Laparoscopic radical resection of gastric cancer and metachronous colon cancer—a case report
Introduction
Gastric cancer (GC) is the fourth most common cancer among both men and women in developed countries (1). In China, GC is the second most common cancer among men, the third among women, and the second leading cause of cancer-related mortality (2). With the advancements in GC diagnosis and treatment, the overall survival of GC patients has been prolonged; which has led to the rise in incidences of second primary cancer (SPC) accompanied with GC. Few literatures have reported that patients with metachronous GC and SPC can receive laparoscopic surgery successfully. In this study, we report a case with metachronous GC and colon cancer who received successful laparoscopic surgery for these two cancers. Billroth (3) first reported multiple primary cancer (MPC) in 1889, and the first systematic study of MPC was delivered by Warren and Gates in 1932 (4). The diagnostic criteria of MPC by Warren and Gates have been widely used. These criteria are: (I) each cancer must be determined as malignant by histologic evaluation; (II) each cancer must be geographically separate and distinct; (III) the possibility of metastasis of each cancer must be eliminated. The incidences of MPC in patients with GC range from 1.1–8.0% (5-7) in previous study. According to the interval of diagnosis between MPC, multiple cancers may develop metachronous or synchronously. Synchronous MPC was defined as those with an interval within 6 months. On the other hand, metachronous MPC are defined as those with an interval of more than 6 months (8-10). In this study, we report a metachronous MPC, as the colorectal cancer (CRC) which was diagnosed 49 months later after the diagnosis of GC. The histopathological examination revealed that both of them were primary cancers. Many metachronous primary cancers developed within 5 years after the diagnosis of first primary cancer. A Korean study reported that the mean occurrence interval for metachronous cancer was 25.5 months in GC patients (8,11). As a result, it is important to summarize a regular standard follow-up procedure for post-operation management of GC patients, and individualized as well. Also, minimal invasive treatments are needed to optimize their prognosis. We present the following case in accordance with the CARE guideline (12).
Case presentation
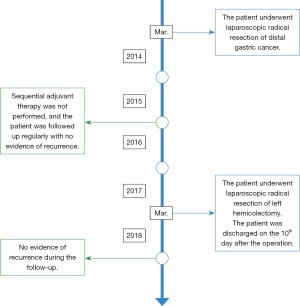
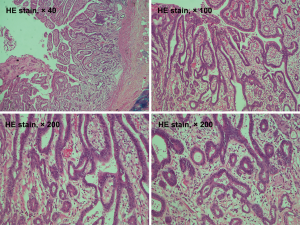
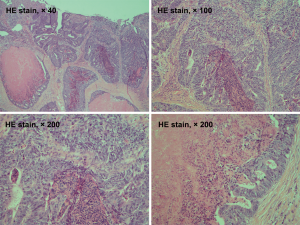
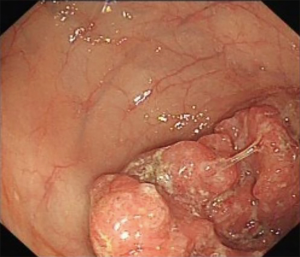
A 69-year-old man was admitted to our hospital for further evaluation and treatment of colon cancer. The patient’s diagnosis and treatment process were briefly outlined in the timeline (Figure 1). Four years ago, he had been diagnosed with early-stage GC and undergone laparoscopic radical resection of distal GC. The pathologic specimen revealed a well-differentiated intramucosal adenocarcinoma with no lymph nodes metastasis (0/9), and with negative resection margins (T1M0N0, stage IA) (Figure 2). Sequential adjuvant therapy was not performed, and the patient was followed up regularly with no evidence of recurrence. Forty-nine months later, the patient had undertaken a colonoscopy due to a symptom of left upper abdomen colic, which revealed a cauliflower-like mass in the splenic flexure of colon (Figure 3). Histopathological examination revealed that the patient had developed adenocarcinoma. Laboratory test showed white blood cell (WBC) 5.2×109/L, hemoglobin 101 g/L, total protein (TP) 56.7 g/L, albumin (ALB) 33.2 g/L, carcinoembryonic antigen (CEA) 1.62 (normal range, 0–5) ng/mL, and carbohydrate antigen 19-9 (CA19-9) 11.62 (normal range, 0–37) IU/mL. Fecal occult blood test was negative. Further, abdominal ultrasound, abdominal computed tomography (CT) scan and gastroscopy found no evidence of distant metastasis or recurrence of GC. The patient underwent laparoscopic radical resection of left hemicolectomy. The pathologic specimen revealed a moderately differentiated adenocarcinoma, which had invaded the subserosa, with no lymph nodes metastasis (0/9), and with negative resection margins (T3M0N0, stage IIA) (Figure 4). The patient recovered well, with no obvious complications, and was discharged on the 10th day after the operation. There was no family history related to the patient’s case.
