FoxP3 promotes lymph node metastasis in patients with papillary thyroid carcinoma complicated with Hashimoto’s thyroiditis
Introduction
In the past 10 years, the important role of inflammation in the process of tumorigenesis and its potential mechanism have become hotspots in oncology research. At the beginning of the 19th century, Japanese surgeon Hashimoto described thyroid pathology with fibrosis and diffuse lymphocytic infiltration firstly, and named Hashimoto’s thyroiditis (HT) (1). Dailey et al. (2) firstly proposed in 1955 that papillary thyroid carcinoma (PTC) was evolved from HT, and many subsequent reports confirmed this view.
Immune cells play an important role in the intricate tumor microenvironment, CD4+CD25+ Regulatory T cells (Tregs) are an important subset of cells in helper T cells. It has specific immune control functions, regulates the function of autoreactive T cells efficiently, and plays a role in preventing the occurrence of autoimmune diseases and maintaining immune stability. Forkhead/winged helix transcription factor (FoxP3) is also regarded as an important molecule and key marker for Treg cell development and function (3). CD4+CD25+ Treg cells are elevated in local tumor tissues and peripheral blood of various tumor patients, and there is a negative correlation between the number of cells and the progression and prognosis of the tumor (4).
We found that PTC in Yunnan Plateau was closely related to HT in our previous case retrospective study (5,6). HT is characterized by massive lymphocytes infiltrating thyroid tissue. There is also a large amount of inflammatory lymphocyte infiltration in the tumor microenvironment of PTC patients complicated with HT, but their type and function are still unclear. This study intended to find clues from thyroid paraffin-embedded tissues of PTC patients complicated with HT after surgery. We established a Transwell model that simulated the co-action environment of umbilical cord blood initial T lymphocytes and thyroid papillary carcinoma cells. FoxP3 transcription factor was used as a marker to explore the distribution and quantity of Treg cells in PTC with HT thyroid tumor tissues, and elucidate the changes and significance of Treg cells in the evolution of HT-PTC.
Methods
Thyroid tissue, paraffin tissue and serum specimens of patients
Fresh tissue specimens from patients undergoing thyroid tumor resection were collected in the Department of Breast and Thyroid Tumor Surgery, Yunnan First People’s Hospital from June 2013 to June 2014. All of them had no family history of cancer, and did not receive radiotherapy or chemotherapy when collecting specimens. We excluded patients who had recently taken drugs (anti-thyroid drugs, thyroxine, iodine-containing drugs, glucocorticoids and sex hormones) and radioactive iodine treatments that might affect thyroid function. The patient’s informed consent was obtained before the sample was collected. This study was approved by the Ethics Committee of the First People’s Hospital of Yunnan Province. All patients were divided into three groups: papillary thyroid carcinoma (PTC) group, papillary thyroid carcinoma with Hashimoto’s thyroiditis (PTC-HT) group and Hashimoto’s thyroiditis (HT) group. Paraffin-embedded tissues were collected in the pathology department after PTC, PTC-HT and HT were confirmed after operation.
Neonatal umbilical cord blood
Neonatal umbilical cord blood were collected from the obstetrics operating room, pregnant women were 22–30 years old, all of them received caesarean sections and had no infectious diseases or blood diseases. Healthy neonatal heparinized cord blood samples were collected from the obstetrics department of the First People’s Hospital of Yunnan Province from March to August 2014. The mother’s inclusion criteria included normal pregnancy; HIV, hepatitis B virus and hepatitis C virus tests were negative. Exclusion criteria: donors who experience pre-eclampsia, fever, acute infection, diabetes and/or chronic disease. Written consent was got from the mother. The study was approved by the Ethics Committee of the First People’s Hospital of Yunnan Province.
Cell lines
Two thyroid papillary carcinoma cell lines TPC-1 (7) (human highly differentiated thyroid papillary carcinoma) and K1 (human highly differentiated metastatic thyroid papillary carcinoma) (8) were selected as the experimental group. The human thyroid follicular epithelial cell line Nthy-ori 3-1 (9) was regarded as a control group which purchased from Guangzhou Ginuo Biotechnology Co., Ltd.
Immunohistochemical staining
Envision immunohistochemical method
The detection kit was Dako product; FoxP3 Mouse anti-Human Monoclonal antibody was purchased from Abcam Company (ab20034). The staining steps included routine dewaxing and antigen repair, and incubation overnight at 4 °C with anti-I (1:250 dilution). Anti-II, DAB, Mayer hematoxylin and neutral gum seals were added the next day. In order to demonstrated the specificity of the immunohistochemical detection of FoxP3 protein antibody, murine IgG was used instead of IgI as a control group in the experiment, and the result was negative. At the same time, the positive staining sections of FoxP3 protein were selected, and the result of PBS instead of IgI staining was negative, which served as a reliable experimental basis for the results.
