MicroRNA-21 mediates bone marrow mesenchymal stem cells protection of radiation-induced lung injury during the acute phase by regulating polarization of alveolar macrophages
Introduction
Radiation-induced lung injury (RILI) often occurs after radiotherapy in patients with non-small cell lung cancer (NSCLC). The prognosis of patients with RILI is usually poor (1). Reports have indicated that lung injury, with symptoms only during radiotherapy for lung cancer, has an incidence rate reaching 30% (2). Currently, RILI treatment options are rather limited, and many of which lead to higher recurrence rates (3). Therefore, finding markers that can indicate the occurrence of RILI, and finding effective therapeutic method to prevent or treat RILI, are all very important goals on the way to improved prognosis of patients receiving radiotherapy.
The mechanisms through which microRNAs (miRNAs) regulate physiological and pathological reactions have recently been extensively studied (4). The specific expression patterns of miRNAs in certain diseases indicate that many of them can be used as diagnostic markers (5). Considering that many miRNAs function by suppressing their target genes, dis-regulated miRNAs can often play a regulatory role in the development of their associated diseases (6). In such cases, introduction of exogenous miRNA or suppression of endogenous miRNA can usually be used as an effective treatment approach.
Bone marrow mesenchymal stem cells (BMSCs) are multipotent cells that can differentiate into osteoblasts, chondrocytes and adipocytes (7). Infusion of BMSCs that overexpress specific miRNAs can effectively introduce exogenous miRNAs to target cells via the paracrine system and achieve therapeutic purposes (8).
In the present study, the correlation between miR-21 expression and the severity and prognosis of NSCLC patients with RILI was investigated. A rat model demonstrated that BMSCs overexpressing miR-21 could protect radiation-induced lung injury during the acute phase. Furthermore, cell-based studies showed that BMSCs overexpressing miR-21 could inhibit M1 polarization of alveolar macrophages (AMs) by suppressing TNF-α expression in these cells, and thus achieve therapeutic purposes.
Methods
Patients and samples
Totally 61 non-small cell lung cancer (NSCLC) patients before, during, and at the end of a treatment course of conventional fractionated radiotherapy were included in this study. Patients inclusion criteria, radiation therapy and classification criteria of RILI were in according with our previous description (2). The serum samples and bronchoalveolar lavage fluid (BALF) samples from all the patients (each patient was collected at various time points were collected for the subsequent assays in according with our previous reports (2). Pulmonary function markers, including diffusion capacity for carbon monoxide (DLCO), forced expiratory volume at 1 s (FEV1), and forced vital capacity (FVC), were recorded and used as baselines in all patients before radiotherapy, and would be tested at 4, 8, 16 and 24 weeks for follow-up treatment. The prospective clinical study was performed from January 2014 to December 2018 in the Eighth Medical Center of PLA General Hospital, Beijing, China. The experimental study was performed in the laboratory of PLA General Hospital from June 2016 to March 2019.
RNA extraction and qRT-PCR assay
Serum samples were collected and centrifuged at a speed of 3,000 g for 5 min at 4 °C. Next, 200 mL serum supernatant was extracted for RNA extraction. Similarly, BALF samples were centrifuged for 10 min at a speed of 3,000 g at 4 °C, and were absorbed 200 mL supernatant for RNA extraction. Total RNA was obtained from the above samples using TRIzol reagent (Invitrogen, New York Island, USA).
Subsequently, qRT-PCR for mRNA was performed using the PrimeScript RT-PCR kit (Takara, Bio, Inc., Shiga, Japan). qRT-PCR for miRNA was performed using TaqMan miRNA kit. All the qRT-PCR experiments were performed on Ependorf quantitative PCR detector. All the primer sequences of the target were from the previous literature (9). The parameters of reverse transcription for mRNA were set as follows: first, 65 °C reaction was set for 5 min, then 37 °C reaction was set for 15 min, and finally 98 °C reaction was set for 5 min. The parameters of qPCR for mRNA were set as follows: first 95 °C reaction for 30 s, then 40 cycles of 95 °C reaction for 5 s, and 60 °C reaction for 5 s, and finally 72 °C reaction for 30 s. The human and rat miR-21 primers were purchased from Qiagen [catalog numbers MS00009079 (Hs-miR-21-5p) and MS00013216 (Rn-miR-21-5p)].The parameters of reverse transcription for miRNA were set as follows: first, 16 °C reaction was set for 30 min, then 42 °C reaction was set for 30 min, and finally 84 °C reaction was set for 5 min. The parameters of q PCR for mRNA were set as follows: First 95 °C reaction for 2 min, then 40 cycles of 95 °C reaction for 15 s, and 60 °C reaction for 30 s.
