
LncRNA XIST acts as a ceRNA sponging miR-185-5p to modulate pancreatic cancer cell proliferation via targeting CCND2
Introduction
Due to a high tendency for aggressive invasion and distant metastasis, the lethality rate of pancreatic cancer (PC) is the fourth highest among all cancers (1). It is reported that PC has a high incidence with >40,000 cases diagnosed each year and a very low 5-year survival rate estimated to be <5% (2,3). Despite the advancement of surgical technologies including chemotherapy and radiotherapy, the efficient remission of PC treatment has not been emerged. Therefore, finding novel biomarkers for identifying biological characteristics of PC is urgent to further explore.
Long non-coding RNAs (lncRNAs), the RNA without the function of protein coding, consist of more than 200 of nucleotide units (4). Emerging evidence has shown the crucial roles of lncRNAs in various bioprocesses and diseases, not only participate in epigenetic modification and transcription of normal cell, but also regulate the proliferation, differentiation and metastasis of cancer cells (5). It has been demonstrated that the dysregulation of lncRNAs can be potentially functioned as important regulators in the development and progression of PC (6). For example, lncRNA UCA1 was considered to impact the proliferation, invasion and migration of PC via regulating miR-96/FOXO3 (7). And knockdown of MEG3 enhanced the PC cell proliferation, migration and invasion, as well as induce epithelial-mesenchymal transition (EMT) (4).
X inactive-specific transcript (XIST), one of the lncRNA affecting the expression of gene, can mediate the transcription silence of X chromosome via a complex mechanism (8). Besides, XIST has been proved to be associated with development of multiple cancers including non-small cell lung cancer (NSCLC), gastric cancer, and hematoma (9-11). Furthermore, XIST was also found to be elevated and could enhance the proliferation PC cell via interacting with miR-40/miR-124/iASPP or miR-133a/EGFR (12,13). However, deeper insights for XIST in the development of PC still need to be further explored.
MicroRNAs (miRNAs), which bind to the 3’-untranslated region of mRNAs and regulate mRNA expression, are small non-coding RNAs (14). It has been demonstrated that miR-185-5p could act as a tumor-suppressive gene involved in mediating the formation of various tumor tissues, like hepatocellular carcinoma, colorectal cancer and NSCLC (15,16). Consistently, miR-185-5p was down-regulated in PC and could also inhibit the proliferation of PC cell via targeting transcriptional co-activator (15,17). Taken together, we hypothesized that miR-185-5p may also exert an anti-tumor function in PC.
We provided insights in this study into the promotive function of lncRNA XIST and the regulatory network of XIST, miR-185-5p, CCND2 in PC. Our findings suggested that lncRNA XIST could exert its oncogenic function as a ceRNA for miR-185-5p to modulate CCND2 expression, which might provide some new targets for PC treatment.
Methods
Tissue samples
PC specimens and adjacent normal tissues were collected from 70 patients who received surgery in the Second Xiangya Hospital of Central South University from 2012 to 2016. All tissues were immediately collected after resection and stored under −80 °C after washing with sterile phosphate-buffered saline (PBS). All patients were informed to write consent and all the experiments involved in this study were approved by the Ethics Committee of the Second Xiangya Hospital of Central South University (No. 201103301).
Cell culture and transfection
PC cell lines PANC-1, ASPC-1, HPAC, BxPC-3 cells and normal pancreatic cell line HPDE cells were all purchased from ATCC (Manassas, VA, USA). Briefly, cells were cultured in RPMI 1640 medium (Thermo Fisher Scientific, USA) containing 10% Gibco® fetal bovine serum (FBS) and 100 µg/mL penicillin-streptomycin (Sigma-Aldrich Co, USA) at 37 °C and 5% CO2. Lentivirus for shRNA for XIST (sh-XIST), negative control (NC) and overexpression plasmids for XIST (pcDNA3.1-XIST) were purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China). miR-185-5p mimics, miR-185-5p inhibitor and their negative controls were purchased from RiboBio (Guangzhou, China). After cultured to 70–80% confluence, cells were transfected with sh-XIST, pcDNA3.1-XIST, or miR-185-5p mimics, miR-185-5p negative control at a concentration of 50 nmol/L using Lipofectamine 3000 (Invitrogen, USA) according to the manufacturer’s instruction.
