
ALDH1+ stem cells demonstrate more stem cell-like characteristics than CD44+/CD24–/low stem cells in different molecular subtypes of breast cancer
Introduction
Breast cancer is the most common malignancy among women in the United States, accounting for 29% of new cases (1). In China, the incidence of breast cancer also ranks first among female malignant tumors, and has been increasing annually (2). With the popularization of breast cancer screening and improvement of treatment methods, the overall survival of breast cancer patients has been greatly improved. However, approximately one-third of the patients still die of recurrence and distant metastasis after treatment. A recent theory on cancer stem cell (CSC) suggested that recurrence and distant metastasis of breast cancer are related to the residual occult CSC.
Breast cancer stem cells (BCSCs) are a small group of cells having the characteristics of stem cells. They have the potential of self-renewal and differentiation as well as are resistant to traditional adjuvant treatment, thereby playing a crucial role in the occurrence, development, and distant metastasis of breast cancer. In 2003, Al-Hajj et al. confirmed for the first time that the CD44+/CD24–/low breast cancer cells presented with properties of CSC. These cells could renew themselves, and were able to form tumors with phenotypic heterogeneity in the subcutaneous fat pads of NOD/SCID mice, whereas the non-CD44+/CD24–/low cells could not (3). In 2005, Ponti et al. confirmed the stem cell-like characteristics of CD44+/CD24–/low breast cancer cells via Mammosphere (MS) assay (4). Subsequently, CD44+/CD24–/low cells were widely used as BCSC in several studies (5-9). Other biomarkers have also been used to identify BCSCs. Acetaldehyde dehydrogenase 1 (ALDH1) is a member of the acetaldehyde dehydrogenase families, which catalyzes oxidation of acetic acid to acetaldehyde in the cell and participates in stem cell differentiation as well as in the transformation of retinol and retinoic acid (10). In 2007, Ginestier et al. suggested that even ALDH1+ breast cancer cells presented with characteristics of BCSCs and could form tumors with phenotypic heterogeneity in the subcutaneous fat pads of NOD/SCID mice, whereas ALDH1-cells could not (11). Other authors further confirmed that the ALDH1+ breast cancer cells presented with stem cell-like characteristics, and that ALDH1+ phenotype was a reliable marker for BCSC (7,8,12-19). CD44+/CD24–/low and ALDH1+ were both widely used biomarkers for identifying BCSCs in several studies.
Some studies pertaining to the different characteristics of CD44 +/CD24–/low and ALDH1+ BCSCs. Through a NOD/SCID mouse xenograft experiment, Ginestier et al. suggested that ALDH1+ BCSC had better tumorigenic ability than CD44+/ CD24–/low BCSC (11). Breast cancer is a heterogeneous disease and can be divided into four subtypes [Luminal A, Luminal B, human epidermal receptor-2 (HER2) overexpression, and triple-negative] based on intrinsic molecular portraits. Each subtype presents with different clinicopathological characteristics, treatment response and prognosis (20,21). However, few studies have compared the biological characteristics of CD44+/CD24–/low and ALDH1+ BCSCs in different molecular subtypes of breast cancer.
Here, we compared the self-renewal and tumorigenic abilities of CD44+/CD24–/low with those of ALDH1+ BCSCs in four molecular subtypes of breast cancer using fresh surgical specimens.
Methods
This study was approved by the Ethics Committee of Sichuan Provincial People’s Hospital in China. Human samples were collected after obtaining informed written consents from all participants.
Patients and tumors
We included 4 female patients with primary breast cancer in the Department of Breast Surgery of Sichuan Provincial People’s Hospital from June 2017 to September 2017, with a median age of 48 years old. All patients were diagnosed with infiltrating ductal carcinoma by preoperative core needle biopsy, there was one case in each molecular subtype. Tumor samples were obtained within 30 minutes after surgery and washed by the D-Hanks solution containing streptomycin for three times. The necrotic tissue, fat tissue and blood clots were carefully removed with the ophthalmic scissors and the tumor samples were cut into 1 mm × 1 mm × 1 mm blocks.
The digestion was performed using trypsinase and type I collagenase for 5 min in a constant-temperature bath at 37 °C, and terminated by fetal bovine serum (FBS). Cell filtrate was filtered and collected using a 200-mesh stainless steel filter. After low-speed centrifugation (69.4 ×g) for 5 min, the supernatant was discarded and the cells were collected. The primary breast cancer cell suspension was prepared with serum-free DMEM-F12 medium (1:50 B27, 20 ng/mL EGF, 10 ng/mL bFGF, 5 µg/mL insulin, GIBICO, US) and cultured in a constant temperature box at 37 °C with 5% CO2 and 95% humidity.
