
Liver kinase B1 correlates with prognosis and epithelial-mesenchymal transition of resectable early stage non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide, and non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for approximately 85% of all diagnosed lung cancers (1). The early stage non-small cell lung cancer (ES-NSCLC) in our study was defined as completely resectable NSCLC in pathological stage T1-4N0-1M0, according to the seventh edition of the American Joint Committee on Cancer staging manual. In general, it is believed that surgical resection is a key element of ES-NSCLC treatment. And the leading cause of treatment failure is metastasis, which accounts for 74% of all treatment failure (2).
Metastasis is a multistep process characterized by the ability of cancer cells to invade into adjacent tissue, blood vessels, lymphatic vessels, and distant tissue. Recent evidences support that epithelial-mesenchymal transition (EMT) allows the tumor cells to acquire invasive properties and to develop metastatic growth characteristics (3-5). Accordingly, EMT is also associated with the progression and metastasis of many types of tumors, particularly lung cancer (6). EMT is a process in which a non-motile epithelial cell changes to a motile mesenchymal (fibroblast-like) phenotype. The hallmark of EMT is the loss of epithelial surface markers, most notably E-cadherin, and the acquisition of mesenchymal markers, such as vimentin.
The tumor suppressor liver kinase B1 (LKB1), also known as serine/threonine kinase 11 (STK11), was first reported in Peutz-Jeghers syndrome patients (7). LKB1 is considered to be involved in the regulation of multiple biological processes by activating a group of downstream kinases, including AMP-activated protein kinase (AMPK) or AMPK-related kinases (8). Salt-inducible kinase1 (SIK1), a member of the AMPK family, is a downstream target of LKB1. Suppression of SIK1 increases the expression of the transcriptional repressor zinc finger E-box binding homeobox 1 (ZEB1), and inhibits the function and expression of E-cadherin in lung alveolar epithelial cell line and human renal proximal tubule cell line (9). ZEB1 has been identified as a key regulator of EMT (10) and the low expression of LKB1 triggers EMT through induction of ZEB1 expression, which downregulates E-cadherin in human lung cancer cell lines (10,11). However, the clinicopathological significance of LKB1, EMT, SIK1, ZEB1 and their relationship in ES-NSCLC patients remain to be determined. Therefore, we carried out this study to explore the role of LKB1 in ES-NSCLC tissues and defined its association with clinicopathological features and survival of the patients. On the basis, we investigated the potential relationship between LKB1 and EMT and the possible effects of SIK1-ZEB1 signaling.
Methods
Patients
We retrospectively reviewed 103 NSCLC patients who underwent radical surgery between August 2012 and December 2013 at the Affiliated Hospital of Xuzhou Medical University. All the patients who were enrolled in the study need to meet the following criteria: pathologically confirmed ES-NSCLC (pT1-4N0-1M0); complete pulmonary resection and systematic node dissection. Patients were excluded from the study due to the following reasons: preoperative chemotherapy or radiotherapy; positive surgical margins; a history of lung cancer; a second primary cancer diagnosed within 5 years of lung cancer duration; loss to follow-up or death within 1 month after surgery. The patients with TXN2-3M0 NSCLC were also excluded because it was highly heterogeneous, and the optimal initial treatment of these patients was possibly not surgery (10). Tumor tissues and matched surrounding normal tissues (at least 5 cm away from the tumor lesions) were obtained from the Pathology Department of the Affiliated Hospital of Xuzhou Medical University. All of the specimens were examined and evaluated by two certified pathologists. Moreover, TTF-1 and P40 staining were performed to confirm the histological type (12). The pathological stage of each patient was diagnosed according to the seventh tumor-node-metastasis (TNM) classification of lung cancer. Whether the patient received adjuvant chemotherapy and the choice of chemotherapy regimen were determined by attending physicians according to relevant guidelines, without any effect on patient enrollment. The patients were followed until 8 December 2017. The follow-up period ranged from 1 to 64 months (average: 47.1 months; median: 51.5 months). Disease-free survival (DFS) and overall survival (OS) were calculated from the date of initial surgery to the date of clinical or pathological relapse or death. The patients were censored at the time of their last cancer-free clinical follow-up or their date of death not related to the lung cancer. This study was approved by the Clinical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (No. XYFY2016-KL021). The study outcomes will not affect the future management of the patients. Personal data of all patients have been secured and prior informed consent was obtained from each patient.