Flow cytometry assay
Flow cytometry was performed to assess the cell cycle and apoptosis. For the detection of cell cycle, briefly, cells were trypsinized and fixed with 70% absolute ethyl alcohol overnight at 4 °C. And then 100 µL RNase A Reagent (Keygen Biotech, Nanjing, China) was added into cells. Propidium iodide reagent (Keygen Biotech) was used to stain the cells at the concentration of 20 µg/mL for 20 minutes. Then DNA content was analyzed by FACScalibur (BD Bioscience, USA). For the detection of apoptosis, cells were harvested and then re-suspended with buffer. The FITC Annexin V Apoptosis Detection Kit I (Ruibo, Guangzhou, China) was applied for staining the cells. A FACScan flow cytometer was performed for analysis cells apoptosis. All the experiments were in triplicated.
Colony formation assay
Cell proliferation was detected using clonogenic formation assay. Briefly, cells were seeded into a 6-well plate at a density of 500 cells per well. The cells were fixed with 4% paraformaldehyde and stained by crystal violet (1 mg/mL) after 14 days of culture. Then the formed number of colonies in culture plate seeded with different cells was counted (>50 cells) under microscope.
Dual luciferase reporter assay
To verify the prediction, dual luciferase reporter assay was conducted as reported elsewhere (18). The predicted binding mode was obtained using bioinformatic prediction by software targetscan 5.1 (http://www.targetscan.org; Whitehead Institute for Biomedical Research, Cambridge, MA, USA). PCR was performed to amplify the 3’-end fragment of lncRNA XIST containing the predicted miR-185-5p-binding site and to subclone into a pmirGLOluciferase target expression vector (Promega, USA) as the XIST wild-type vector. And the mutated miR-185-5p-binding site was constructed as XIST Mutant vector. 293T cells were co-transfected with 200 ng of either pmirGLO-XIST-wide type (WT) or pmirGLO-XIST-mutant (MUT) vector and 80 ng of miR-185-5p mimics or inhibitor and their negative controls with Lipofectamine 3000 (Invitrogen). The verification of combination between miR-185-5p and CCND2 was realized via co-transfection with pmirGLO-CCND2-WT or pmirGLO-CCND2-MUT and miR-185-5p mimics or negative control. And the relative luciferase activity was measured using Dual-luciferase Reporter Assay Kit (Progema, USA) after 48 h of transfection.
Quantitative real time PCR (qRT-PCR)
The expression of XIST and miR-185-5p was determined using qRT-PCR. Briefly, total RNA was extracted using Trizol reagent (Takara, Otsu, Japan) according to the manufacturer’s instructions. And then, 1 µg RNA was converted into cDNA using Reverse Transcription Kit (Takara). SYBR Premix Ex Taq (Takara) was applied for qRT-PCR assays. The PCR reactions were conducted in an ABI 7500 Fast RealTime PCR System (Life Technologies).
Primers used in PCR were listed below: XIST: sense: 5’-AGCTCCTCGGACAGCTGTAA-3’, anti-sense: 5’-CTCCAGATAGCTGGCAACC-3’; miR-185-5p: sense: 5’-TGGAGAGAAAGGCAGTTCCTGA-3’, anti-sense: 5’-GCTTCGGCAGCACATATACTAAAAT-3’; U6: sense: 5’-CTCGCTTCGGCAGCACA-3’, anti-sense: 5’-AGATAGGATTACTACAC-3’ GAPDH: sense: 5’-CAAGGTCATCCATGACAACTTTG-3’, anti-sense: 5’-GTCCACCACCCTGTTGCTGTAG-3’. Relative RNA levels were calculated by the 2−ΔΔCq method. Relative expression was normalized to GAPDH and U6, respectively.
Western blot analysis
Briefly, total protein extracted from the cells was loaded on 10% SDS-PAGE and transferred to PVDF membranes (Millipore, USA). The membranes were probed with the specific primary antibodies (All purchased from Abcam, USA) as listed: CCND2 (ab207604, 1:1,000), CDC2 (ab32444, 1:1,000), CDC4 (ab12292, 1:500), CDC6 (ab109315, 1:1,000), CCND1 (ab134175, 1:10,000), Caspase-3 (ab13585, 1:500), Bcl-2 (ab32124, 1:1,000), Bax (ab32503, 1:1,000), GAPDH (ab8245, 1:500). After incubation with primary antibodies at 4 °C overnight, membranes were incubated with corresponding secondary horseradish peroxidase-conjugated anti-rabbit IgG antibody (ab6721, 1:5,000) at room temperature for 2 h. The target bands were then scanned using Super Signal West Pico Chemiluminescent Substrate Kit (Pierce, Rockford, IL, USA). GAPDH was served as an internal control.