RILI rat model
The steps and methods of the RILI rat model constructed in this study refer to our previous publication (10). In short, female, 8-week-old Sprague-Dawley (SD) rats weighing about 240 g were used to construct RILI rat models. After being fixed, rats were anesthetized by intravenous injection of pentobarbital (40 mg/kg). Rats’ heads and abdomen were protected from lead radiation. The irradiation parameters were as follows: (I) 6 mV photons (Siemens, Germany) were generated and (II) were focused to the surface at a distance of 100 cm. (III) Single irradiation of 20 Gy (2.5 Gy/min), and (IV) irradiation area of 4.5 cm × 4.5 cm. After irradiation, rats were euthanized with carbon dioxide for a certain time (2 h and 4 weeks, 8 weeks, 16 weeks and 24 weeks). Flow rate of CO2 displaced no more than 30% of the chamber volume per minute. The mice were observed until they were found to have stopped breathing, and then stopped injecting carbon dioxide. The rats were taken out and observed for another 5 min to confirm apnea. Between animals, the chamber is turned over to completely empty the carbon dioxide chamber. Subsequently, the serum, BALF and tissues samples from the rats were collected for the subsequent assays in according with our previous reports (10). At 28 weeks after irradiation, all the remaining mice were euthanized with CO2 as human endpoints.
Isolation, purification, and culture of BMSCs
Carbon dioxide was used to euthanize rats, and then the bone marrow cavity of rats was washed with full culture medium. The washed medium was filtered by 200 mesh filter. The filtrate was repeatedly blown to form a uniform single cell suspension and then cultured on a 10 cm disc. After removing non-adherent cells, the medium was replaced every 1–2 days. After 10–14 days, 90% of the cells were fused and passaged. Such repeated third generation cells are used for subsequent experiments.
Stable transfection of BMSCs with miR-21 overexpressing vector
MiR-21 overexpressing vector were purchased from Ribobio, Guangzhou. According to the manufacturer’s instructions, Lipofectamine® 2000 (Invitrogen; Thermo Fisher Science, Inc.) was used to transfer the over-expressing miRNA-21 vector into BMSC, and were select stable transfectants by G-418. BMSCs that were stable transfected with miR-21 overexpressing vector were termed BMSC-miR-21-OV and control BMSCs were termed BMSC-NC.
Transplantation of the BMSCs into the rats
BMSCs were injected into rats at the dose of 2×106 by tail vein injection. The same dose of saline was injected into the control group. Every three rats were killed at 3, 7 and 14 days after BMSC injection to determine whether BMSC was successfully transplanted.
Isolation and culture of rat peritoneal macrophages
After RILI model was successfully established in rats, peritoneal macrophages were subsequently extracted. Briefly, 20 mL of PBS, together with 10% FBS, were injected into the abdominal cavity of rats. Subsequently, rat peritoneal fluid was extracted and centrifuged at a speed of 192 ×g for 10 min at 4 °C. The precipitated cells were re-dispersed and inoculated into a six-well plate and cultured in complete culture medium. After 6 hours, the uncoated cells were removed. Fresh media should be replaced every 1–2 days. Two days later, the cells were sorted by CD68 and CD11.
In vitro co-culture of peritoneal macrophages with BMSCs
Totally 1×106 macrophages were inoculated into 6-well plates. Twelve hours later, the cells were replaced with a medium containing 1×106 BMSCs. Macrophages and BMSC were co-cultured with Transwell inserts (Corning, Inc.).
