The molecular mechanism of platelet lysate promotes transformation of non-union cells into osteoblasts
Introduction
Platelet lysate (PL) was a liquid component obtained from whole blood by density gradient centrifugation to concentrate platelets, and then passed through three times freeze-thaw to lyse platelets (1). PL was characterized by the removal of platelet membranes and other cell debris, in order to reduce immunogenicity and retain many of the cell growth factors, which could create conditions for allogeneic or xenogeneic transplantation (2,3). In recent years, PL had been applied to the research of bone tissue engineering, and significantly promoted bone regeneration and repair. PL is administered to treat many diseases such as bone regeneration, oral mucositis, osteoarthritis, and ocular diseases (4). The injectable tissue-engineered bone, which could improve the osteogenesis rate of osteogenic traction, was constituted by PL, autologous bone, allogeneic bone or hydroxyapatite, and autologous bone matrix stem cells. In addition to procoagulant effects, PL also had the function of promoting tissue wound repair. PL contained many important cell growth factors and was removed of platelet surface antigens. Cell growth factors could promote the division and proliferation of various cells, collagen synthesis, stimulate the growth of blood vessel and induce cell differentiation, which were essential for bone repair and regeneration (5). When PL is activated to gelate by thrombin, cell growth factors are released. PL is a solution saturated by proteins, growth factors and chemokines. PL mainly contains cell growth factors, such as platelet derived growth factor (PDGF), transforming growth factor-β (TGF-β), insulin-like growth factor (IGF), vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF), etc. (6). In recent years, studies had shown that RANKL/RANK/OPG played an important role in bone metabolism (7). RANK was a receptor of RANKL, which involved in the differentiation of osteoclasts and promoting bone resorption. OPG was another receptor for RANKL, which inhibited osteoclast development and bone resorption (8).
In this study, nine cases of PL treatment for bone healing patients were reported, and revealed the molecular mechanism of PL on nonunion cell proliferation. We found that PL had a remarkable therapeutic effect on bone repair related diseases, such as delayed fracture healing, femoral head necrosis and meniscal tear. also revealed the molecular mechanism of PL on bone repair. Early/late osteoblastic differentiation and osteoblastic proliferation could be assessed by the expression levels of OPG, OCN, OPN and ALP (9-12). The 5% PL had a significantly positive effect on the proliferation of nonunion cells; 10% PL had a significantly positive effect on the expression levels of OPG, OPN, OCN and ALP proteins in nonunion cells, improved the protein expression ratio of OPG/RANKL, but had no effect on RANKL protein expression.
Methods
Clinical data
From June 2016 to June 2018, nine patients were treated with PL, patient information were shown in Table 1.
Table 1
| Serial number | Gender | Age (year) | Disease | Course (month) | Follow-up time (month) | Fracture healing |
|---|---|---|---|---|---|---|
| 1 | Female | 40 | Nonunion of humerus fracture | 6 | 12 | Bony union |
| 2 | Female | 60 | Nonunion of femoral fracture | 6 | 12 | Bony union |
| 3 | Female | 17 | Nonunion of radius and ulna fracture | 7 | 17 | Bony union |
| 4 | Male | 53 | Delayed healing after femoral osteotomy | 6 | 12 | Improved |
| 5 | Male | 47 | Nonunion of tibial fracture | 4 | 9 | Improved |
Clinic treatment
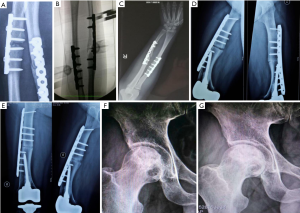
Nonunion of the radius and ulna fractures (Figure 1A), peripheral injection was performed under the guidance of the C-arm (Figure 1B). The injection angle was 45° to the forearm plane and the injection lasted for 15–30 minutes. Two injections per course, once every six months. Each treatment was injected twice, one injection for each of half year. Postoperative treatment, no wound infection, foreign body rejection and other complications, the suffering limb insisted on doing functional exercises everyday after surgery. Regular dressing change after surgery, weight-bearing functional exercise of the suffering limb, regular monthly review. After bilateral femoral osteotomy (Figure 1C,D,E), under the guidance of the C-arm, the lesion was injected around the thigh plane, the injection lasted 15–30 minutes. Each treatment was injected twice, one injection for each of two months. Postoperative treatment, no wound infection, foreign body rejection and other complications, the suffering limbs insisted on doing functional exercises every day after surgery (Figure 1F,G).