Statistical analysis
SPSS 18.0 and Graph Pad Prism6.0 software were used for statistical analysis. All data was presented by mean ± Standard deviation (SD). Comparison between two groups was performed using the Student’s t-test. Comparison among three or more groups was conducted using one-way analysis of variance (ANOVA). The correlation between XIST and miR-185-5p was determined using Spearman’s analysis. It was considered to be statistically significant when P-value was less than 0.05.
Results
Negative correlation between lncRNA XIST and miR-185-5p in PC tissues and cell lines
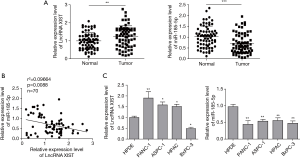
To determine the expression of XIST and miR-185-5p both in PC and normal tissues, firstly, we collected PC tissues and paired normal tissues from 70 patients and then the expression of XIST and miR-185-5p was measured using qRT-PCR. The results showed that lncRNA XIST was up-regulated in PC tissues, while miR-185-5p was down-regulated, compared with adjacent normal tissues (Figure 1A). Spearman’s analysis also displayed negative correlation between XIST and miR-185-5p (Figure 1B). Meanwhile, four PC cell lines PANC-1, ASPC-1, HPAC, BxPC-3 cells and normal pancreatic cell line HPDE cells were applied for further confirmation. Similarly, as shown in Figure 1C, increasing expression of XIST and decreasing of miR-185-5p were also displayed in PC cell lines. Among all PC cells, PANC-1 cells with the highest expression of XIST and BxPC-3 cells with the lowest expression of XIST were selected for further experiments.
Effects of XIST on cell proliferation, cell cycle and apoptosis in PC cells
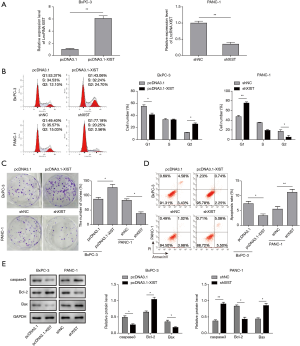
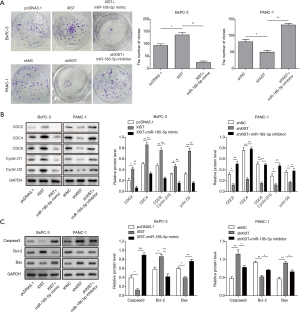
To further explore whether XIST acts as an oncogene in PC, we established PANC-1 cells in which XIST was stably knocked down with sh-XIST transfection and BxPC cells in which XIST was stably overexpressed with pcDNA3.1-XIST transfection (Figure 2A), indicating the successful establishment of lncRNA XIST knockdown or overexpression cell model. Then, we observed that cell cycle was arrested in G0/G1 phase with knocking down XIST using flow cytometry assay. Whereas, cell cycle of BxPC-3 cells transfected with pcDNA3.1-XIST suggested a opposite result (Figure 2B). In parallel, colony formation assay also indicated that knockdown of XIST significantly suppressed colony formation of PANC-1 cells, while overexpression of XIST promoted cell proliferation of BxPC-3 cells (Figure 2C). Moreover, flow cytometry assay showed a significant increase of cell apoptosis after knockdown of XIST in PANC-1 cells, while cell apoptosis showed a reduction with overexpression of XIST in BxPC-3 cells (Figure 2D). In addition, western blot analysis also indicated that overexpression of XIST induced the up-regulation of Bcl-2 and down-regulation of Caspase-3, Bax, while XIST knockdown showed the opposite tendency (Figure 2E). Taken together, these data indicated that lncRNA XIST might function as an oncogene to enhance the tumorigenesis of PC.