Discussion
In patients with GC, the most common SPC is CRC, followed by lung cancer (5-7,9,13,14). The high incidence of CRC in GC patients may be attributed to the genetic and environmental factors. Several studies have revealed higher incidences of SPC in GC patients with microsatellite instability (MSI) (15,16). Nevertheless, Kim et al. (17) reported that MSI or p53 overexpression was not effective in predicting GC patients developing metachronous or synchronous CRC. This is so because GC and CRC have different etiologic pathways; which make it tough to evaluate the relationship between them. Sonnenberg et al. (18) reported that 2.35-fold risk for CRC was detected in patients with H. pylori gastritis. Since H. pylori infection is one of the recognized risk factors for GC, it may be another risk factor for GC patients to develop CRC (19).
Previous studies have revealed that age, male gender, blood type, tobacco, alcohol, chemotherapy, radiotherapy, an early stage of GC, multiplicity of GC, and family tumor history are all risk factors of SPC development (6,8,11,20-22). Male and elderly GC patients tend to develop SPC more frequently. A Polish study (6) reported that GC patients had synchronous or metachronous cancers. Patients with synchronous cancer were older (68.0±10.3) years while the metachronous cancer patients were 59.9±11.1) years. Furthermore, GC patients with blood type O were more likely to develop SPC (56.2% vs. 31.6%, respectively, P=0.002). Cigarette smoking and alcohol drinking were also reported as risk factors for SPC development in GC patients; which may be explained by the field cancerization effect (5,8,23,24). Chemotherapy and radiotherapy of initial cancer were considered to increase the incidence of SPC (6,22). However, some studies reported that adjuvant chemotherapy may not be an independent risk factor for developing SPC (25,26). Ławniczak et al. (6) and Muela Molinero et al. (27) found that most GC patients with MPC, had either their first- or second-degree relatives as cancer victims; particularly GC. Due to the favorable prognosis of early-stage GC, there is a high risk of SPC development in patients with early-stage GC (8,11,20).
In GC patients with SPC, a comparison of synchronous SPC, and metachronous SPC revealed that the latter had a better prognosis (14,21). Ikeda et al. (28) reported that the 10-year survival rate of GC patients without SPC was 69.3%. The survival rate in synchronous SPC was 40.1%; while in metachronous SPC was 75.2%. This difference may be due to routine follow-up after treatment for first primary cancer, which increases the opportunity of early detection. Also, metachronous SPC occurs in early-stage and more frequently among GC. The main cause of death for MPC, either in first primary or second cancer is still unclear. For metachronous MPC patients, the first primary cancer seems to be the major cause of death. In contrast, for synchronous MPC patients, SPC seems to be the major cause of death (8,28).