Cytokine assay
Cytokine levels and nitric oxide production in culture supernatants or in the serum samples from both clinical patients and rat model were determined using commercial ELISA kits (R&D Systems and BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer’s protocol. Each value represents the mean of triplicate values.
Statistical analysis
All statistics were conducted using SPSS software package (version 19.0; SPSS Corporation). The clinical characteristics of the two groups were compared by Pearson Chi-square test or Fisher exact test. Student t-test or one-way ANOVA were used for continuous data analysis. The overall survival rate (OS) or disease-free survival rate (DFS) was assessed by Kaplan-Meier method.
Results
MiR-21 in sera and bronchoalveolar lavage fluid (BALF) supernatant is significantly increased in NSCLC patients with RILI
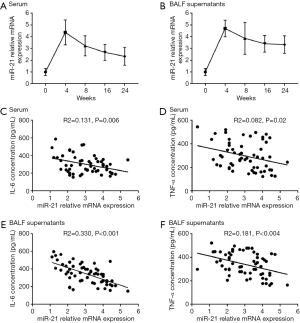
Before and on the day of intensity-modulated radiotherapy (IMRT), miR-21 expression characteristics in the serum of every patient were measured and considered as the baseline. Serum levels were also recorded at weeks 4, 8, 16, and 24 after treatment (Figure 1A). Serum miR-21 level has increased at the early stage after irradiation therapy in all patients, peaked at week 4 and then gradually decreased through weeks 8, 16, and 24. Similar results were also observed in BALF supernatant samples (Figure 1B). Moreover, we found negative correlations between miR-21 level and that of IL-6 and TNF-α at week 4 in both sera (Figure 1C,D) and BALF supernatants (Figure 1E,F).
MiR-21 level at week 4 could be considered a potential biomarker for predicting RILI severity
We assigned the average expression of miR-21 at week 4 as the cutoff value (cutoff value =3.0) and then divided the patients into miR-21 high group (32 patients) and miR-21 low group (30 patients). As shown in Table 1, at weeks 8 and 24 after treatment, patients with high mIR-21 suffered RILI-related effects significantly less than that those in the low MIR-21 group. This was reflected in lower RILI incidence and RILI grade in the high miR-21 group (P=0.008 and P=0.031, respectively). After comparing lung function markers between the two groups, we found that there was no difference in carbon monoxide diffusion capacity (DLCO), forced expiratory volume in one second (FEV1), and forced vital capacity (FVC) values between the high and low miR-21 groups. After IMRT, the DLCO, FEV1, and FVC values in both groups decreased relative to baseline values (Table 1). No significant differences in DLCO, FEV1, and FVC values were found when the two groups were evaluated on week 8. At week 24, significant decrease in FVC and FEV1 values was observed in miR-21 low group, when compared with the miR-21 high group. The above data indicate that miR-21 expression increased in NSCLC patients with RILI, and that the level of miR-21 at week 4 could be considered a potential biomarker for predicting the severity of RILI.
Table 1
| Parameters | Post-radiation therapy 8 weeks | P value | Post-radiation therapy 24 weeks | P value |
|---|---|---|---|---|
| Grade of RILIa | ||||
| Grade 1 (n) | 0.008 | 0.031 | ||
| miR-21 low | 7 | 10 | ||
| miR-21 high | 3 | 8 | ||
| Grade 2 (n) | ||||
| miR-21 low | 4 | 7 | ||
| miR-21 high | 1 | 4 | ||
| Grade 3 (n) | ||||
| miR-21 low | 3 | 5 | ||
| miR-21 high | 1 | 3 | ||
| Pulmonary functionb | ||||
| FVC (%) | 0.262 | 0.005 | ||
| miR-21 low | −3.48±0.89 | −5.15 ±0.94 | ||
| miR-21 high | −3.24±0.77 | −4.33 ±1.27 | ||
| FEV1 (%) | 0.224 | 0.029 | ||
| miR-21 low | −3.52±1.03 | −5.88±1.23 | ||
| miR-21 high | −3.17±1.21 | −5.17±1.28 | ||
| DLCO (%) | 0.308 | 0.069 | ||
| miR-21 low | −4.93±1.22 | −4.65±1.53 | ||
| miR-21 high | −4.62±1.14 | −4.02±1.10 |
Patients in miR-21 high group (32 patients) and low group (30 patients) were examined at 8 and 24 weeks after commencement of radiation therapy for grade of RILI and pulmonary function. a, the values are shown as the number of patients with each grade (n). P value was calculated by Pearson Chi-Square test; b, Baseline values of FEV1, DLCO and FVC are set as 0 in trial and control groups. Percentage changes in FEV1, DLCO and FVC at 8 and 24 weeks are calculated from baseline. P value was calculated by student t test. FVC, forced vital capacity; FEV1, forced expiratory volume at 1 s; DLCO, diffusion capacity for carbon monoxide.