Immunohistochemistry
Formalin-fixed paraffin-embedded blocks from cancerous tissues were cut into 4-µm sections. Immunostaining was performed by the PV-9001 Polink-2 plus® Polymer HRP Detection System. The tissue sections were first deparaffinized with xylene, following by rehydration with serially decreasing concentrations of ethanol and immersion in 3% H2O2 for 10 minutes to abolish endogenous peroxidase activity. Antigen retrieval was performed using 10mM sodium citrate buffer (pH =6.0) followed by diluting in phosphate-buffered saline (PBS). Sections were incubated overnight at 4 °C with the primary antibodies, including anti-LKB1 (D60C5F10; 1:250 dilution; Cell Signaling Technology, Danvers, MA, USA), anti-SIK1 (bs-5252R; 1:150 dilution; Biotechnology, Beijing, China), anti-ZEB1 (E2A7414-1; 1:150 dilution; enogene, Nanjing, China), anti-E-cadherin (BS1097; 1:250 dilution; Bioworld Technology Co., Ltd., MN, USA), anti-vimentin (BS6007M; 1:200 dilution; Bioworld Technology Co., Ltd., MN, USA). The sections were washed and incubated with Polymer Helper for 30 minutes. Then, the poly-HRP anti-Rabbit IgG was used for 30 minutes at room temperature. After the sections were washed three times with PBS, color visualization was performed using 3,3'-diaminobenzidine (DAB) and hematoxylin.
Evaluation of immunohistochemical staining
Immunohistochemical staining was scored by two pathologists independently and blind to the clinical and pathological data. Disagreements between pathologists were discussed jointly and resolved by consensus. The score of immunoreactivity for the LKB1, SIK1, ZEB1 and E-cadherin was evaluated semiquantitatively using the product of staining intensity and the percentage of positive-stained tumor cells (13,14). Staining intensity was graded as follows: non-staining as 0, weak stain as 1, moderate stain as 2, and marked stain as 3. The percentage of stained tumor cells was divided into five levels: non-staining as 0, 1–25% as 1, 26–50% as 2, 51–75% as 3, and >75% as 4. The results were divided into 2 groups: high expression when the results were ≥4, and low expression when the results were <4 (13,14). Vimentin was a special marker protein which expressed in stromal cells, but it did not express in the epithelial tumor cells. Therefore, the protein expression of vimentin was considered high if the percentage of positive cells >0% (15).
Statistical analysis
All statistical analysis was performed using SPSS version 19 (IBM SPSS Statistics; IBM Corporation, NY, USA). The association between LKB1 status and clinicopathological parameters were analysed by chi-squared test and Mann-Whitney U rank sum test. Correlation among LKB1, SIK1, ZEB1 and EMT-related proteins was analysed by chi-squared tests and Pearson correlation coefficient. All statistical tests were two-tailed. Kaplan-Meier method with the log-rank test and Cox proportional hazards regression analysis were used to evaluate the survival data. A P value of <0.05 was considered to be statistically significant for all analyses.
Results
Correlations of LKB1 expression with clinicopathologic characteristics and risk of distant metastases
Immunohistochemical staining revealed that LKB1 protein was moderately or strongly expressed, and a total of 72.82% (75/103) of the ES-NSCLC patients had high expression in tumor tissues (Figure 1). In the 103 cases of ES-NSCLC samples, the correlations among LKB1 expression, clinicopathologic characteristics and risk of distant metastases after treatment were shown in Table 1. We found that LKB1 expression was associated with tumor histology (χ2=5.176, P=0.023), differentiation (χ2=5.507, P=0.019), lymph node metastases (χ2=5.864, P=0.015) and distant metastases after treatment (χ2=5.697, P=0.017) but not with T stage, TNM stage, smoking status, patient gender and age (Table 1).
