 1.031 (0.370–2.869) | 0.003 | 0.954 |
| Surgery for HNSCC only | 16.9 | 0.909 (0.314–2.635) | 0.031 | 0.861 |
| No surgery for both cancer | 26.8 |
OS, overall survival; HNSCC, squamous cell carcinoma of head and neck; ESCC, squamous cell carcinoma of esophagus.
Table 4
| Variables | HR (95% CI) | χ2 | P |
|---|---|---|---|
| Clinical stage of ESCC | |||
| Stage I–II | 0.470 (0.262–0.843) | 6.416 | 0.011 |
| Stage III–IV | |||
| Synchronous or metachronous cancer | |||
| Synchronous | 1.564 (0.854–2.866) | 2.098 | 0.147 |
| Metachronous | – | – | – |
| Receiving surgery | 12.131 | 0.007 | |
| Surgery for both cancer | 0.303 (0.108–0.847) | 5.183 | 0.023 |
| Surgery for ESCC only | 0.773 (0.272–2.200) | 0.233 | 0.629 |
| Surgery for HNSCC only | 0.933 (0.315–2.764) | 0.016 | 0.901 |
| No surgery for both cancer | – | – | – |
OS, overall survival; HNSCC, squamous cell carcinoma of head and neck; ESCC, squamous cell carcinoma of esophagus.
Discussion
There has no consensus of treatment strategy for these patients until now. It is hard to decide whether to treat each cancer separately or not, whether to perform chemotherapy and/or radiation therapy, and whether to perform simultaneous or staged operations if operations are possible. The choice of treatment strategy should take into consideration the interval time between two kinds of cancer (synchronous or metachronous), the location and clinical stages of two different cancer, the previous treatment method of the first cancer, patient conditions, and even the oncologist’s expertise. Therefore, a multidisciplinary team management approach is essential for customized treatment strategies in these patients. The treatment strategy of all patients in present study were determined by the multidisciplinary team. Most of ESCC in these patients received surgery. We divided the patients into three groups: synchronous group, ESCC followed by HNSCC group and HNSCC followed by ESCC group, and found that there were no significant differences in percent of patients receiving surgery among these groups. It suggested that the possibility of receiving surgery for ESCC may not be affected whether or not synchronous or metachronous HNSCC existed (24). However, the possibility of receiving surgery for HNSCC may be affected by the existence of ESCC. The rate of receiving surgery for HNSCC in HNSCC followed by ESCC group is higher than in synchronous group. The main reason is that the synchronous cancer is complicated and the surgical indications of HNSCC are often more limited than for solitary HNSCC.
We reviewed previous published literature (Table 5), and found that the 2-year OS for these patients were 16.7–44.0%. Our results showed that the 1-, 2- and 3-year OS for these patients were 77.1%, 57.1% and 37.1%, respectively, and the median survival time was 33.5 months. The multivariate analysis showed that the clinical stage of ESCC was one of the independent prognostic factors for OS while the clinical stage of HNSCC was not. Park et al. (10) also found that the long-term treatment results were closely related to the severity of ESCC, but not related to HNSCC. Shinoto et al. (18) found that the advanced clinical stage (III–IV) of ESCC was one of the unfavorable prognostic factors of OS for these patients with double primary cancer. The main reason may be that ESCC has a much poorer outcome than HNSCC, and the prognosis would generally be determined by the clinical stage of ESCC (10).
Table 5
| Study | Patient number | Treatment method | PFS (%) | OS (%) | Median survival time (months) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1-year | 2-year | 1-year | 2-year | 3-year | |||||
| Park (10) | 27 (synchronous) | Surgery/radiotherapy/chemotherapy | – | 57.5 | – | 39.6 | – | 28.2 | |
| Yoshino (17) | 21 (metachronous) | Surgery/radiotherapy | – | – | – | 42.0 | 30.0 | – | |
| Shinoto (18) | 34 (synchronous) | Radiotherapy/chemotherapy | – | 33.0 | – | 44.0 | – | – | |
| Lim (11) | 37 (synchronous) | Surgery/radiotherapy/chemotherapy | – | – | – | – | 48.2 | – | |
| 11 (metachronous) | Surgery/radiotherapy/chemotherapy | – | – | – | – | – | – | ||
| Welza (7) | 24 (synchronous) | Surgery/radiotherapy/chemotherapy | – | – | – | – | – | 37.0 | |
| Hsu (19) | 12 (synchronous) | Radiotherapy/chemotherapy | – | – | 41.7 | 16.7 | – | 10.3 | |
| Fan (20) | 41 (synchronous) | Surgery/radiotherapy/chemotherapy | 37.0 | 10.0 | 62.0 | 18.0 | – | – | |
| Present study | 21 (synchronous) | Surgery/radiotherapy/chemotherapy | – | – | 77.1 | 57.1 | 37.1 | 33.5 | |
| 49 (metachronous) | Surgery/radiotherapy/chemotherapy | – | – | – | – | – | – | ||
OS, overall survival; PFS, progression-free survival; HNSCC, squamous cell carcinoma of head and neck; ESCC, squamous cell carcinoma of esophagus.