Cell isolation and culture
Slag bone was placed in a centrifuge tube, added 20 mL DMEM medium +10% FBS, and discarded the precipitate after centrifugation. Suspension cells appeared in the medium, then were placed in a cell culture flask at 37 °C and 5% CO2. The culture medium was renewed every 24 h in order to remove all non-adherent bone nonunion cells. When the adherent cells were grown to 80% confluency, 1% trypsin was used for digestion, the adherent cells were digested to suspension cells, then were cultured and passaged.
Collection of PL
According to Landesberg method, PL was prepared from intracardiac blood of twelve male Wistar rats (body weigh 250–300 g) (13). Centrifuged at 200 g for 10 min, then the liquid in the centrifuge tube was divided into two layers, the upper supernatant was plasma, and the lower layer was platelet concentrate. Absorbed all supernatant, then transferred to another centrifuge tube and centrifuged at 200 g for 10 min. The supernatant was discarded and the remaining mix was PL. The platelet concentration of PL was adjusted to 1×109/mL with PBS. The mixed PL was frozen in liquid nitrogen, and then thawed in 37 °C water bath, for 3 consecutive times, 10 min for each time interval. The supernatant was obtained by centrifugation at 2,500 g for 2 min and 4 °C to harvest PL. PL was filtered through a 0.22 µm sterile filter and stored at −80 °C until use. The study was approved by the Laboratory Animal Ethics Committee of the First Affiliated Hospital of Harbin Medical University (No. 2019029).
MTT assay
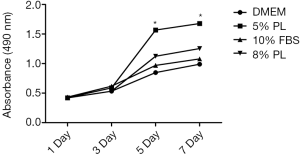
The nonunion cells were inoculated and cultured for 24 hours, and then adjusted the cell concentration to 4×105/mL, cultured for 24h. Adjusted cell concentration to 1×107/mL and transferred into 96-well plates, 100 µL per well. Nonunion cells were cultured with different concentrations (5% PL, 8% PL, 10% FBS and DMEM medium) of PL in the carbon dioxide incubator (37 °C, 10% CO2). Cells were incubated with 20 µL MTT (5 g/L) solution for 4 hours. After removal of liquid, 100 µL DMSO was added to every well to dissolve the crystal, plate was shaken at 600 r/min for 10 min. The absorbance of each well was detected by a microplate reader at a wavelength of 490 nm (SuPerMax 3100, Shanghai Flash Spectrum Biotechnology Co., Ltd.).
Immunofluorescence
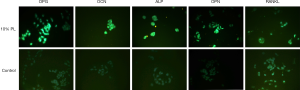
The nonunion cells density was about 5×105 cells/mL, 1.5 mL/well in a 6-well plate, and then cultured for 48 hours. The experiment was divided into 2 groups, the control group added 0% PL, and the experimental group added 10% PL in the culture medium; 48 h after the treatment, the culture medium was discarded, and the nonunion cells were washed 3 times with PBS in a 6-well plate. The nonunion cells were fixed with 4% paraformaldehyde for 15 min, washed 3 times with PBS, permeabilized with 0.5% Triton X-100 for 5 min at room temperature, washed 3 times with PBS, blocked with 5% BSA at room temperature for 30 min. Two hundred µL 1% BSA +2 µL primary anti-RANKL antibody, 200 µL 1% BSA +2 µL primary anti-OPG antibody, 200 µL 1% BSA +2 µL primary anti-ALP antibody, 200 µL 1% BSA +2 µL primary anti-OPN antibody, 200 µL 1% BSA +2 µL primary anti-OCN antibody, mixed and added to each well respectively, incubated at 4 °C overnight. Added 1:1,000 diluted fluorescence secondary antibody and incubated in dark for 1 h at room temperature. Sealed the slides liquid with anti-fluorescence quencher, observated under fluorescent microscope.