Evaluation of immunohistochemical results
Positive criteria: The color of nuclei of FoxP3 positive cells was turn into brown granules from brown-yellow. Immunohistochemical staining sections were photographed using an upright microscope (Leica, DM500, ICC50), and the whole staining was observed under low magnification (10×). The lymphocyte-intensive areas were selected and 10 images were randomly selected. Five of the most representative visual fields with clear background were selected from 10 images of each slice. Image Pro-Plus 6.0 image analysis software was used to record macro and count positive expression cells (individual/HPF) (10), The area which we obtained from JPEG images was 0.87 mm2. These spots were also captured in digital images of each group of FoxP3 stained sections. Average values of 5 groups data were obtained for each case. Then the number of FoxP3 positive cancer cells was manually excluded and the corrected number of FoxP3 positive cells was expressed as the number of positive cells per field of vision. Subsequently, the average of each group was taken as the criterion and divided into low expression group (< average) and high expression group (> average).
Transwell cell chamber co-culture
The umbilical cord blood came from the caesarean section parturients in the obstetrics department. The average age of the parturients was 25.6±3.1 years old. The disposable aseptic plastic blood collection bag was used. After the newborn was delivered, the umbilical cord was double-ligated and cut at 1/3 of the navel. The umbilical cord was disinfected for umbilical vein puncture, collecting the cord blood and the blood collection bag was sealing immediately after the blood collection. The whole process was strictly aseptic operation.
Isolation of umbilical cord blood mononuclear cells
Diluted and mix the umbilical cord blood with the 0.9% physiological saline in equal volume, and then added it to the Ficoll separation solution (the density of the separation solution is 1.079 g/mL) according to the ratio of 1:1, and slowly put it in to maintain the interface clearly. After centrifugation at 2,000 rpm/min for 30 minutes, the liquid was divided into 4 layers after centrifugation: the red blood cell layer, the Ficoll layer, the mononuclear cell layer, and the plasma layer were sequentially from bottom to top. Absorbed the layer of mononuclear cells slowly which appear as white clouds or clusters on the interface, adding PBS solution, centrifuging for 5 minutes with 2,000 rpm/min, and repeated operation twice. The number of mononuclear cells was counted, then the number of active and dead cells was counted by trypan blue staining, and then the whole mononuclear cells were inoculated in 10% FBS culture medium.
Cell culture of thyroid cancer cell line and normal follicular epithelial cell line
Removed the cryotube containing K1, TPC-1, Nthy ori 3-1 cells from the liquid nitrogen tank, immediately put it in a 37 °C constant temperature water bath pan and shake it to melt quickly. The frozen tube was removed and disinfected with 75% alcohol, and then the cell suspension was aspirated by a pipette and transferred to a tube, centrifuged at 1,500 rpm for 5 minutes, and the supernatant was discarded. The cells were then resuspended in fresh complete medium and inoculated into a culture flask and cultured in a cell culture incubator containing 5% CO2 and a relative humidity of 90% at 37 °C. The complete medium consisted of 85% RPMI1640 basic medium, 10% inactivated fetal bovine serum and double antibodies. When the degree of cell fusion reached 90%, the supernatant was discarded, fresh medium was added, the cells were blown with a pipette and the cells were blown as much as possible, and divided evenly into two flasks.
Cell co-culture
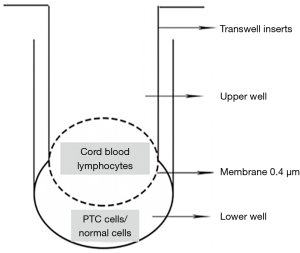
Two human thyroid papillary carcinoma cell lines TPC-1 and K1 and human normal thyroid follicular epithelial cell line Nthy-ori 3-1 were obtained. The cell concentration was regulated to 1×105/mL. Pipetted 4 mL into a 6-well plate and waited for it to adhere to the wall. Co-cultured of umbilical cord blood lymphocytes and tumor cells in a ratio of 3:1. Pipetting 2 mL of the cord blood initial T lymphocyte suspension with a concentration of 6×105/mL in the chamber, and no air bubbles should be generated. Incubated at 37 °C, 5% CO 2 in an incubator for 24, 36, 48, and 60 h (Figure 1). Initial T lymphocytes which were cultured individually from cord blood were added as control group. The extracted Treg cells labeled with flow cytometric antibodies were analyzed by flow cytometry.
The ratio of Treg cell analyzed by flow cytometry
The cells cultured at 37 °C in a constant temperature incubator for 0, 24, 36, 48, and 60 h were collected and stained on the cell surface and inside the cells. CD45+CD4+CD25+CD127− labeled Treg cells were analyzed.
- Cell surface staining: The cultured cells stimulated by 2,000 rpm ×5 min were collected, centrifuged and the supernatant was discarded, washed with PBS once, and then centrifuge at 2,000 rpm × 5 min to discard the supernatant, added with ECD-anti-CD45 (15 µL), FITC-anti-CD4− (15 µL), PC5-anti-CD25 (15 µL), PE-anti-CD127 (15 µL), and incubated for 30 min at room temperature in the dark.
- Polyformaldehyde-PBS resuspension cells with a concentration of 1% after 400 µL precooling were added and stored at 4 °C for reserved.
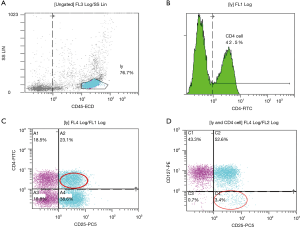
- The percentage of CD45+CD4+CD25+CD127-expression in cord blood was analyzed by flow cytometry: location of lymphocyte population by CD45+ and side scatter angle (SSC) (Figure 2A). CD4+ was used to set the gate (Figure 2B), then CD4+CD25+ was set as a double gate in the CD4+ cell population (Figure 2C), and the percentage of CD4+CD25+CD127−/CD4+ was analyzed finally (Figure 2D).