In present study, these patients were divided into four groups according to whether surgery is received or not: receiving surgery for both cancer, receiving surgery for ESCC only, receiving surgery for HNSCC only, and no receiving surgery for both cancer. We found that the patients with receiving surgery for both cancer had better OS compared to the other patients. Multivariate analysis showed that it was the independent prognostic factor for OS. This result is similar to the previous studies (22,23,27,28). Furthermore, univariate analysis revealed that the 3-year OS of patients with metachronous cancer was higher than the patients with synchronous cancer (50.7% vs. 23.8%, P=0.035). The main reason may be that the synchronous cancer is more complicated and serious than the metachronous cancer. It means that the patients with metachronous cancer have more opportunities to receive aggressive treatment than the patients with synchronous cancer. Our study revealed that there were no significant differences in rates of ESCC receiving surgery among different groups. However, the rate of HNSCC receiving surgery in metachronous group is higher than in synchronous group.
In conclusion, our results suggested that the treatment outcome of patients with synchronous or metachronous HNSCC and ESCC was acceptable, especially for patients with early clinical stage ESCC and with chance to receiving surgery for both cancer. It is very necessary to determine the appropriate treatment strategy through multidisciplinary team to improve the outcomes of these diseases. However, this retrospective study is potentially limited by the relatively small number of patients, and it was very difficult to analyze the effect of different treatment strategies on prognosis according to tumor sites of HNSCC and ESCC.
Acknowledgments
I wanted to take this chance to thank my teachers: Xueguan Lu and Yongxue Zhu. In the process of composing this paper, they gave me many academic and constructive advice, and helped me to correct my paper. And I was also very grateful for the other authors involved in this article.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.12.81). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Fudan University Shanghai Cancer Center (No. 1905202-11). Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Poon RT, Law SY, Chu KM, et al. Multiple primary cancers in esophageal squamous cell carcinoma: incidence and implications. Ann Thorac Surg 1998;65:1529-34. [Crossref] [PubMed]
- Nagasawa S, Onda M, Sasajima K, et al. Multiple primary malignant neoplasms in patients with esophageal cancer. Dis Esophagus 2000;13:226-30. [Crossref] [PubMed]
- Kumagai Y, Kawano T, Nakajima Y, et al. Multiple primary cancers associated with esophageal carcinoma. Surg Today 2001;31:872-6. [Crossref] [PubMed]
- Lee GD, Kim YH, Kim JB, et al. Esophageal cancer associated with multiple primary cancers: surgical approaches and long-term survival. Ann Surg Oncol 2013;20:4260-6. [Crossref] [PubMed]
- Shirai K, Tamaki Y, Kitamoto Y, et al. Prognosis was not deteriorated by multiple primary cancers in esophageal cancer patients treated by radiotherapy. J Radiat Res 2013;54:706-11. [Crossref] [PubMed]
- Fukuzawa K, Noguchi Y, Yoshikawa T, et al. High incidence of synchronous cancer of the oral cavity and the upper gastrointestinal tract. Cancer Lett 1999;144:145-51. [Crossref] [PubMed]
- Welz S, Schmid A, Hehr T, et al. Treatment-outcome for synchronous head-and-neck and oesophageal squamous cell carcinoma. Radiother Oncol 2005;77:267-70. [Crossref] [PubMed]
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953;6:963-8. [Crossref] [PubMed]
- Strong MS, Incze J, Vaughan CW. Field cancerization in the aerodigestive tract--its etiology, manifestation, and significance. J Otolaryngol 1984;13:1-6. [PubMed]