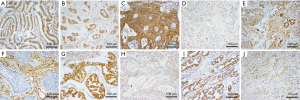
Table 1
| Variables | n | LKB1 expression | P | ||
|---|---|---|---|---|---|
| H | L | Statistics | |||
| Sex | χ2=1.794 | 0.180 | |||
| male | 76 | 58 | 18 | ||
| female | 27 | 17 | 10 | ||
| Age(years) | χ2=1.923 | 0.166 | |||
| <60 | 30 | 19 | 11 | ||
| ≥60 | 73 | 56 | 17 | ||
| Smoking status | χ2=0.146 | 0.702 | |||
| No | 51 | 38 | 13 | ||
| Yes | 52 | 37 | 15 | ||
| Histological type | χ2=5.176 | 0.023 | |||
| Scc | 52 | 43 | 9 | ||
| Ad | 51 | 32 | 19 | ||
| Tumour stage | U=848 | 0.104 | |||
| T1 | 34 | 27 | 7 | ||
| T2 | 50 | 37 | 13 | ||
| T3 | 12 | 8 | 4 | ||
| T4 | 7 | 3 | 4 | ||
| Lymph node metastasis | χ2=5.864 | 0.015 | |||
| No | 67 | 54 | 13 | ||
| Yes | 36 | 21 | 15 | ||
| Distant metastasis after treatment | χ2=5.697 | 0.017 | |||
| No | 70 | 56 | 14 | ||
| Yes | 33 | 19 | 14 | ||
| Differentiation | χ2=5.507 | 0.019 | |||
| Well | 76 | 60 | 16 | ||
| Poor | 27 | 15 | 12 | ||
| TNM stage | U=928 | 0.328 | |||
| I | 49 | 32 | 17 | ||
| II | 27 | 24 | 3 | ||
| IIIA (excluding N2 disease) | 27 | 19 | 8 | ||
LKB1, liver kinase B1; n, number; H, high expression; L, low expression; Scc, squamous cell carcinoma; Ad, adenocarcinoma.
Univariate and multivariate analyses indicate an independent role of LKB1 in the prognosis of ES-NSCLC
According to the immunohistochemistry results, all patients with ES-NSCLC were divided into 2 groups: LKB1-high expression group and LKB1-low expression group. Log-rank test revealed that high expression group had longer OS (P=0.000) and DFS (P=0.000) than low expression group (Figure 2) in Kaplan-Meier curves. Besides, 3-year OS (65.33% vs. 35.71%, P=0.007) and 3-year DFS (50.67% vs. 25.00%, P=0.019) in high expression group were respectively higher than that in LKB1-low expression group. The univariate Cox regression analysis indicated that LKB1 expression together with lymph node metastasis, distant metastases after treatment, and TNM stage were significantly associated with OS and DFS respectively (Table 2). In the multivariate Cox regression analysis, LKB1 expression (HR =0.442; 95% CI: 0.253 to 0.771, P=0.004) and TNM stage (HR =1.52; 95% CI: 1.108 to 2.091, P=0.010) were independent prognostic factors for OS. Accordingly, LKB1 expression (HR =0.339; 95% CI: 0.209 to 0.551, P=0.000) and distant metastasis after treatment (HR =2.480; 95% CI: 1.546 to 3.978, P=0.000) were independent prognostic factors for DFS (Table 2).
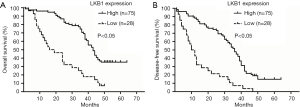
Table 2
| Variables | Category | OS | DFS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| HR [95% CI] | P | HR [95% CI] | P | HR [95% CI] | P | HR [95% CI] | P | |||||
| Sex | Male vs. female | 1.140 [0.692–1.879] | 0.606 | NA | – | 0.907 [0.568–1.449] | 0.684 | NA | – | |||
| Age (years) | <60 vs. ≥60 | 1.008 [0.615–1.650] | 0.976 | NA | – | 1.031 [0.651–1.635] | 0.895 | NA | – | |||
| Smoking status | Yes vs. no | 0.926 [0.586–1.461] | 0.743 | NA | – | 0.946 [0.625–1.431] | 0.793 | NA | – | |||
| Histological type | Scc vs. Ad | 1.191 [0.754–1.881] | 0.453 | NA | – | 1.130 [0.747–1.710] | 0.563 | NA | – | |||
| Tumour stage | T1 vs. T2 vs. T3 vs. T4 | 1.375 [1.043–1.814] | 0.024 | 1.076 [0.767–1.508] | 0.672 | 1.192 [0.921–1.541] | 0.182 | NA | – | |||
| Lymph node metastasis | No vs. yes | 2.138 [1.346–3.397] | 0.001 | 0.684 [0.316–1.482] | 0.336 | 1.840 [1.197–2.827] | 0.005 | 0.843 [0.422–1.686] | 0.632 | |||
| Distant metastasis after treatment | No vs. yes | 2.032 [1.261–3.273] | 0.004 | 1.399 [0.836–2.343] | 0.202 | 3.002 [1.904–4.732] | 0.000 | 2.480 [1.546–3.978] | 0.000 | |||