Western blot
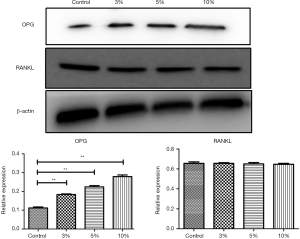
The nonunion cells (5×105 cells/well) were seeded into the 6-well plates and incubated until 90% confluence. Cells were treated with 0% PL (control group), 3% PL, 5% PL and 10% PL for 7days. Then lysed using radioimmunoprecipitation (RIPA) buffer after incubation. Proteins were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred to a polyvinylidene difluoride (PVDF) membrane. The desired proteins were stained for appropriate primary and secondary antibodies and detected by LAS-3000 after soaking with enhanced chemiluminescence (ECL) reagents. Chemiluminescent intensity of each protein was normalized with glyceraldehyde 3-phosphate dehydrogenase (β-actin).
Statistical analysis
GraphPad Prism 7.0 software were used for statistical analysis. The measured parameters were presented as means ± SD. The t-test was used for comparison between two groups, P<0.05 was considered statistically significant.
Results
PL promotes bone healing
After 12 months of treatment with PL, five patients were followed up without complications, three of five patients with nonunion and delayed healing achieved clinical bone healing. The criteria of fracture healing: at least 3 cortical bone grafts were achieved by X-ray review, and without pain after weight bearing. One patient with nonunion had improved, and the formation of osteophytes was observed by reexamination, bone marrow cavity was not closed. Reexamination showed callus growth, and lower limb patients were still unable to bear weight. Two cases were femoral head necrosis, one case was osteoarthritis, one case was meniscus tear (see Figure 1 and Table 1).
Effect of PL on the proliferation of rat nonunion cells
PL had just been applied to clinical applications and lacks abundant clinical comparative cases. Reasonable scientific basis and a suitable volume fraction of PL should be found preliminarily through experimental methods. MTT assay showed that the proliferation rates of the four groups were not significant (P>0.05) at 3 day. The proliferation rates of the 5% PL group were significantly higher than those of the other groups at 5 and 7 day (P<0.05). The proliferation rates of 8% PL were not significantly different from those of 10% FBS group at any time point (P>0.05) (see Figure 2). PL showed a positive effect on nonunion cell proliferation, 5% PL was especially significant, but the proliferation effects of 8% PL and 10% FBS were not obvious.
Effect of PL on the protein expression levels of osteogenic related genes
In the western blot analysis, the results showed that 3% PL, 5% PL and 10% PL could significantly up-regulate the level of OPG protein expression, but had no effect on RANKL protein expression. As the PL concentration increased, the expression level of OPG protein also increased (see Figure 3). Immunofluorescence also confirmed that 10% PL increased the protein expression level of OPG and could not increase the protein expression of RANKL. In addition, 10% PL could also increase the protein expression of osteogenic related genes, such as OCN, ALP, and OPN (see Figure 4).
Discussion
The 5% PL was a commonly used volume fraction in clinical practice, and the result of MTT assay showed that 5% PL had a significant positive effect on the proliferation of bone nonunion cells (P<0.05); 3%, 5% and 10% PL significantly increased OPG protein expression in nonunion cells (P<0.05), especially the effect of 10% PL was the most obvious. But 3%, 5% and 10% PL had no effect on RANKL protein expression. Immunofluorescence assay confirmed that 10% PL could improve OPG protein expression and had no effect on that of RANKL; 10% PL could also increase OPN, OCN and ALP protein expression.
Platelet-rich plasma was mainly used for autologous transplantation due to its strong immunogenicity. Treatment with platelet-rich plasma for patients with knee osteoarthritis presented beneficial effects in repairing joint tissue and alleviating bone damage, cartilage destruction (14). The specialists of RegenLab® USA developed the RegenKit® BCT, which allows the isolation of platelet-rich plasma from less whole blood sample in an easy way (15). PL was a liquid derivative of platelets after cell lysis, was not only removed residual solid cell components and reduced immunogenicity, but also retained many of growth factors, which created conditions for future allogeneic or xenogeneic transplantation. In the early stages of trauma, evoked coagulation reactions and released growth factors by platelets played a crucial role in regulation of tissue repair and bone healing. PDGF, IGF and TGF-β, which were growth factors released by platelets, were important migration, proliferation and differentiation regulators of nonunion cell (16). PL could promote the growth and proliferation of nonunion cells and bone marrow mesenchymal stem cells, inhibited excessive differentiation. PL was beneficial to early bone healing.