Detection of FoxP3 protein expression in co-cultured T lymphocytes by Western blot
The total protein of the cells was extracted by grouping, the cells were collected and washed, and the cells were lysed by RIPA, centrifuged at 4 °C, 14,000 r/min for 15 min. The supernatant was taken and stored at −80 °C for use. Bradford protein quantitative kit was used to determine protein concentration. SDS-PAGE electrophoresis was carried out at 50 µg per porin sample volume, and the voltage-stabilized ice bath electrophoresis was transferred to cellulose nitrate membrane. 5% skim milk powder was blocked at room temperature for 1 h, primary antibody was incubated at 4 °C overnight (FoxP3 1:100, β-actin 1:1,000), rinsed, and secondary antibody (1:5,000) was incubated at 37 °C for 2 h. The membrane was transferred to a medical X-ray developing box and fixed and developed in a dark room to obtain a target protein band. The ratio of the target protein to the OD value of β-actin represents the relative expression level of the protein. The same amount of chemiluminescent substrates A and B were mixed evenly and then immersed in PVDF membrane for incubation for 3 min.
Statistical analysis
The original data was formed into an Excel table to establish a database. SPSS17.0 software was used for statistical analysis. The measurement data was represented by . T T-test was used to compare the mean of the two groups. The three groups were compared by one-way ANOVA. S-N-K test was used to compare the two groups. Rate was used fot representing enumeration data, compare it with χ2 test. Spearman was used to analyze the correlation between the factors. The test level was α=0.05. Gray scale analysis of the protein target bands was performed using Image J software (Version 1.38).
Results
Relationship between expression of FoxP3 and pathological features in tumor microenvironment of PTC patients complicated with HT
In order to observe the distribution characteristics of Treg in PTC/HT microenvironment, we used immunohistochemistry to observe the specific expression of FoxP3 in PTC/HT tumor tissues. No positive expression of FoxP3 and RORγt was found in nodular goiter tissue (Figure 3A). FoxP3 is mainly expressed in the nucleus of lymphocytes infiltrated in tumor tissues, and the nuclei of positive cells might show brown or even brown. The number of FoxP3+ lymphocyte infiltration in tumor tissues of 46 patients with PTC/HT ranged from 0 to 130/HPF, the average was 74/HPF (Figure 3), 24 in the low expression group and 22 in the high expression group. The expression of FoxP3 in the PTC/HT tumor microenvironment is associated with lymph node metastasis. The proportion of lymph node metastasis in the FoxP3 high expression group was significantly higher than that in the low expression group (68.2% vs. 37.5%, P=0.037). The expression level of FoxP3 in tumor microenvironment of PTC/HT patients was not related to age and gender (P>0.05) (Table 1).
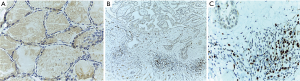
Table 1
| Clinical characteristics | Low expression (%), n=24 | High expression (%), n=22 | P |
|---|---|---|---|
| Age | |||
| <45 years | 11 (45.8) | 14 (63.6) | 0.226 |
| ≥45 years | 13 (54.2) | 8 (36.4) | |
| Gender | |||
| Male | 6 (25.0) | 4 (18.2) | 0.575 |
| Female | 18 (75.0) | 18 (81.8) | |
| Lymph node metastasis | |||
| Negative | 15 (62.5) | 7 (31.8) | 0.037* |
| Positive | 9 (37.5) | 15 (68.2) | |
| Tumor diameter (cm) | 1.52±0.90 | 1.92±0.71 | 0.10 |
*P<0.05.
Expression of Treg cells after co-acting with umbilical cord blood initial T lymphocytes and TPC-1
The proportion of Treg cells in umbilical cord blood initial T lymphocytes was 4.23%±1.61%. After the interaction between thyroid papillary carcinoma cell line TPC-1 and umbilical cord blood initial T lymphocytes, the initial CD4+ T lymphocytes coulg be induced differentiate into CD4+CD25+CD127− Treg cells, the proportion of Treg cells increases over time. After they worked together for 36 hours, the proportion of Treg cells reached to 19.83%±1.41%, which was significantly higher than the proportion of 24, 48 and 60 h, the difference was statistically significant (P<0.001). After co-acting with the thyroid follicular epithelial cell line Nthy-ori 3-1 and the umbilical cord blood initial T lymphocytes in the control group, they could also differentiate into Treg cells. The proportion of Treg cells also rose over time, the proportion of Treg cells reached to 15.67%±2.06% at 36 h, which was lower than the co-acting with TPC-1, the difference was statistically significant (P<0.001) (Table 2, Figure 4, Figure 5).