miR-185-5p is a direct target of XIST
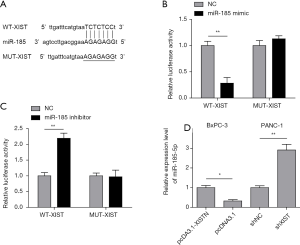
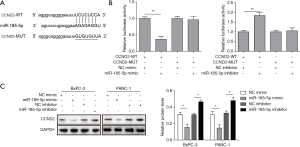
Recently, accumulating evidence has proved that lncRNAs may participate in the ceRNA regulatory network. Bioinformatic analysis suggested that there is the complementary base pairing between XIST and miR-185-5p (Figure 3A). To confirm the direct binding relationship between XIST and miR-185-5p, a luciferase reporter assay was performed. We found that miR-185-5p mimic markedly reduced the luciferase activities of pmirGLO-XIST-WT, while there was no obvious difference in cells transfected with miR-185-5p mimic and pmirGLO-XIST-MUT (Figure 3B). Consistently, the luciferase activities in cells transfected with miR-185-5p inhibitor and pmirGLO-XIST-WT or pmirGLO-XIST-MUT showed the same result (Figure 3C). Additionally, an inverse correlation of XIST and miR-185-5p in PC cells was also revealed. As shown in Figure 3D, up-regulation of XIST significantly suppressed miR-185-5p expression in BxPC-3 cells, while down-regulation of XIST markedly increased miR-185-5p expression. These findings implied that XIST directly targets and negatively regulates miR-185-5p.
Effects of miR-185-5p on cell proliferation, cell cycle and apoptosis in PC cells
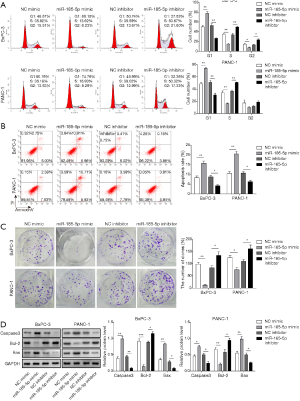
To further investigate the potential role of miR-185-5p in PC cells, miR-185-5p was overexpressed or knocked down in both BxPC-3 and PANC-1 cells. As shown in Figure 4A, in both BxPC-3 and PANC-1 cells, when miR-185-5p was overexpressed, cells in G0/G1 were significantly increased and cells in G2/M and S were significantly decreased, while cells transfected with miR-185-5p inhibitor showed opposite results. In addition, high expression of miR-185-5p suppressed cell proliferation and induced apoptosis using the colony formation and flow cytometry assays, respectively. On the contrary, inhibition of miR-185-5p presented the opposite results (Figure 4B,C). Besides, the expression of Bcl-2 was significantly increased within miR-185-5p inhibitor, but Caspase-3 and Bax expression were repressed. Similarly, miR-185-5p mimic displayed the same trend (Figure 4D). All these results indicated miR-185-5p acted as a tumor-suppressive mediator in the development of PC.
CCND2 is a direct target of miR-185-5p
To study the role of miR-185-5p in the mechanisms of PC, bioinformatics analysis was performed to predict the target of miR-185-5p and found that CCND2 may be related to miR-185-5p (Figure 5A). And then the luciferase reporter assay was subjected to validate the effect of miR-185-5p on CCND2 expression. The results showed the luciferase activity was significantly decreased when cells were co-transfected with miR-185-5p mimic and CCND2 wild-type vector, but not CCND2 mutant vector, while the luciferase activity was increased within miR-185-5p inhibitor co-transfected with CCND2 wild-type vector (Figure 5B). In addition, western blot analysis was performed to measure the expression of CCND2. And the results showed that transfection with miR-185-5p mimic decreased CCND2 protein expression, while transfection with miR-185-5p inhibitor increased CCND2 expression level (Figure 5C).