PL short-term application could significantly promote nonunion cell proliferation, migration and adhesion, but long-term application could inhibit ALP activity and matrix mineralization (14). Growth factors, such as TGF-β and PDGF, played important roles in bone remodeling. They could activate osteoclasts to induce bone resorption, also activate chemotaxis, proliferation and differentiation of osteogenic progenitor cells to promote osteogenesis. Both processes were simultaneously promoted each other to complete bone reconstruction. Its main molecular signaling mechanism was coupling bone resorption and bone formation through the RANK/RANKL/OPG pathway to maintain the integrity of the skeletal system (17). In animal experiments of transgenic mice, OPG could inhibit the formation of osteoclasts before the differentiation of early stem cells, effectively reducing the incidence of osteoporosis in ovariectomized mice, and also increased bone mass and bone density. RANKL stimulated the differentiation and maturation of osteoclasts, activated osteoclasts, and enhanced the function of osteoclasts (18). In this study, we found that after the addition of PL, the expression levels of OPG, OCN, OPN, ALP protein were increased, but the expression level of RANKL protein did not change, the ratio of OPG/RANKL was increased. Researchers isolated various types of cells from bone delayed healing tissues, such as long fusiform or short fusiform, which were similar to nonunion cells and could differentiate to nonunion cells (19). But female nonunion cells were affected by estrogen and progesterone, influenced the reliability of experimental results. We chose the nonunion cells of male rats to gain more convincing. At present, bone grafting was an effective method for the treatment of fracture nonunion. We believed that PL therapy had the advantages of convenient material extraction, simple preparation, small rejection reaction and positive effect. The biological characteristics of PL and the interaction of its different growth factors were still not understood. Bone healing was a tissue repair process with complex regulatory mechanisms, similar to embryonic or postnatal bone development, which required the cooperation of multiple cells and internal environmental components (20). In the early stage of fracture, blood coagulation and its accompanying inflammatory response constituted a chemical microenvironment for healing and repair. Many growth factors play an important role in regulating cell biological behavior and accelerating bone tissue healing and repair. But these potential repair mechanisms were still not fully understood. However, it has been confirmed by clinical applyment that PL has a definite therapeutic effect in the treatment of fracture nonunion. Delayed bone healing was still a relatively difficult problem in current orthopedic diseases. Reasonable and effective measures should be made according to the differences of patients. PL Combined with bone grafting could be used as a treatment option, but the clinical application period was not a long time. The clinical effects of PL still required further researches and experiments. According to the results of this experiment, different concentrations of PL had different effects on the proliferation of nonunion cells. In future researches, we will reveal the PL effects of different treating times on the proliferation of nonunion cells, aim to reveal the appropriate injection treating time.