Table 2
| Groups | 0 h | 24 h | 36 h | 48 h | 60 h | F | P |
|---|---|---|---|---|---|---|---|
| With Nthy ori 3-1 | 4.23±1.61 | 11.13±1.92 | 15.67±2.06 | 10.83±1.31 | 5.80±1.23 | 22.92 | <0.001 |
| With TPC-1 | 4.94±2.54 | 6.37±1.11 | 19.83±1.41* | 13.50±1.91 | 12.47±1.81 | 32.52 | <0.001 |
| With K1 | 4.83±1.51 | 12.56±1.71* | 30.97±3.01* | 16.80±2.19 | 17.97±1.16* | 66.89 | <0.001 |
| F | 0.12 | 12.10 | 36.95 | 7.92 | 54.35 | ||
| P | 0.893 | 0.008 | <0.001 | 0.021 | <0.001 |
24 h: with K1 vs. with TPC-1, P=0.006; 36 h: with K1 vs. with TPC-1, P=0.004; 60 h: with K1 vs. with Nthy ori 3-1, P=0.0002; 60 h: with TPC-1 vs. with Nthy ori 3-1, P=0.006; 60 h: with TPC-1 vs. with K1, P=0.015.
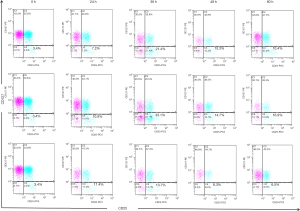

Expression of Treg cells after co-acting with umbilical cord blood initial T lymphocytes and K1
Co-acting with the thyroid papillary carcinoma cell line K1 and umbilical cord blood initial T lymphocytes can induce initial CD4+ T lymphocytes differentiate into CD4+CD25+CD127− Treg cells. The proportion of Treg cells increased over time, and after they worked together for 36 hours, the proportion of Treg cells reached to 33.63%±0.51%, which was significantly higher than the proportion of 24, 48 and 60 h, the difference was statistically significant (P<0.001). The proportion of Treg cells after co-acting 36 h with K1 and the initial T lymphocytes was higher than the control group, and also higher than the co-acting with TPC-1, the difference was statistically significant (P<0.001) (Table 2, Figure 4, Figure 5).
Expression of FoxP3 protein in lymphocytes after co-culture umbilical cord blood lymphocytes with PTC cell lines by Semi-quantitative Western blot
We examined the expression of FoxP3 protein in lymphocytes after co-culture of umbilical cord blood lymphocytes and PTC cell lines by Western blotting, which was also confirmed by flow cytometry. The results indicated that after co-culture with the thyroid papillary carcinoma cell line TPC-1 and K1, umbilical cord blood initial lymphocytes expressed low levels of FoxP3 protein immediately, the expression level would gradually increase and fluctuate, and by 36 h, the proportion of FoxP3 would be the highest. The proportion of FoxP3 protein induced by the K1 cell line with lymph node metastasis was higher than that of the TPC-1 cell line without lymph node metastasis. Normal thyroid follicular epithelial cells could also induce cord blood T cells to express FoxP3 protein, but the expression level was stable and there was no significant fluctuation (Figure 6).

Gray value of each target protein band could be obtained by Image J gray scale scanning, the relative expression levels of FoxP3 protein could be calculated by the gray value of the respective housekeeping gene bands. Calculation formula: relative expression level of the target protein = (gray value of target protein − gray value of background) / (gray value of β-actin − gray value of background). T-lymphocyte at different time points of co-culture could be used as abscissa, and the relative expression level of target protein could be used as ordinate to draw a curve, the lowest expression level of FoxP3 protein in T lymphocyte was 1 at 0 h, and the relative expression level of FoxP3 protein in T lymphocyte was obtained at each time point of co-culture (Figure 6).
The ratio of Th17/Treg cells after co-acting with umbilical cord blood initial T lymphocytes and thyroid papillary carcinoma cell lines for 36 hours
The ratio of Th17 cells after co-acting with umbilical cord blood initial T lymphocytes and thyroid papillary carcinoma cell lines (TPC-1 and K1) was detected by flow cytometry, which could be seen in the published research of our group (6), The proportion of Th17 cells after co-culture for 36 h with two cell lines was the highest. So we calculated the ratio of Th17/Treg cells at 36 h time to evaluate the balance between the both. The ratio of Th17/Treg cells in the initial T lymphocytes induced by normal thyroid follicular epithelium after co-acting for 36 h was found to be <1 (0.68±0.03). The Th17/Treg balance was clearly shifted toward the Treg direction. After 36 h of co-culture, the ratio of Th17/Treg cells in the initial T lymphocytes induced by TPC-1 or K1 was higher than the normal control group (Nthy-ori 3-1) (1.21±0.06 vs. 0.68±0.03; 1.40±0.10 vs. 0.68±0.03), the difference was statistically significant (P<0.001). The ratio of Th17/Treg cells in the initial T lymphocytes induced by cancer cell lines was >1, The Th17/Treg balance was clearly shifted toward the Th17 direction. K1 was a cancer cell line with lymph node metastasis. The ratio of Th17/Treg cells induced by K1 in the initial T lymphocytes was also higher than that of the TPC-1 cancer cell line without lymph node metastasis (1.21±0.06 vs. 1.40±0.10), the difference was statistically significant by LSD test (P<0.05) (Table 3).