For most MPC patients, surgery is recommended for curative option. However, traditional open radical resection is feasible but traumatic; especially for MPC patient who may have undergone two or more surgeries before. In this case, we performed laparoscopic surgery for both GC and metachronous colon cancer. The patient recovered smoothly with no obvious complications, and was discharged on the 10th day after the operation. Intraperitoneal adhesions caused by previous operations are always a concern for surgeons. The advantage of laparoscopic surgery is that it is minimally invasive and causes less postoperative adhesions; which make it feasible for performing repeated operations for MPC patients. Furthermore, laparoscopic surgery has been identified as feasible and safe for metachronous CRC, regardless of whether previous surgery was minimally invasive or open, and with short-term benefits (29,30). Otherwise, with the development of diagnostic techniques, the rate of detection of early-stage GC has been improved. Endoscopic resection has been widely accepted for curing early-stage GC in Japan and Korea, which has been proved feasible, convenient, and minimally invasive (31,32).
Most SPC are always diagnosed due to more symptoms during the follow-up examination (33). Thus, a study (8), which included 3,066 GC patients, was used to develop a monogram for predicting metachronous MPC. The selected parameters include gender, age, the multiplicity of GC, stage of GC, and tumor size as 5. The c-index was 0.72, which means that this nomogram had a certain predictive accuracy. Compared with other predicted methods, this nomogram had better prediction. However, the small incidence of MPC in GC patients (70 in 3,066 patients) limits the generalization of the study result. Just as the author indicated, further external validation with independent patient cohorts is required to improve the accuracy of prediction.
Conclusions
A regular standard follow-up program should be established for GC patient, in order to determine the MPC. Treatment for MPC should be more individualized and comprehensive. Minimally invasive surgery may be an appropriate option. An efficient predictive tool is demanded, which can improve the prognosis of GC survivors.
Acknowledgments
We are grateful to Prof. Tian-Hong Fu (Department of Pathology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University) for her contribution to the case.
Funding: The present study supported by a grant from
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.01.44). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was ethically approved by the Ethics Committee of Sir Run Shaw Hospital of Zhejiang University (No. 20180518-021). Written informed consent was obtained from the patient for publication of this case report and accompanying figures.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Blakely AM, Miner TJ. Surgical considerations in the treatment of gastric cancer. Gastroenterol Clin North Am 2013;42:337-57. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Billroth T, von Winiwarter A. General surgical pathology and therapeutics in 51 Vorlesungen: a textbook for students and physicians in fifty-one lectures. Berlin: Rerimer, 1889.
- Warren S. Multiple primary malignant tumors. A survey of the literature and a statistical study. Am J Cancer 1932;16:1358-414.
- Buyukasik O, Hasdemir AO, Gulnerman Y, et al. Second primary cancers in patients with gastric cancer. Radiol Oncol 2010;44:239-43. [Crossref] [PubMed]
- Ławniczak M, Gawin A, Jaroszewicz-Heigelmann H, et al. Synchronous and metachronous neoplasms in gastric cancer patients: a 23-year study. World J Gastroenterol 2014;20:7480-7. [Crossref] [PubMed]
- Kim JY, Jang WY, Heo MH, et al. Metachronous double primary cancer after diagnosis of gastric cancer. Cancer Res Treat 2012;44:173-8. [Crossref] [PubMed]
- Kim C, Chon H, Kang B, et al. Prediction of metachronous multiple primary cancers following the curative resection of gastric cancer. BMC Cancer 2013;13:394. [Crossref] [PubMed]