Flow cytometry
The above primary breast cancer cell suspension was stained with trypan blue, and the number of living cells was counted to be over 90%. CD44+/CD24−/low cells were collected with reference to the method of Al-Hajj et al. (3). Anti-CD44-PE/CY7 (Anti-CD44 antibody, Abcam, English) and CD24-FITC (Anti-CD24 antibody, Abcam, English) antibodies were added into the test tube, and homologous control antibodies were added into the control tube, which were mixed well and incubated at room temperature for 30 min in the dark. The mixtures were washed twice with PBS and centrifuged at a low speed (69.4 ×g) for 5 min. Then, the supernatant was discarded, and the cells were re-suspended by adding PBS solution with cell concentration adjusted to 1×106/mL, and sorted immediately on the machine. The main steps of ALDH1+ cell sorting were as follows: 5 µL/mL activated ALDELUORTM (ALDELUORTM Kit, STEMCELL, Canada) substrates were added, mixed well and incubated at 37 °C for 30 min. After low-speed centrifugation (69.4 ×g) for 5 min, the supernatant was discarded, ALDELUORTM buffer was added for re-suspension with cell concentration adjusted to 1×106/mL, and the cells were immediately sorted on the machine. The selected cells were cultured in serum-free DMEM-F12 medium in a constant temperature box at 37 °C with 5% CO2 and 95% humidity.
Mammosphere culture
Single-cell suspensions after flow sorting of four molecular subtypes of breast cancer were collected, including CD44+/CD24−/low, ALDH1+ and non-BCSC (ALDH1-) cells, which were re-suspended in DMEM-F12 medium, with the cell concentration adjusted to 1×103/mL. Cells in each group were cultured on a 96-well plate with 5 duplicated wells and 100 µL per well. Then, each well was incubated in DMEM-F12 medium with a total volume of 200 µL in a constant temperature box at 37 °C with 5% CO2 and 95% humidity.
Animal model
All NOD/SCID mice were divided into Luminal A group, Luminal B group, HER2 overexpression group and Triple-negative group, with 3 subgroups in each group, namely CD44+/CD24−/low subgroup, ALDH1+ subgroup and non-BCSCs (ALDH1−) subgroup, with five NOD/SCID mice in each subgroup. The CD44+/CD24−/low subgroup, ALDH1+ subgroup and non-BCSCs (ALDH1−) subgroup were inoculated with selected CD44+/CD24−/low cells, ALDH1+ cells and non-BCSCs (ALDH1−) cells, respectively. The NOD/SCID mouse xenografted experiment was performed with reference to Al-Hajj (3) and Ginestier (11). After collecting well-growing cells from each group, single-cell suspensions were prepared with PBS, with the cell concentration adjusted to 1×106/mL. The cells of each group were mixed with Matrigel matrix at a volume ratio of 1:1, with the final cell concentration of 0.5×106/mL. The number of cells inoculated in each group was set at 0.5×105. Each NOD/SCID mouse was inoculated with 0.1 mL mixture into the subcutaneous fat pad of the forechest using a 1-mL medical syringe for 8 weeks under standard conditions. Once a week, the inoculated sites of mice were examined by touching, and the longest diameter of the tumors was measured and recorded. The mice were sacrificed by dislocation of the cervical spine 8 weeks later.
Statistical procedures
All data in this study was expressed as mean ± SD. T test was used for the comparison of two independent samples. All statistical analyses were performed by SPSS (IBM SPSS Statistics, Chicago, IL, US). All statistical analyses were 2-sided and P<0.05 was defined as statistically significant.
Results
Proportion of CD44+/CD24–/low and ALDH1+ BCSCs in four molecular subtypes of breast cancer after flow cytometry
Primary cells of Luminal A, Luminal B, HER2 overexpression and triple-negative subtypes were successfully cultured, and CD44+/CD24–/low and ALDH1+ BCSCs were sorted using flow cytometry. The proportion of CD44+/CD24–/low and ALDH1+ BCSCs in four molecular subtypes of breast cancer is shown in Figures 1,2 and Table 1.
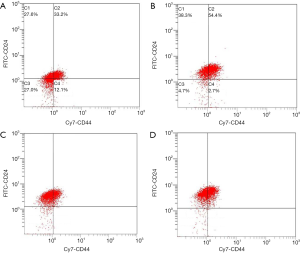
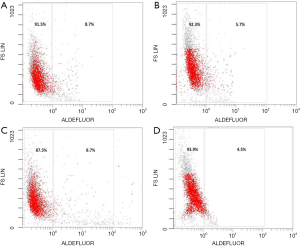
Table 1
| Subtypes | ALDH1+ (%) | CD44+ /CD24–/low (%) |
|---|---|---|
| Luminal A | 8.7 | 12.1 |
| Luminal B | 5.7 | 2.7 |
| HER2 overexpression | 8.7 | 0.8 |
| Triple-negative | 4.5 | 0.7 |
BCSC, breast cancer stem cell.