Table 3
| Groups | With Nthy ori 3-1 | With TPC-1 | With K1 | F | P |
|---|---|---|---|---|---|
| Treg (%) | 15.67±2.06 | 19.83±1.41 | 30.97±3.01 | 36.95 | <0.001 |
| Th17 (%) | 10.70±1.45 | 24.00±1.68 | 43.30±2.86 | 184.46 | <0.001 |
| Th17/Treg | 0.68±0.03 | 1.21±0.06 | 1.40±0.10 | 99.76 | <0.001 |
Discussion
Pathologists have known far past that there are innate and acquired infiltration of immune cells around tumors, i.e., tumor infiltrating lymphocytes (TIL), which can reflect inflammation in non-neoplastic tissues (11,12) and play the role of immune system in eradicating cancer cells (12). TIL represents the host immune response and is directly related to the microinvasive state and has been widely reported to play an important role in the tumor microenvironment (13). In TIL, CD4+ T helper T lymphocytes are widely recognized as important participants in tumor immunity and have important effect for tumor progression (14). They depend on the action of different cytokines and transcription factors and can differentiate into four immunotype subtypes (Th17, Treg, Th1, Th2) (15), playing an important role in the immune response. The phenotypic and functional results of TIL are complex. Some TILs are expressed as effector cells, which are beneficial to host anti-tumor immunity. Others are shown to inhibit tumor immune response, and they have different phenotypes and effects in different tumor microenvironments. Therefore, the prognostic significance of TIL may be dynamic. Tumor development and prognosis may be determined by the intricate interactions between immune cells and tumor cells, which can reflect the efforts of the immune system to eradicate tumor cells. Research on immune response to papillary thyroid carcinoma has been around for a long time (16,17). The relationship between HT and PTC has also been reported repeatedly, and TIL infiltration is also found in and around PTC tumor tissues (17,18). All of these support the hypothesis that an immune response may influence the progression of PTC. Previous studies have also shown that local inflammatory response and concurrent HT can predict a more favorable prognosis in patients with PTC (19,20). But the actual impact of the immune system, the existence of autoimmune thyroid disease and its relationship with the development of cancer is still controversial (18,21). We investigated the correlation of T lymphocyte subset infiltration in PTC with or without HT tumor microenvironment, and explored the role of Treg-assisted CD4+ T lymphocytes in the evolution of HT-PTC.
Regulatory T cells (Treg cells), also known as CD4+CD25+FoxP3+ T lymphocytes, destroyed anti-tumor immunity by inhibiting the function of various immune cells, and constantly weakened the surveillance of tumor immunity (22). Treg has been shown to be involved in down-regulating immune responses in autoimmune, transplant rejection and tumor immunity (23,24). The Forkhead box P3 was a key marker for CD4+CD25+ regulatory T cells (Tregs) (25). High levels of Tregs were firstly reported in peripheral blood of various types of cancer patients (26). The proportion of Tregs in various tumor tissues and metastatic lymph nodes was elevated subsequently (27). The study found that FoxP3+ Treg cells were associated with poor prognosis in many human malignancies (e.g., colon, ovarian, gastric, thyroid, and melanoma) (18,26,28,29). There have been reported that increase of the number of FoxP3+ Tregs was associated with late stage of TNM and PTC lymph node metastasis (18,30). Liu et al. (31) have showed that the percentage of CD4+CD25+CD127− Treg cells in peripheral blood CD4+ T cells of PTC patients was significantly higher than that of patients with nodular goiter (MNG). There is a large amount of FoxP3+ Treg infiltrated around the tumor in PTC tumor and metastatic lymph node tissues, while there is no FoxP3 expression in MNG tissues. A higher percentage of Treg cells in peripheral blood and tumor tissues is associated with extrathyroid expansion and lymph node metastasis. Studies in children have also shown that FoxP3 has a statistically significant high expression in thyroid cancer compared to thyroid tumors, which may indirectly prove the role of FoxP3 in the process of carcinogenesis (32). Cunha et al. (33) found that FoxP3+ lymphocytes were more common in PTC tumors smaller than 2 cm by tissue immunohistochemistry. Patients without extrathyroid invasion and patients with chronic lymphocytic thyroiditis were more common. The expression of FoxP3 in differentiated thyroid carcinoma (DTC) cells was confirmed and found to be important for tumor invasiveness. As early as 2012, there were studies (34) showing that immune cells are more susceptible to infiltration into malignant tumors than benign lesion. Our study also confirmed that there was no distribution of FoxP3+ Tregs in benign thyroid tumors, and a large number of such cells were observed around PTC tumor tissues. The simultaneous presence of CD68+, CD4+, CD8+, CD20+, FoxP3+ and Th17 lymphocytes in the tumor microenvironment of patients with DTC complicated with chronic lymphocytic thyroiditis (CLT) was associated with low-invasiveness of the tumor and more favorable prognosis and pathological features. The role of FoxP3+ Tregs in various human cancers remains controversial. In this study, we found that the proportion of lymph node metastasis in the high expression of FoxP3 in the tumor microenvironment of PTC patients with HT was significantly higher than that in patients with low expression. The difference was statistically significant. It is suggested that high expression of FoxP3 in PTC/HT patients may promote lymph node metastasis. Cunha et al. (33) reported that the nucleus FoxP3 staining of differentiated thyroid cancer cells was stronger in young patients and lymph node metastasis, indicating that FoxP3 expression might be associated with aggressive thyroid cancer aggressiveness, which was similar in our study.