Treatment with miR-21-knockout BMSCs reduces mortality in rats with RILI
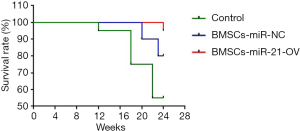
The use of miRNA-overexpressing stem cells is a newly developed therapeutic strategy for the treatment of various diseases (8). Thus, we aimed to determine whether treatment with miR-21-overexpressing BMSCs was effective against RILI. Rats with RILI were randomly split into three groups. The control group received saline injection to the tail vein before and after the irradiation treatment. The miR-21-overexpressing BMSCs (BMSC–miR-21-OV) group and non-treated wildtype BMSCs (BMSC–NT) group were treated with BMSC–miR-21-OV or BMSC–NT, respectively, for 3 d before and 4 d after irradiation treatment. The rats showed signs of physical weakness, and several died within 24 weeks post-irradiation. The survival curves for the three groups are shown in Figure 2. Mortality rates were 45% (9/20) for the control group, 20% (4/20) for the BMSC–NT group, and 5% (1/20) for the BMSC–miR-21-OV group. These mortality rates differed significantly between the three groups.
Treatment with miR-21-knockout BMSCs decreases RILI-induced acute inflammation.
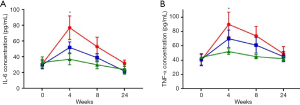
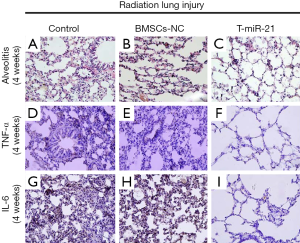
Studying histological sections, acute inflammation was found in all experimental groups at week 4 post-irradiation. Compared with the control group, the BMSC–NT exhibited the following weaknesses: (I) inflammatory cells throughout the lung mesenchyme, (II) collapsed alveoli with significant inflammatory exudates, and (III) pulmonary hyaline membranes in the lung tissue. These findings indicate that BMSCs treatment can significantly inhibit the development of RILI in rats. Moreover, the inhibition effect of BMSCs was markedly enhanced in the BMSC–miR-21-OV group. In addition, immunohistochemical staining revealed that the BMSC–miR-21-OV group exhibited the lowest expression of the proinflammatory factors IL-6 and TNF-α, followed by the BMSC–NT group. Meanwhile, the control group showed the highest IL-6 and TNF-α expression levels (Figure 3).
ELISA analysis revealed significant increase in the serum level of IL-6 and expression of TNF-α in the irradiated control group. These peaked at week 4 and then decreased gradually. Compared with the BMSC–NT group, BMSC–miR-21-OV treatment was more effective in inhibiting the expression of IL-6 and TNF-α (Figure 4). The combined aforementioned data indicate that injection of miR-21-overexpressing BMSCs is an effective treatment strategy against RILI.