Conclusions
PL had a significantly positive effect on the clinical treatment of delayed fracture healing and nonunion, and rat cranial nonunion cell proliferation, also had a significantly positive effect on the expression levels of OPG, OPN, OCN and ALP proteins in nonunion cells, improved the protein expression ratio of OPG/RANKL.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.12.95). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Laboratory Animal Ethics Committee of the First Affiliated Hospital of Harbin Medical University (No. 2019029). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chevallier N, Anagnostou F, Zilber S, et al. Osteoblastic differentiation of human mesenchymal stem cells with PL. Biomaterials 2010;31:270-8. [Crossref] [PubMed]
- Dhillon MS, Behera P, Patel S, et al. Orthobiologics and platelet rich plasma. Indian J Orthop 2014;48:1-9. [Crossref] [PubMed]
- Andia I, Abate M. Platelet-rich plasma: underlying biology and clinical correlates. Regen Med 2013;8:645-58. [Crossref] [PubMed]
- Zamani M, Yaghoubi Y, Movassaghpour A, et al. Novel therapeutic approaches in utilizing platelet lysate in regenerative medicine: Are we ready for clinical use? J Cell Physiol 2019;234:17172-86. [Crossref] [PubMed]
- Ranzato E, Boccafoschi F, Mazzucco L, et al. Role of ERK1/2 in platelet lysate-driven endothelial cell repair. J Cell Biochem 2010;110:783-93. [Crossref] [PubMed]
- Burnouf PA, Juan PK, Su CY, et al. A novel virally inactivated human platelet lysate preparation rich in TGF-beta, EGF and IGF, and depleted of PDGF and VEGF. Biotechnol Appl Biochem 2010;56:151-60. [Crossref] [PubMed]
- Song Y, Du ZW, Yang QW, et al. Association of Genes Variants in RANKL/RANK/OPG Signaling Pathway with the Development of Osteonecrosis of the Femoral Head in Chinese Population. Int J Med Sci 2017;14:690-7. [Crossref] [PubMed]
- González-Galván MC, Mosqueda-Taylor A, Bologna-Molina R, et al. Evaluation of the osteoclastogenic process associated with RANK/RANK-L/OPG in odontogenic myxomas. Med Oral Patol Oral Cir Bucal 2018;23:e315-9. [PubMed]
- Emami A, Larsson A, Petrén-Mallmin M, et al. Serum bone markers after intramedullary fixed tibial fractures. Clin Orthop Relat Res 1999;220-9. [PubMed]
- Jules J, Shi Z, Liu J, et al. Receptor activator of NF-{kappa}B (RANK) cytoplasmic IVVY535-538 motif plays an essential role in tumor necrosis factor-{alpha} (TNF)-mediated osteoclastogenesis. J Biol Chem 2010;285:37427-35. [Crossref] [PubMed]
- Stoffel K, Engler H, Kuster M, et al. Changes in biochemical markers after lower limb fractures. Clin Chem 2007;53:131-4. [Crossref] [PubMed]
- Oni OO, Mahabir JP, Iqbal SJ, et al. Serum osteocalcin and total alkaline phosphatase levels as prognostic indicators in tibial shaft fractures. Injury 1989;20:37-8. [Crossref] [PubMed]
- Sabarish R, Lavu V, Rao SR. A Comparison of Platelet Count and Enrichment Percentages in the Platelet Rich Plasma (PRP) Obtained Following Preparation by Three Different Methods. J Clin Diagn Res 2015;9:ZC10-2. [PubMed]
- Huang G, Hua S, Yang T, et al. Platelet-rich plasma shows beneficial effects for patients with knee osteoarthritis by suppressing inflammatory factors. Exp Ther Med 2018;15:3096-102. [PubMed]
- Santos SCNDS, Sigurjonsson ÓE, Custódio CA, et al. Blood Plasma Derivatives for Tissue Engineering and Regenerative Medicine Therapies. Tissue Eng Part B Rev 2018;24:454-62. [Crossref] [PubMed]
- Ota K, Quint P, Weivoda MM, et al. Transforming growth factor beta 1 induces CXCL16 and leukemia inhibitory factor expression in osteoclasts to modulate migration of osteoblast progenitors. Bone 2013;57:68-75. [Crossref] [PubMed]
- Lo KW, Kan HM, Gagnon KA, et al. One-day treatment of small molecule 8-bromo-cyclic AMP analogue induces cell-based VEGF production for in vitro angiogenesis and osteoblastic differentiation. J Tissue Eng Regen Med 2016;10:867-75. [Crossref] [PubMed]
- Park JH, Lee NK, Lee SY. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol Cells 2017;40:706-13. [PubMed]
- Zong S, Zeng G, Fang Y, et al. The effects of α-zearalanol on the proliferation of bone-marrow-derived mesenchymal stem cells and their differentiation into osteoblasts. J Bone Miner Metab 2016;34:151-60. [Crossref] [PubMed]
- Zigdon-Giladi H, Rudich U, Michaeli Geller G, et al. Recent advances in bone regeneration using adult stem cells. World J Stem Cells 2015;7:630-40. [Crossref] [PubMed]