A large number of Treg cells have been observed to infiltrate the PTC/HT tumor microenvironment in the aforementioned immunohistochemical study of PTC combined with HT tumor tissue. This phenomenon is the result of a long-term game between tumor and chronic inflammation. However, when the risk factors induce PTC, the anti-tumor cell immunity responds immediately, and the initial CD4+ T (Th0) cells in the peripheral blood are induced to enter the PTC tumor microenvironment. At the initial stage of the interaction between the both, can PTC cancer cells induce the initial CD4+T (Th0) cells to become Treg cells to exert anti-tumor effects? What effect does this immune cell have on the prognosis of PTC? Our group has found that the existence of Th17 cells in the tumor microenvironment of PTC/HT patients, and it is associated with better prognosis of patients (6). The presence of Treg and Th17 cells in the tumor microenvironment of PTC/HT patients, what is the relationship between the them? These issues have not been explicitly studied. Therefore, we used the Transwell chamber to design a model for the interaction between umbilical cord blood initial T lymphocytes and thyroid papillary carcinoma cell lines, to evaluate the effect of PTC cells on the induction of differentiation of naive T lymphocytes and predict the effect of induced initial T lymphocytes on PTC prognosis.
Mayer et al. (35) showed that umbilical cord blood contained a small number of CD4+CD25+ Treg cell subsets. It needs antigen-stimulated amplification and becomes a functional inhibitory Treg, CD4+CD25+ T cells from umbilical cord blood are more sensitive than T cell allergens and endotoxin-induced proliferative responses derived from adult peripheral blood (36). It is believed that since human umbilical cord blood contains primary T cells mainly, they represent a perfect initial T lymphocyte model system that can be used to assess the effects of cancer cells on naive T lymphocytes. In this study, the Transwell isolation co-culture model allowed for the isolation of sufficient active lymphocytes to obtain high-quality expression data for proteins, mRNAs, and cell supernatants. So our short-term co-culture system is ideal for studying the early and dynamic interactions between thyroid cancer cells and TIL. Neonatal immunity is characterized by an initially immature immune system, which is an immunogenic weak and Th2-polarized immunity, and lacked of T-helper type 1 (Th1) ability to resist infection (37). So we selected the initial T lymphocytes isolated from the cord blood of newborns with weaker immunogenicity as the experimental subjects, and have them co-cultured with PTC cell lines with or without lymph node metastasis. To observe the induced effect of cancer cells on T lymphocytes by PTC more accurately.
Fujimaki et al. (38) found that umbilical cord blood CD4+CD25+ T cells expressed FoxP3 weakly and did not have the ability to inhibit activity. Darrasse-Jèze et al. (39) found that T regulatory cells with inhibitory regulatory functions in umbilical cord blood and expressed FoxP3 mRNA. Treg cells in umbilical cord blood are incapable, they do not proliferate and do not produce interleukins when stimulated (40).
These Tregs play an important role in controlling autoimmunity (41) and avoiding fetal immune rejection (42). In this study, we found that the low expression of CD4+CD25+CD127− Treg cells in neonatal cord blood by flow cytometry. Western blotting also demonstrated the low expression of the Treg cell-specific nuclear transcription factor FoxP3 protein in cord blood which was consistent with the Published literature.
We investigated the effects of neonatal cord blood T cells Differentiate into immunosuppressive Tregs and inflammatory Th17 cells by normal thyroid follicular epithelial cell line (Nthy-ori 3-1) and the thyroid papillary carcinoma cell line (TPC-1 and K1). Combined with our published research about the effect of specific nuclear transcription factor RORγ t of Th17 on the umbilical cord blood initial T lymphocyte (6). First, we found that PTC cells have a positive effect on the differentiation of human primary T cells into FoxP3-expressing Treg cells and RORγ t-expressing Th17 cells. Umbilical cord blood T cells are highly responsive to TPC-1 and K1 in this process, while cord blood T cells are hyporesponsive to normal control thyroid follicular epithelial cells. Second, after the co-acting of PTC cells with cord blood T cells, the induced differentiation of Th17 cells and Treg cells fluctuated increasing. At 36 h, the proportion of CD3+CD8−IL-17+ Th17 cells and CD4+CD25+CD127− Treg cells reached to the highest. The ratio of Th17 cells was higher than that of Treg cells. The ratio of Th17/Treg cells induced by cancer cells was greater than 1 and the balance of Th17/Treg was significantly biased toward Th17. The large proportion of Th17 cells might be the reason for the better prognosis of patients with PTC complicated with HT. Finally, the proportion of Treg cells induced by K1 cell line with lymph node metastasis was higher than that of TPC-1 cell line without lymph node metastasis. It might be suggested that the immune effect of Treg is mainly related to PTC lymph node metastasis. Combined with tumor tissue immunohistochemistry, the expression of FoxP3 was positively correlated with lymph node metastasis in patients with PTC complicated with HT. We hypothesized that Treg cells present around the tumor of PTC patients complicated with HT may promoting lymph node metastasis.