Co-culture of miR-21-knockout BMSCs plays an important role in inhibiting AMs M1 polarization
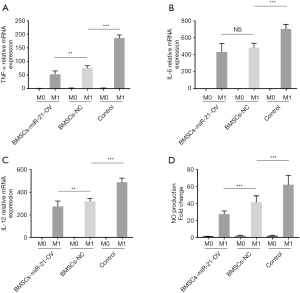
In RILI patients and animal model, the secretion of proinflammatory factors is closely related to polarization of lung macrophages (11). Previous reports have demonstrated that a number of important macrophage M1 polarization markers, including IL-12p35 and TNF-α, are miR-21 targets in vitro (12,13). We therefore explored whether BMSCs-miR21-OV could change the polarization of lung macrophages. AMs from the rats were cultured in 12-well plates. BMSCs-miR-21-OV or BMSCs-NT were then added as coculture. The cell cultures were stimulated with 200 ng/L LPS for 24 h to induce the macrophages into M1 polarization (9,14). We found that BMSCs-NT coculture could not alter the mRNA expressions of M1 markers, such as IL-6, IL-12, and TNF-α. their expression was comparable to the control AMs. BMSCs-miR-21-OV coculture significantly inhibited the induction of the macrophages into M1 polarization (Figure 5A,B,C). BMSCs-miR-21-OV coculture also decreased production of Nitric oxide in AMs (Figure 5D). These results suggest that miR-21 may regulate RILI by controlling macrophage polarization.
Discussion
The overall prognosis of NSCLC patients after radiotherapy varies greatly among individuals. A more accurate assessment of the incidence of RILI in lung cancer patients during radiotherapy will help reduce treatment-related mortality, thereby improving the overall lung cancer survival (1,2). At present, several research groups are engaged in the search for promising RILI biomarkers. Groves et al. found that CCSP/SP-D ratio may be useful as a biomarker of radiation-induced pulmonary fibrosis (15). Wang et al. suggested that IL-8 and TGF-β1 could predict RILI toxicity in NSCLC patients (16). Sun et al. have shown that serum miRNA signature could predict response to radiation therapy in NSCLC (17). Generally, these targets are closely related to the development of acute inflammation (11). Our study demonstrated that low miR-21 levels could predict higher RILI incidence and grade, lower pulmonary function in patients, and enhancement of IL-6 and TNF-α expressions, especially during the acute phase. These findings suggest that miR-21 can be used as a potential marker for RILI diagnosis and prognosis. Based on our findings, we also suggest that miR-21 may play a regulatory role in the pathogenesis of RILI.
Injection of miRNA-overexpressing BMSCs is considered a viable method for introducing exogenous miRNAs into the body because BMSCs can deliver miRNAs to the target cells via exosomes (18). In the present study, pretreatment with BMSC–miR-21-OV was more effective at preventing radiation-induced pulmonary injury during the acute inflammatory phase, when compared to the injection of BMSC–NT. Rats in the BMSC–miR-21-OV group exhibited more attenuated inflammation than did those in the BMSC–NT treatment group, suggesting a protective effect for miR-21. Furthermore, cell-based experiments confirmed that this therapeutic effect was achieved by targeting inflammatory factors, such as TNF-α, and regulating M1 polarization of pulmonary macrophages. These findings suggest that pretreatment with BMSC–miR-21-OV may be a novel and effective management strategy for patients undergoing radiotherapy at the early acute inflammatory stage. However, owing to the high prevalence of pulmonary interstitial fibrosis at late stages of RILI (19,20), we should further explore whether miR-21 can regulate pulmonary interstitial fibrosis formation. In addition, data on the systematic toxicity of MSC-miR-21 treatment are currently missing. Therefore, long-term analysis of the outcome of MSC-miR-21 treatment is necessary to realize its potential.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.12.77). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the ethics committee of the Eighth Medical Center of PLA General Hospital and all the experiments were carried out according to principles of Helsinki Declaration. Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bernchou U, Christiansen RL, Asmussen JT, et al. Extent and computed tomography appearance of early radiation induced lung injury for non-small cell lung cancer. Radiother Oncol 2017;123:93-8. [Crossref] [PubMed]