lncRNA XIST acts as a ceRNA sponging miR-185-5p to regulate CCND2 modulating cell proliferation and apoptosis
In order to explore whether XIST exerted its oncogenic role through miR-185-5p/CCND2 axis in PC, cells were transfected with pcDNA3.1-XIST and miR-185-5p mimic or sh-XIST and miR-185-5p inhibitor in combination. Results showed that in BxPC-3 cells, when transfected with pcDNA3.1-XIST, cell proliferation was significantly increased, while this effect was blocked with miR-185-5p mimic (Figure 6A). On the contrary, in PANC-1 cells transfected with sh-XIST, cell proliferation was significantly inhibited but the effect was significantly recovered with miR-185-5p inhibitor (Figure 6A). Besides, expression levels of CCND2 and cell cycle-related proteins CDC2, CDC4, CDC6, CCND1 were significantly up-regulated when XIST was overexpression in BxPC-3 cells and were markedly down-regulated when XIST was knocked down in PANC-1 cells. And these effects were significantly reversed by overexpression or inhibition of miR-185-5p, respectively (Figure 6B). Meanwhile, overexpression of XIST, decreased the expression of Caspase-3 and Bax, but induced Bcl-2 up-regulation, and when XIST was knocked down, the alteration trends were opposite. Altogether, all these effects were reversed by additional transfection of miR-185-5p mimic or inhibitor (Figure 6C). These results suggested that miR-185-5p could be involved in XIST-mediated biological functions of PC, indicating that XIST could modulate cell proliferation and apoptosis through miR-185-5p/CCND2 axis in the development of PC.
Discussion
Despite the development of treatment and diagnosis, the prognosis of PC is still very poor and thus deeper understanding for molecular mechanisms of PC development is urgent (19). Currently, the dysregulation of non-coding RNAs have been demonstrated in various cancers, including PC (20,21). For example, Huang et al. showed that up-regulation of lncRNA PVT1 in PC patients was closely associated with the patients’ poor prognosis (22). Gu et al. also found lncRNA MEG3 exerted its anti-cancer effects contributing to the suppression on PC development (23). It was also observed lncRNA HOTTIP could modulate cancer stem cell properties by regulating HOXA9 in PC (24). Additionally, lncRNA XIST was also reported to be involved in the progression of PC. A recent study demonstrated that XIST could regulate proliferation of PC cells through regulation of miR-133a/EGFR (13). However deeper insights for how XIST influence PC process are still unknown.
In the present study, we also found XIST was up-regulated in PC cells. Moreover, we found that XIST contributed to cell proliferation and inhibited cell apoptosis of PC cells, and the effects might be through inhibiting miR-185-5p. Roles for XIST in PC and other cancers as an oncogene have been reported in several researches. It has been proved that up-regulation of XIST was associated with the development of PC (12). Sun et al. showed XIST exerted oncogenic functions in PC by miR-34a-5p (18). Yao et al. indicated that the inhibition of XIST showed tumor-suppressive functions in human glioblastoma stem cells by up-regulating miR-152 (25). On the contrary, several studies also revealed the anti-tumor function of XIST in the cancer development. It was found that loss of XIST could promote brain metastasis in breast cancer (26). Huang et al. also found knockdown of XIST resulted in enhanced cell viability (27). Thus, these findings suggested that XIST might demonstrate different roles in different cancers.
miR-185-5p has been proven as a tumor suppressor in several cancers. It was found in prostatic cancer tissue and cell lines, miR-185-5p was down-regulated (28). And miR-185-5p could also inhibit the epithelial mesenchymal transition of breast cancer cells (29). LncRNAs such as H19 and PDIA3P were also reported to regulate miR-185-5p in cancer development (30,31). Moreover, a recent study demonstrated XIST could promote progression of gastric cancer by targeting miR-185 (32). In the present study, we verified that miR-185-5p was also a downstream target of XIST in PC. Meanwhile, miR-185-5p was closely associated with XIST-mediated biological functions in PC cells. Our results showed that XIST exerts its oncogenic functions on cell proliferation, cell cycle and apoptosis, thus contributing to PC progression, while these effects were significantly reversed by miR-185-5p. Our finding indicated that miR-185-5p might be a tumor suppressor in the development of PC.
Role of CCND2 in cancer development has been noticed in many studies. Evron et al. demonstrated CCND2 expression was down-regulated in the majority of breast cancers (33). Zhang et al. showed miR-206 could inhibit gastric cancer proliferation by repressing CCND2 (34). Besides, several studies also showed the relationship of miR-185-5p and CCND2. Sun et al. found lncRNA PDIA3P could interact with miR-185-5p by targeting CCND2 in oral squamous cell carcinoma (35). Bibaki et al. showed miR-185 could target on CCND2 in lung cancer (36). In the present study, we also found miR-185-5p could inhibit proliferation and migration of PC cells by down-regulation of CCND2. Besides, we confirmed that CCND2 was a downstream target of miR-185-5p, which could directly target CCND2 in PC cells, and the effects were regulated by XIST. The present study also has some limitations. First CCND2 is not the only downstream target of XIST in cancer development, thus the XIST/miR-185-5p/CCN2 axis is also one of the regulation ways for PC. Other signaling pathways and deeper insights are still needed.