| Differentiation | Well vs. poor | 1.568 [0.951–2.586] | 0.078 | NA | – | 1.425 [0.896–2.265] | 0.135 | NA | – | |||
| TNM stage | I vs. II vs. IIIa | 1.867 [1.409–2.473] | 0.000 | 1.522 [1.108–2.091] | 0.010 | 1.586 [1.224–2.055] | 0.000 | 1.171 [0.875–1.566] | 0.288 | |||
| LKB1 expression | High vs. low | 0.308 [0.189–0.501] | 0.000 | 0.442 [0.253–0.771] | 0.004 | 0.281 [0.176–0.450] | 0.000 | 0.339 [0.209–0.551] | 0.000 | |||
LKB1, liver kinase B1; OS, overall survival; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; NA, not adopted; Scc, Squamous cell carcinoma; Ad, Adenocarcinoma
LKB1 expression is negatively correlated with EMT
E-cadherin expression was observed at membrane. Vimentin was localised to stromal structures as well as the cytoplasm of neoplastic tissues. In the 103 cases of ES-NSCLC samples, the high expression of E-cadherin was observed in 76.70% (79/103) of lung cancer samples (Figure 1), whereas E-cadherin-low expression was observed in the other 23.30% (24/103) of cases. Vimentin-high expression was observed in 35.92% (37/103) lung cancer samples (Figure 1), whereas it was low in the other 64.08% (66/103) of cases. LKB1 expression was positively correlated with E-cadherin (r=0.231; P=0.019) but negatively correlated with vimentin (r=−0.225; P=0.022).
LKB1 expression is positively correlated with SIK1 but negatively correlated with ZEB1
SIK1 expression was observed at cytoplasm and nuclear, and ZEB1 was localised to nuclear of the neoplastic tissues. In the 103 cases of NSCLC samples, the high expression of SIK1 was observed in 38.83% (40/103) of lung cancer samples (Figure 1), whereas SIK1-low expression was observed in the other 61.17% (63/103) of cases. As shown in Table 3, LKB1 expression was positively correlated with SIK1 (r=0.218; P=0.027) but negatively correlated with ZEB1 (r=−0.242; P=0.014).
Table 3
| Variables | SIK1 | ZEB1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| H | L | r | P | H | L | r | P | ||
| LKB1 | 0.218 | 0.027 | −0.242 | 0.014 | |||||
| H | 34 | 41 | 36 | 39 | |||||
| L | 6 | 22 | 21 | 7 | |||||
| SIK1 | −0.206 | 0.037 | |||||||
| H | – | – | – | – | 17 | 23 | |||
| L | 40 | 23 | |||||||
LKB1, liver kinase B1; SIK1, salt-inducible kinase 1; ZEB1, Zincfinger E-box Binding Homeobox 1; H, high expression; L, low expression; r, correlation coefficient.
Discussion
The primary treatment for ES-NSCLC is surgical resection, and metastasis has been recognized as the leading cause of treatment failure and poor clinical outcomes in ES-NSCLC patients after surgery (16). Evidences suggest that EMT is a crucial progress conferring malignant cells invasion and metastasis abilities with low expression of polarity and adhesion and acquisition of a mesenchymal phenotype and mobility (17,18). In this study, we elucidated the clinical significance of LKB1 in ES-NSCLC by studying the EMT marker protein and its regulatory signaling.
LKB1 plays an important role in multiple biological processes, including cell growth, cell cycle progression, cell polarity and metabolism (19,20). According to the data from the TCGA cohort, inactivation of LKB1 was detected in approximately 2% lung squamous cell carcinoma and 17% lung adenocarcinoma (21,22). Ji et al. reported that LKB1 alteration was detected in 19% lung squamous cell carcinoma and 34% lung adenocarcinoma (23). In this study, the results in lung squamous cell carcinoma and lung adenocarcinoma are 17.31% (9/52) and 37.25% (19/51) respectively, similarly to what Ji et al. reported. The data from our study and TCGA cohort are inconsistent, possibly because of ethnic difference and sample size.