- Sun LC, Tai YY, Liao SM, et al. Clinical characteristics of second primary cancer in colorectal cancer patients: the impact of colorectal cancer or other second cancer occurring first. World J Surg Oncol 2014;12:73. [Crossref] [PubMed]
- López ML, Lana A, Díaz S, et al. Multiple primary cancer: an increasing health problem. Strategies for prevention in cancer survivors. Eur J Cancer Care (Engl) 2009;18:598-605. [Crossref] [PubMed]
- Kim JW, Jang JY, Chang YW, et al. Clinical features of second primary cancers arising in early gastric cancer patients after endoscopic resection. World J Gastroenterol 2015;21:8358-65. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Suh BJ. Synchronous and metachronous colon cancers in patients with gastric cancer: report of 2 cases. Case Rep Oncol 2016;9:752-9. [Crossref] [PubMed]
- Watanabe M, Kochi M, Fujii M, et al. Dual primary gastric and colorectal cancer: is the prognosis better for synchronous or metachronous? Am J Clin Oncol 2012;35:407-10. [Crossref] [PubMed]
- Cho I, An JY, Kwon IG, et al. Risk factors for double primary malignancies and their clinical implications in patients with sporadic gastric cancer. Eur J Surg Oncol 2014;40:338-44. [Crossref] [PubMed]
- Yun HR, Yi LJ, Cho YK, et al. Double primary malignancy in colorectal cancer patients--MSI is the useful marker for predicting double primary tumors. Int J Colorectal Dis 2009;24:369-75. [Crossref] [PubMed]
- Kim HJ, Kim N, Choi YJ, et al. Clinicopathologic features of gastric cancer with synchronous and metachronous colorectal cancer in Korea: are microsatellite instability and p53 overexpression useful markers for predicting colorectal cancer in gastric cancer patients? Gastric Cancer 2016;19:798-807. [Crossref] [PubMed]
- Sonnenberg A, Genta RM. Helicobacter pylori is a risk factor for colonic neoplasms. Am J Gastroenterol 2013;108:208-15. [Crossref] [PubMed]
- Jones M, Helliwell P, Pritchard C, et al. Helicobacter pylori in colorectal neoplasms: is there an aetiological relationship? World J Surg Oncol 2007;5:51. [Crossref] [PubMed]
- Lee JH, Bae JS, Ryu KW, et al. Gastric cancer patients at high-risk of having synchronous cancer. World J Gastroenterol 2006;12:2588-92. [Crossref] [PubMed]
- Eom BW, Lee HJ, Yoo MW, et al. Synchronous and metachronous cancers in patients with gastric cancer. J Surg Oncol 2008;98:106-10. [Crossref] [PubMed]
- Chen SC, Liu CJ, Hu YW, et al. Second primary malignancy risk among patients with gastric cancer: a nationwide population-based study in Taiwan. Gastric Cancer 2016;19:490-7. [Crossref] [PubMed]
- Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer 2009;124:2406-15. [Crossref] [PubMed]
- Braakhuis BJ, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter's concept of field cancerization: evidence and clinical implications. Cancer Res 2003;63:1727-30. [PubMed]
- Nio Y, Hirahara N, Minari Y, et al. Second malignancies after a gastrectomy for gastric cancers: the effects of adjuvant therapies. Anticancer Res 1999;19:3591-9. [PubMed]
- Kinoshita Y, Tsukuma H, Ajiki W, et al. The risk for second primaries in gastric cancer patients: adjuvant therapy and habitual smoking and drinking. J Epidemiol 2000;10:300-4. [Crossref] [PubMed]
- Muela Molinero A, Jorquera Plaza F, Ribas Ariño T, et al. Multiple malignant primary neoplasms in patients with gatric neoplasms in the health district of León. Rev Esp Enferm Dig 2006;98:907-16. [Crossref] [PubMed]
- Ikeda Y, Saku M, Kawanaka H, et al. Features of second primary cancer in patients with gastric cancer. Oncology 2003;65:113-7. [Crossref] [PubMed]
- Park SY, Choi GS, Jun SH, et al. Laparoscopic salvage surgery for recurrent and metachronous colorectal cancer: 15 years' experience in a single center. Surg Endosc 2011;25:3551-8. [Crossref] [PubMed]
- Nagasaki T, Akiyoshi T, Ueno M, et al. Feasibility and safety of laparoscopic surgery for metachronous colorectal cancer. Surg Today 2015;45:434-8. [Crossref] [PubMed]
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011;74:485-93. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19. [Crossref] [PubMed]
- Kim SH, Kim HJ, Lee JI, et al. Multiple primary cancers including colorectal cancer. J Korean Soc Coloproctol 2008;24:467-72. [Crossref]