Th17 and Treg cells share a common origin. Under normal circumstances, both of them are connected, affected and restricted in the process of differentiation, and they are in a state of balance to jointly maintain the stability of the internal environment. Once cell numbers and states of them are confused, the balance is broken and multiple diseases will follow. It has been confirmed that Th17/Treg imbalance is closely related to the occurrence and development of various tumors. Maruyama et al. (43) found that Th17 and Treg cells accumulated in the early stage of the disease in the microenvironment of gastric cancer. In the disease progresses, the infiltration of Th17 cells decrease while the Treg cells increase. Chen et al. (44) have shown that there is a significant Th17/Treg imbalance in peripheral blood of patients with cervical cancer, which may contribute to the early diagnosis of cervical cancer. Although there is no report about the relationship between Th17/Treg imbalance and PTC combined with HT. We hypothesized that after external stimulation, the body can induce Th17 differentiating quickly and blocking the inhibition of Treg cells. Thus, Th17/Treg is biased toward Th17 cells. Many papers regarding Th17 cells indicate that they exert a two-way immune function in tumors. Under different conditions, it can both promote (45,46) and inhabit (47,48) the development of tumors. Th17 cells have been shown to inhibit lymph node metastasis in previous studies, and are associated with a good prognosis for PTC with HT, so the shift in Th17/Treg balance is also an objective condition that contributes to a good prognosis. In the tumor microenvironment co-acting by thyroid cancer and inflammation, although Treg cells are related to promoting lymph node metastasis, it is possible that the production of Th17 cells impairs the cancer-promoting effect of Treg cells. The lower the ratio of Th17/Treg, the invasive ability and metastatic ability of the tumor become stronger. Moreover, if the balance drifts toward the Treg, it will result in worse prognosis. Since this experiment simulates the transient effect of the initial T lymphocytes in the cancer cell line, the number of transiently acting lymphocytes cannot be compared with the long-term effect. The effects of Th17 and Treg on PTC lymph node metastasis need to be further determined by experiments. The plasticity of the CD4+ T cell subset has been demonstrated, and these cells have been previously considered to be the final state of differentiation, but after so many years of research, this conclusion has been denied. These cells usually have the ability to reorient their functions, including Th17 cells and Treg cells, which are capable of transforming each other in a specific microenvironment. Therefore, whether these changes in PTC with HT are the cause of tumorigenesis or the result of tumor development, we need more research and exploration. Reducing the proportion of Treg cells in patients of PTC complicated with HT, increasing the proportion of Th17 cells, and restoring the dynamic balance of Th17 cells and Treg cells may provide a new immunological target treatment and help for improving the prognosis of PTC complicated with HT.
Acknowledgments
Funding: The present study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.01.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee of the First People’s Hospital of Yunnan Province (NO.2014YYGJ071) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baretić M. 100 years of Hashimoto thyroiditis, still an intriguing disease. Acta Med Croatica 2011;65:453-7. [PubMed]
- Dailey ME, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg 1955;70:291-7. [Crossref] [PubMed]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 2005;6:345-52. [Crossref] [PubMed]
- Beyer M, Schultze JL. Regulatory T cells in cancer. Blood 2006;108:804-11. [Crossref] [PubMed]
- Zeng R, Shou T, Yang KX, et al. Papillary thyroid carcinoma risk factors in the Yunnan plateau of southwestern China. Ther Clin Risk Manag 2016;12:1065-74. [Crossref] [PubMed]
- Zeng R, Lyu Y, Zhang G, et al. Positive effect of RORgammat on the prognosis of thyroid papillary carcinoma patients combined with Hashimoto's thyroiditis. Am J Transl Res 2018;10:3011-24. [PubMed]
- Tanaka J, Ogura T, Sato H, et al. Establishment and biological characterization of an in vitro human cytomegalovirus latency model. Virology 1987;161:62-72. [Crossref] [PubMed]
- Challeton C, Branea F, Schlumberger M, et al. Characterization and radiosensitivity at high or low dose rate of four cell lines derived from human thyroid tumors. Int J Radiat Oncol Biol Phys 1997;37:163-9. [Crossref] [PubMed]
- Liu H, Zeng Q, Cui Y, et al. The role of the IRE1 pathway in excessive iodide- and/or fluoride-induced apoptosis in Nthy-ori 3-1 cells in vitro. Toxicol Lett 2014;224:341-8. [Crossref] [PubMed]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671-5. [Crossref] [PubMed]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986;315:1650-9. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest 2007;117:1137-46. [Crossref] [PubMed]
- Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol 2010;40:1830-5. [Crossref] [PubMed]
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood 2008;112:1557-69. [Crossref] [PubMed]
- Matsubayashi S, Kawai K, Matsumoto Y, et al. The correlation between papillary thyroid carcinoma and lymphocytic infiltration in the thyroid gland. J Clin Endocrinol Metab 1995;80:3421-24. [PubMed]
- Modi J, Patel A, Terrell R, et al. Papillary thyroid carcinomas from young adults and children contain a mixture of lymphocytes. J Clin Endocrinol Metab 2003;88:4418-25. [Crossref] [PubMed]
- French JD, Weber ZJ, Fretwell DL, et al. Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J Clin Endocrinol Metab 2010;95:2325-33. [Crossref] [PubMed]
- Gupta S, Patel A, Folstad A, et al. Infiltration of differentiated thyroid carcinoma by proliferating lymphocytes is associated with improved disease-free survival for children and young adults. J Clin Endocrinol Metab 2001;86:1346-54. [PubMed]
- Huang BY, Hseuh C, Chao TC, et al. Well-differentiated thyroid carcinoma with concomitant Hashimoto's thyroiditis present with less aggressive clinical stage and low recurrence. Endocr Pathol 2011;22:144-9. [Crossref] [PubMed]
- Muzza M, Degl'Innocenti D, Colombo C, et al. The tight relationship between papillary thyroid cancer, autoimmunity and inflammation: clinical and molecular studies. Clin Endocrinol (Oxf) 2010;72:702-8. [Crossref] [PubMed]
- Ding ZC, Zhou G. Cytotoxic chemotherapy and CD4+ effector T cells: an emerging alliance for durable antitumor effects. Clin Dev Immunol 2012;2012:890178.
- Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell 2000;101:455-8. [Crossref] [PubMed]
- Baecher-Allan C, Anderson DE. Regulatory cells and human cancer. Semin Cancer Biol 2006;16:98-105. [Crossref] [PubMed]
- Fontenot JD, Rasmussen JP, Williams LM, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 2005;22:329-41. [Crossref] [PubMed]
- Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942-9. [Crossref] [PubMed]
- Leffers N, Gooden MJ, de Jong RA, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother 2009;58:449-59. [Crossref] [PubMed]
- Schatton T, Schutte U, Frank NY, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res 2010;70:697-708. [Crossref] [PubMed]
- Merlo A, Casalini P, Carcangiu ML, et al. FOXP3 expression and overall survival in breast cancer. J Clin Oncol 2009;27:1746-52. [Crossref] [PubMed]
- Gogali F, Paterakis G, Rassidakis GZ, et al. Phenotypical analysis of lymphocytes with suppressive and regulatory properties (Tregs) and NK cells in the papillary carcinoma of thyroid. J Clin Endocrinol Metab 2012;97:1474-82. [Crossref] [PubMed]
- Liu Y, Yun X, Gao M, et al. Analysis of regulatory T cells frequency in peripheral blood and tumor tissues in papillary thyroid carcinoma with and without Hashimoto's thyroiditis. Clin Transl Oncol 2015;17:274-80. [Crossref] [PubMed]
- Szylberg Ł, Bodnar M, Harasymczuk J, et al. Expression of FoxP3 protein plays a key role in thyroid tumors in children. Fetal Pediatr Pathol 2014;33:84-91. [Crossref] [PubMed]
- Cunha LL, Morari EC, Nonogaki S, et al. Foxp3 expression is associated with aggressiveness in differentiated thyroid carcinomas. Clinics (Sao Paulo) 2012;67:483-8. [Crossref] [PubMed]
- Cunha LL, Morari EC, Guihen AC, et al. Infiltration of a mixture of immune cells may be related to good prognosis in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2012;77:918-25. [Crossref] [PubMed]
- Mayer E, Bannert C, Gruber S, et al. Cord blood derived CD4+ CD25(high) T cells become functional regulatory T cells upon antigen encounter. PLoS One 2012;7:e29355. [Crossref] [PubMed]
- Eiwegger T, Mayer E, Brix S, et al. Allergen specific responses in cord and adult blood are differentially modulated in the presence of endotoxins. Clin Exp Allergy 2008;38:1627-34. [Crossref] [PubMed]
- Adkins B. Peripheral CD4+ lymphocytes derived from fetal versus adult thymic precursors differ phenotypically and functionally. J Immunol 2003;171:5157-64. [Crossref] [PubMed]
- Fujimaki W, Takahashi N, Ohnuma K, et al. Comparative study of regulatory T cell function of human CD25CD4 T cells from thymocytes, cord blood, and adult peripheral blood. Clin Dev Immunol 2008;2008:305859.
- Darrasse-Jèze G, Marodon G, Salomon BL, et al. Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetuses. Blood 2005;105:4715-21. [Crossref] [PubMed]
- Takahashi T, Kuniyasu Y, Toda M, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol 1998;10:1969-80. [Crossref] [PubMed]
- Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004;22:531-62. [Crossref] [PubMed]
- Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 2004;5:266-71. [Crossref] [PubMed]
- Maruyama T, Kono K, Mizukami Y, et al. Distribution of Th17 cells and FoxP3(+) regulatory T cells in tumor-infiltrating lymphocytes, tumor-draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer. Cancer Sci 2010;101:1947-54. [Crossref] [PubMed]
- Chen Z, Ding J, Pang N, et al. The Th17/Treg balance and the expression of related cytokines in Uygur cervical cancer patients. Diagn Pathol 2013;8:61. [Crossref] [PubMed]
- Tosolini M, Kirilovsky A, Mlecnik B, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011;71:1263-71. [Crossref] [PubMed]
- Liu J, Duan Y, Cheng X, et al. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun 2011;407:348-54. [Crossref] [PubMed]
- Muranski P, Borman ZA, Kerkar SP, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 2011;35:972-85. [Crossref] [PubMed]
- Martin-Orozco N, Muranski P, Chung Y, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 2009;31:787-98. [Crossref] [PubMed]