A recent study showed that distant metastasis is a major cause of treatment failure of ES-NSCLC, which is closely associated with PFS and OS (2). Several studies in vivo and in vitro suggest that LKB1 is a critical barrier to lung cancer metastasis (24,25). In this study, our results indicated the risk of distant metastasis in LKB1-low expression group (50.00% vs. 25.33%, P=0.017) was higher than that in high expression group, consistent with previous studies (24,25). Calles et al. reported that patients with LKB1 loss had higher number of metastatic sites at the time of diagnosis of stage IV disease and developed brain metastasis more frequently (26). The correlation between LKB1 expression and location of distant progression needs further investigation.
LKB1 has been shown to be a valid prognostic marker of lung cancer (26,27). However, its clinical significance in ES-NSCLC remains unknown. In this study, we semiquantitatively examined the expression of LKB1 and analysed the correlations among LKB1 expression, clinicopathological parameters in postoperative patients with ES-NSCLC for the first time. Furthermore, we demonstrated that patients with LKB1-high expression had higher 3-year OS and DFS than LKB1-low expression patients. Multivariate Cox regression analysis confirmed that LKB1 expression was independent predictor for longer OS and DFS. These data suggest LKB1 is a clinical biomarker independently associated with prognosis in ES-NSCLC.
EMT is a critical event in the invasion, progression and metastasis of epithelial cancers. A hallmark of EMT is down-regulation of epithelial markers such as E-cadherin and up-regulation of mesenchymal markers like vimentin (28). Roy et al. (11) blocked the expression of LKB1 gene in lung cancer cells and observed up-regulated expression of ZEB1 concurrently with induction of EMT; while silencing ZEB1 expression showed opposite effects, demonstrating that LKB1 inactivation triggers EMT in lung cancer cells through the induction of ZEB1. Yao et al. (24) reported that Re-expression of LKB1 in radioresistant NSCLC cells line A549R caused increased expression of SIK1, decreased expression of ZEB1 and a reversal of EMT, while knockdown of LKB1 in H1299R cells caused decreased expression of SIK1 and increased expression of ZEB1 and promoted the EMT phenotype. These results suggest that LKB1-SIK1 signaling suppresses EMT by repressing the expression of transcriptional factor ZEB1. Accordingly, our results about the association among LKB1, EMT markers, SIK1 and ZEB1 were partly compatible with abovementioned findings, and association between EMT and expression level of SIK1 and ZEB1 should be further investigated.
For the first time to our knowledge, we revealed that LKB1 expression was an independent favourable prognostic indicator in patients with ES-NSCLC, which was related with lower risk of distant metastasis. Furthermore, our findings demonstrated that the level of LKB1 expression was correlated with EMT and expression of SIK1 and ZEB1. More studies are needed to confirm the underlying mechanism involving LKB1, SIK1, ZEB1 and EMT in ES-NSCLC.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.12.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Clinical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (No. XYFY2016-KL021). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Provencio M, Sanchez A. Therapeutic integration of new molecule-targeted therapies with radiotherapy in lung cancer. Transl Lung Cancer Res 2014;3:89-94. [PubMed]
- Demicheli R, Fornili M, Ambrogi F, et al. Recurrence dynamics for non-small-cell lung cancer: effect of surgery on the development of metastases. J Thorac Oncol 2012;7:723-30. [Crossref] [PubMed]
- Scanlon CS, Van Tubergen EA, Inglehart RC, et al. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J Dent Res 2013;92:114-21. [Crossref] [PubMed]
- Singh M, Yelle N, Venugopal C, et al. EMT: Mechanisms and therapeutic implications. Pharmacol Ther 2018;182:80-94. [Crossref] [PubMed]
- Amawi H, Ashby C, Samuel T, et al. Polyphenolic Nutrients in Cancer Chemoprevention and Metastasis: Role of the Epithelial-to-Mesenchymal (EMT) Pathway. Nutrients 2017;9:911. [Crossref] [PubMed]
- Steinestel K, Eder S, Schrader AJ, et al. Clinical significance of epithelial-mesenchymal transition. Clin Transl Med 2014;3:17. [Crossref] [PubMed]
- Wang Z, Wu B, Mosig RA, et al. STK11 domain XI mutations: candidate genetic drivers leading to the development of dysplastic polyps in Peutz-Jeghers syndrome. Hum Mutat 2014;35:851-8. [Crossref] [PubMed]
- Han D, Li SJ, Zhu YT, et al. LKB1/AMPK/mTOR signaling pathway in non-small-cell lung cancer. Asian Pac J Cancer Prev 2013;14:4033-9. [Crossref] [PubMed]
- Eneling K, Brion L, Pinto V, et al. Salt-inducible kinase 1 regulates E-cadherin expression and intercellular junction stability. FASEB J 2012;26:3230-9. [Crossref] [PubMed]
- Goossens S, Vandamme N, Van Vlierberghe P, et al. EMT transcription factors in cancer development re-evaluated: Beyond EMT and MET. Biochim Biophys Acta Rev Cancer 2017;1868:584-91.