In conclusion, our study provided the first evidence that XIST could promote cell proliferation, inhibit cell apoptosis through modulating miR-185-5p/CCND2 axis. This study might give deeper understanding for role of XIST and miR-185-5p in development of PC, and might provide some new targets for PC treatment. However, our study is limited in vitro experiments and the certain molecular mechanisms of XIST in PC need to be further verified in vivo.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.01.26). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients were informed to write consent and all the experiments involved in this study were approved by the Ethics Committee of the Second Xiangya Hospital of Central South University (No. 201103301).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang Q, Zeng L, Chen Y, et al. Pancreatic Cancer Epidemiology, Detection, and Management. Gastroenterol Res Practice 2016;2016:1-10.
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Kaistha BP, Honstein T, Müller V, et al. Key role of dual specificity kinase TTK in proliferation and survival of pancreatic cancer cells. Br J Cancer 2014;111:1780. [Crossref] [PubMed]
- Ma L, Wang F, Du C, et al. Long non-coding RNA MEG3 functions as a tumour suppressor and has prognostic predictive value in human pancreatic cancer. Oncol Rep 2018;39:1132. [PubMed]
- Tang Y, He Y, Zhang P, et al. LncRNAs regulate the cytoskeleton and related Rho/ROCK signaling in cancer metastasis. Mol Cancer 2018;17:77. [Crossref] [PubMed]
- Duguang L, Jin H, Xiaowei Q, et al. The involvement of lncRNAs in the development and progression of pancreatic cancer. Cancer Biol Ther 2017;18:927-36. [Crossref] [PubMed]
- Zhou Y, Chen Y, Ding W, et al. LncRNA UCA1 impacts cell proliferation, invasion, and migration of pancreatic cancer through regulating miR-96/FOXO3. Iubmb Life 2018;70:276-90. [Crossref] [PubMed]
- McHugh CA, Chen CK, Chow A, et al. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature 2015;521:232. [Crossref] [PubMed]
- Fang J, Sun CC, Gong C. Long noncoding RNA XIST acts as an oncogene in non-small cell lung cancer by epigenetically repressing KLF2 expression. Biochem Biophys Res Commun 2016;478:811-7. [Crossref] [PubMed]
- Chen DL, Ju HQ, Lu YX, et al. Long non-coding RNA XIST regulates gastric cancer progression by acting as a molecular sponge of miR-101 to modulate EZH2 expression. J Exp Clin Cancer Res Cr 2016;35:142. [Crossref] [PubMed]
- Yildirim E, Kirby JE, Brown DE, et al. Xist RNA Is a Potent Suppressor of Hematologic Cancer in Mice. Cell 2013;152:727-42. [Crossref] [PubMed]
- Liang S, Gong X, Zhang G, et al. The lncRNA XIST interacts with miR-140/miR-124/iASPP axis to promote pancreatic carcinoma growth. Oncotarget 2017;8:113701. [Crossref] [PubMed]
- Wei W, Liu Y, Lu Y, et al. LncRNA XIST Promotes Pancreatic Cancer Proliferation through miR-133a/EGFR. J Cellul Biochem 2017;118.
- Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Ann Rev Med 2009;60:167-79. [Crossref] [PubMed]
- Imam JS, Buddavarapu K, Leechang JS, et al. MicroRNA-185 suppresses tumor growth and progression by targeting the Six1 oncogene in human cancers. Oncogene 2010;29:4971-9. [Crossref] [PubMed]
- Zhang Z, Liu X, Feng B, et al. STIM1, a direct target of microRNA-185, promotes tumor metastasis and is associated with poor prognosis in colorectal cancer. Oncogene 2015;34:4808-20. [Crossref] [PubMed]
- Xia D, Li X, Niu Q, et al. MicroRNA-185 suppresses pancreatic cell proliferation by targeting transcriptional coactivator with PDZ-binding motif in pancreatic cancer. Exp Ther Med 2018;15:657. [PubMed]
- Sun Z, Zhang B, Cui T. Long non-coding RNA XIST exerts oncogenic functions in pancreatic cancer via miR-34a-5p. Oncol Rep 2018;39:1591-600. [PubMed]
- Hidalgo M. Pancreatic cancer. N Engl J Med 2010;362:1605-17. [Crossref] [PubMed]
- Yoshida K, Toden S, Ravindranathan P, et al. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis 2017;38:1036-46. [Crossref] [PubMed]
- Previdi MC, Carotenuto P, Zito D, et al. Noncoding RNAs as novel biomarkers in pancreatic cancer: what do we know? Future Oncol 2017;13:443-53. [Crossref] [PubMed]
- Huang C, Yu W, Wang Q, et al. Increased expression of the lncRNA PVT1 is associated with poor prognosis in pancreatic cancer patients. Minerva Medica 2015;106:143. [PubMed]
- Gu L, Zhang J, Shi M, et al. lncRNA MEG3 had anti-cancer effects to suppress pancreatic cancer activity. Biomed Pharmacother 2017;89:1269. [Crossref] [PubMed]
- Fu Z, Chen C, Zhou Q, et al. LncRNA HOTTIP modulates cancer stem cell properties in human pancreatic cancer by regulating HOXA9. Cancer Lett 2017;410:68. [Crossref] [PubMed]
- Yao Y, Ma J, Xue Y, et al. Knockdown of long non-coding RNA XIST exerts tumor-suppressive functions in human glioblastoma stem cells by up-regulating miR-152. Cancer Lett 2015;359:75-86. [Crossref] [PubMed]
- Xing F, Liu Y, Wu SY, et al. Loss of XIST in Breast Cancer Activates MSN-c-Met and Reprograms Microglia via Exosomal miRNA to Promote Brain Metastasis. Cancer Res 2018;78:4316-30. [Crossref] [PubMed]
- Huang YS, Chang CC, Lee SS, et al. Xist reduction in breast cancer upregulates AKT phosphorylation via HDAC3-mediated repression of PHLPP1 expression. Oncotarget 2016;7:43256-66. [PubMed]
- Ostadrahimi S, Fayaz S, Parvizhamidi M, et al. Downregulation of miR-1266-5P, miR-185-5P and miR-30c-2 in prostatic cancer tissue and cell lines. Oncol Lett 2018;15:8157-64. [PubMed]
- Yin C, Yin C, Zhang G, et al. miR-185-5p inhibits F-actin polymerization and reverses epithelial mesenchymal transition of human breast cancer cells by modulating RAGE. Mol Med Rep 2018;18:2621-30. [PubMed]
- Sun CC, Zhang L, Li G, et al. The lncRNA PDIA3P Interacts with miR-185-5p to Modulate Oral Squamous Cell Carcinoma Progression by Targeting Cyclin D2. Mol Ther Nucleic Acids 2017;9:100-10. [Crossref] [PubMed]
- Wu T, Qu L, He G, et al. Regulation of laryngeal squamous cell cancer progression by the lncRNA H19/miR-148a-3p/DNMT1 axis. Oncotarget 2016;7:11553-66. [PubMed]
- Zhang Q, Chen B, Liu P, et al. XIST promotes gastric cancer (GC) progression through TGF-β1 via targeting miR-185. J Cell Biochem 2018;119:2787-96. [Crossref] [PubMed]
- Evron E, Umbricht CB, Korz D, et al. Loss of cyclin D2 expression in the majority of breast cancers is associated with promoter hypermethylation. Cancer Res 2001;61:2782-7. [PubMed]
- Zhang L, Liu X, Jin H, et al. miR-206 inhibits gastric cancer proliferation in part by repressing cyclinD2. Cancer Lett 2013;332:94-101. [Crossref] [PubMed]
- Sun CC, Zhang L, Li G, et al. The lncRNA PDIA3P Interacts with miR-185-5p to Modulate Oral Squamous Cell Carcinoma Progression by Targeting Cyclin D2. Mol The Nucleic Acids 2017;9:100. [Crossref] [PubMed]
- Bibaki E, Tsitoura E, Koutoulaki C, et al. Expression of miR-185 targets DNA methyltransferase 1 and Cyclin D2 in IPF and lung cancer in BALF cells: Preliminary results. Eur Respir J 2015;46:PA3039.