- Bao P, Zhao W, Li Y, et al. Protective effect of ulinastatin in patients with non-small cell lung cancer after radiation therapy: a randomized, placebo-controlled study. Med Oncol 2015;32:405. [Crossref] [PubMed]
- Hanania AN, Mainwaring W, Ghebre YT, et al. Radiation-Induced Lung Injury: Assessment and Management. Chest 2019;156:150-62. [Crossref] [PubMed]
- Domańska-Senderowska D, Laguette MN, Jegier A, et al. MicroRNA Profile and Adaptive Response to Exercise Training: A Review. Int J Sports Med 2019;40:227-35. [Crossref] [PubMed]
- Wu C, Wang X, Zhang J, et al. MicroRNA-224 Expression and Polymorphism Predict the Prognosis of Hepatitis B Virus-Related Hepatocellular Carcinoma Patients After Liver Resection. Clin Lab 2019;65: [Crossref] [PubMed]
- Long Y, Zhu Y. Identification of FBXW7α-regulated genes in M1-polarized macrophages in colorectal cancer by RNA sequencing. Saudi Med J 2019;40:766-73. [Crossref] [PubMed]
- Xu T, Zhang Y, Chang P, et al. Mesenchymal stem cell-based therapy for radiation-induced lung injury. Stem Cell Res Ther 2018;9:18. [Crossref] [PubMed]
- Xu R, Shen X, Si Y, et al. MicroRNA-31a-5p from aging BMSCs links bone formation and resorption in the aged bone marrow microenvironment. Aging Cell 2018;17:e12794. [Crossref] [PubMed]
- Hu Y, Xu F, Zhang R, et al. Interleukin-1β-induced IRAK1 ubiquitination is required for TH-GM-CSF cell differentiation in T cell-mediated inflammation. J Autoimmun 2019;102:50-64. [Crossref] [PubMed]
- Bao P, Gao W, Li S, et al. Effect of pretreatment with high-dose ulinastatin in preventing radiation-induced pulmonary injury in rats. Eur J Pharmacol 2009;603:114-9. [Crossref] [PubMed]
- Tian X, Wang F, Luo Y, et al. Protective Role of Nuclear Factor-Erythroid 2-Related Factor 2 Against Radiation-Induced Lung Injury and Inflammation. Front Oncol 2018;8:542. [Crossref] [PubMed]
- Qiu YF, Wang MX, Meng LN, et al. MiR-21 regulates proliferation and apoptosis of oral cancer cells through TNF-α. Eur Rev Med Pharmacol Sci 2018;22:7735-41. [PubMed]
- Yang X, Pan Y, Xu X, et al. Sialidase Deficiency in Porphyromonas gingivalis Increases IL-12 Secretion in Stimulated Macrophages Through Regulation of CR3, IncRNA GAS5 and miR-21. Front Cell Infect Microbiol 2018;8:100. [Crossref] [PubMed]
- Jeon EJ, Kim DY, Lee NH, et al. Telmisartan induces browning of fully differentiated white adipocytes via M2 macrophage polarization. Sci Rep 2019;9:1236. [Crossref] [PubMed]
- Groves AM, Williams JP, Hernady E, et al. A Potential Biomarker for Predicting the Risk of Radiation-Induced Fibrosis in the Lung. Radiat Res 2018;190:513-25. [Crossref] [PubMed]
- Wang S, Campbell J, Stenmark MH, et al. Plasma Levels of IL-8 and TGF-β1 Predict Radiation-Induced Lung Toxicity in Non-Small Cell Lung Cancer: A Validation Study. Int J Radiat Oncol Biol Phys 2017;98:615-21. [Crossref] [PubMed]
- Sun Y, Hawkins PG, Bi N, et al. Serum MicroRNA Signature Predicts Response to High-Dose Radiation Therapy in Locally Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2018;100:107-14. [Crossref] [PubMed]
- Tian X, Wang F, Luo Y, et al. Protective Role of Nuclear Factor-Erythroid 2-Related Factor 2 Against Radiation-Induced Lung Injury and Inflammation. Front Oncol 2018;8:542. [Crossref] [PubMed]
- Chen L, Lu FB, Chen DZ, et al. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol Immunol 2018;93:38-46. [Crossref] [PubMed]
- Huang Y, Zhang W, Yu F, et al. The Cellular and Molecular Mechanism of Radiation-Induced Lung Injury. Med Sci Monit 2017;23:3446-50. [Crossref] [PubMed]