- Roy BC, Kohno T, Iwakawa R, et al. Involvement of LKB1 in epithelial-mesenchymal transition (EMT) of human lung cancer cells. Lung Cancer 2010;70:136-45. [Crossref] [PubMed]
- Pelosi G, Scarpa A, Forest F, et al. The impact of immunohistochemistry on the classification of lung tumors. Expert Rev Respir Med 2016;10:1105-21. [Crossref] [PubMed]
- Jiang L, Liang X, Liu M, et al. Reduced expression of liver kinase B1 and Beclin1 is associated with the poor survival of patients with non-small cell lung cancer. Oncol Rep 2014;32:1931-8. [Crossref] [PubMed]
- Matsubara D, Kishaba Y, Yoshimoto T, et al. Immunohistochemical analysis of the expression of E-cadherin and ZEB1 in non-small cell lung cancer. Pathol Int 2014;64:560-8. [Crossref] [PubMed]
- Dauphin M, Barbe C, Lemaire S, et al. Vimentin expression predicts the occurrence of metastases in non small cell lung carcinomas. Lung Cancer 2013;81:117-22. [Crossref] [PubMed]
- Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw 2013;11:645-53; quiz 53. [Crossref] [PubMed]
- Li L, Li W. Epithelial–mesenchymal transition in human cancer: Comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharmacology & Therapeutics 2015;150:33-46. [Crossref] [PubMed]
- Creighton CJ, Gibbons DL, Kurie JM. The role of epithelial-mesenchymal transition programming in invasion and metastasis: a clinical perspective. Cancer Manag Res 2013;5:187-95. [Crossref] [PubMed]
- Xu Z, Liu J, Shan T. New Roles of Lkb1 in Regulating Adipose Tissue Development and Thermogenesis. J Cell Physiol 2017;232:2296-8. [Crossref] [PubMed]
- Shan T, Xu Z, Liu J, et al. Lkb1 regulation of skeletal muscle development, metabolism and muscle progenitor cell homeostasis. J Cell Physiol 2017;232:2653-6. [Crossref] [PubMed]
- Cancer Genome Atlas Research N. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519-25. [Crossref] [PubMed]
- Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. [Crossref] [PubMed]
- Ji H, Ramsey MR, Hayes DN, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature 2007;448:807-10. [Crossref] [PubMed]
- Yao YH, Cui Y, Qiu XN, et al. Attenuated LKB1-SIK1 signaling promotes epithelial-mesenchymal transition and radioresistance of non-small cell lung cancer cells. Chin J Cancer 2016;35:50. [Crossref] [PubMed]
- Gao Y, Ge G, Ji H. LKB1 in lung cancerigenesis: a serine/threonine kinase as tumor suppressor. Protein Cell 2011;2:99-107. [Crossref] [PubMed]
- Calles A, Sholl LM, Rodig SJ, et al. Immunohistochemical Loss of LKB1 Is a Biomarker for More Aggressive Biology in KRAS-Mutant Lung Adenocarcinoma. Clin Cancer Res 2015;21:2851-60. [Crossref] [PubMed]
- Xiao J, Zou Y, Chen X, et al. The Prognostic Value of Decreased LKB1 in Solid Tumors: A Meta-Analysis. PLoS One 2016;11:e0152674. [Crossref] [PubMed]
- Xiao W, Tang H, Wu M, et al. Ozone oil promotes wound healing by increasing the migration of fibroblasts via PI3K/Akt/mTOR signaling pathway. Biosci Rep 2017; [Crossref] [PubMed]