- Park JW, Lee SW. Clinical outcomes of synchronous head and neck and esophageal cancer. Radiat Oncol J 2015;33:172-8. [Crossref] [PubMed]
- Lim H, Kim DH, Jung HY, et al. Clinical significance of early detection of esophageal cancer in patients with head and neck cancer. Gut Liver 2015;9:159-65. [Crossref] [PubMed]
- Scherübl H, Steinberg J, Schwertner C, et al. Coincidental squamous cell cancers of the esophagus, head, and neck: risk and screening. HNO 2008;56:603-8. [PubMed]
- Ogata T, Takagi Y, Osaka Y, et al. Endoscopic screening for esophageal cancer in patients with head and neck cancers: is it useful for early detection and treatment of esophageal cancer? Gastrointest Endosc 2005;61:AB139. [Crossref]
- Wang YK, Chuang YS, Wu TS, et al. Endoscopic screening for synchronous esophageal neoplasia among patients with incident head and neck cancer: prevalence, risk factors, and outcomes. Int J Cancer 2017;141:1987-96. [Crossref] [PubMed]
- Winn DM, Blot WJ. Second cancer following cancers of the buccal cavity and pharynx in Connecticut, 1935-1982. Natl Cancer Inst Monogr 1985;68:25-48. [PubMed]
- Tepperman BS, Fitzpatrick PJ. Second respiratory and upper digestive tract cancers after oral cancer. Lancet 1981;2:547-9. [Crossref] [PubMed]
- Yoshino K, Endo M, Ishikawa N, et al. Diagnosis and treatment of metachronous cancers in the esophagus and the head and neck region. J Surg Oncol 1995;58:246-51. [Crossref] [PubMed]
- Shinoto M, Shioyama Y, Sasaki T, et al. Clinical results of definitive chemoradiotherapy for patients with synchronous head and neck squamous cell carcinoma and esophageal cancer. Am J Clin Oncol 2011;34:362-6. [Crossref] [PubMed]
- Hsu CJ, Chun-Wei W. Definitive concurrent chemoradiation therapy via intensity modulated radiation therapy for synchronous esophageal cancer in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys 2013;87:S289-90. [Crossref]
- Fan K, Chang J, Tsang N, et al. Treatment result of advanced synchronous head-and-neck and esophageal cancer. Int J Radiat Oncol Biol Phys 2013;87:S469. [Crossref]
- Schotborgh C, Liu L, Lemmens V, et al. High incidence of a second primary esophageal squamous cell carcinoma in patients with previous head-and-neck cancer: a nationwide population-based study. Gastroenterol 2011;140:S-98-9. [Crossref]
- Matsumoto A, Watanabe M, Mine S, et al. Comparison of synchronous versus staged surgeries for patients with synchronous double cancers of the esophagus and head-and-neck. Dis Esophagus 2017;30:1-6. [PubMed]
- Yoshida R, Morita M, Ando K, et al. Salvage esophagectomy after definitive chemoradiotherapy for synchronous double cancers of the esophagus and head-and-neck. Dis Esophagus 2010;23:59-63. [Crossref] [PubMed]
- Wind P, Roullet MH, Quinaux D, et al. Long-term results after esophagectomy for squamous cell carcinoma of the esophagus associated with head and neck cancer. Am J Surg 1999;178:251-5. [Crossref] [PubMed]
- Zenga J, Kreisel D, Kushnir VM, et al. Management of cervical esophageal and hypopharyngeal perforations. Am J Otolaryngol 2015;36:678-85. [Crossref] [PubMed]
- Roullet MH, Wind P, Zinzindohoué F, et al. Esophagectomy for squamous cell carcinoma of the esophagus isolated or associated with head and neck cancer: long-term survival. Ann Chir 2001;126:526-34. [Crossref] [PubMed]
- Elias D, Mamelle G, el Malt O, et al. Synchronous cancers of the esophagus and of the ORL area: results of combined treatments with esophagectomy (28 cases). Bull Cancer 1991;78:173-8. [PubMed]
- Shimane T, Mori T, Ono T, et al. Effects of concurrent S-1, nedaplatin/radiation therapy for 5 cases of head and neck cancer with esophageal carcinoma. Gan To Kagaku Ryoho 2010;37:1349-52. [PubMed]