Comparison between MS formation ability of CD44+/CD24−/low and that of ALDH1+ BCSCs in different molecular subtypes of breast cancer
Primary breast cancer cells of each molecular subtype were successfully obtained after mechanical and chemical treatments. After 2–4 days of culture in a serum-free medium, the cells began to divide and proliferate, and a small amount of MSs appeared within 5–6 days. After 7–10 days, typical mature MSs could be observed, consisting of hundreds of cells. On continuing the serum-free suspension culture, it was observed that the MSs no longer significantly increased, and most MSs underwent disintegration after 2 weeks. The CD44+/CD24–/low and ALDH1+ BCSCs of four molecular subtypes formed MSs within 2–3 days of serum-free culture, with a higher number of cells and larger MS diameters. Typically, MSs could be observed within 6–7 days, and the morphology remained stable after 2 weeks of culture. The non-BCSCs from four molecular subtypes could not form MS in the serum-free culture medium. The number of MSs was calculated after 7 days of culture. As shown in Figure 3, in the four molecular subtypes, the ALDH1+ BCSCs formed more MSs than those formed by CD44+/CD24–/low BCSCs.
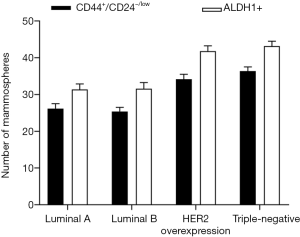
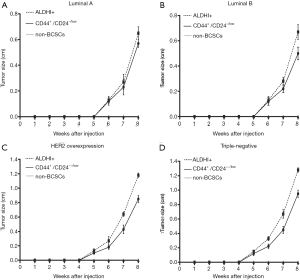
Comparison between the tumorigenic ability of CD44+/CD24–/low and that of ALDH1+ BCSCs in different molecular subtypes of breast cancer
The growth curves of grafted tumors in the three groups of cells of each molecular subtype were shown in Figure 4. After 8 weeks of observation, the non-BCSC groups did not form grafted tumor in the four subtypes. On the eighth week, in the four subtypes of breast cancer, ALDH1+ BCSC showed lager tumor diameters than CD44+/CD24–/low BCSC. The diameters of the grafted tumors of ALDH1+ and CD44+/CD24−/low BCSCs were 0.67±0.04 vs. 0.56±0.04 cm, P=0.002 (Luminal A subtype); 0.68±0.05 vs. 0.50±0.04 cm, P<0.001 (Luminal B subtype); 1.18±0.03 vs. 0.85±0.05 cm, P=0.001 (HER2 overexpression subtype); 1.28±0.03 vs. 0.95±0.05 cm, P=0.001 (triple-negative subtype).
Discussion
BCSCs retain certain key properties of stem cells, including self-renewal that initiates and drives tumorigenesis as well as differentiation that contributes to tumor heterogeneity (22). BCSCs are also heterogeneous and are cells with different phenotypes that share the properties of stem cells. Other studies (4,23) have corroborated the findings reported by Al-Hajj et al. (3) that CD44+/CD24–/low breast cancer cells had the properties of BCSC. In 2007, Ginestier et al. confirmed that ALDH1+ cells had the properties of BCSC (11). Both CD44+/CD24–/low and ALDH1+ phenotypes could be used as universal phenotypes of BCSC and have been used across several studies conducted on BCSC. However, the stem cell-like characteristics of the two phenotypes may be different, and there were few related studies.
In this study, tumor tissues from Luminal A, Luminal B, HER2 overexpression, triple-negative subtypes of breast cancer were obtained; primary breast cancer cells were successfully cultured via serum-free suspension culture; and CD44+/CD24–/low BCSCs, ALDH1+ BCSCs and non-BCSCs were sorted using flow cytometry. This result suggested that BCSCs of different phenotypes exist in breast cancer, and CD44+/CD24–/low and ALDH1+ phenotypes could be used as reliable markers for identifying BCSC. In different molecular subtypes of breast cancer, the proportions of CD44+/CD24–/low and ALDH1+ BCSCs were 0.7% to 12.1% and 4.5% to 8.7%, respectively, which was consistent with those reported in other studies (0.6% to 12%) (4,11).
In this study, three phenotypes of cells were sorted, namely ALDH1+ BCSC, CD44+/CD24–/low BCSC and non-BCSC. The self-renewal and tumorigenic abilities of BCSCs were compared by performing MS formation and NOD/SCID mouse xenograft experiments. In the four molecular subtypes of breast cancer, non-BCSCs did not form MS in the MS formation experiment, and no grafted tumor was formed in the subcutaneous fat pad of NOD/SCID mice, which was consistent with the study conducted by Ginestier (11). In the MS formation experiment, the number of MS formed by ALDH1+ BCSCs in each molecular subtype was significantly higher than that formed by CD44+/CD24–/low BCSCs (P<0.001). In the NOD/SCID mouse xenograft experiment, the diameters of tumors formed by ALDH1+ BCSCs in the four molecular subtypes were significantly larger than those formed by CD44+/CD24–/low BCSCs (P<0.05). The above results suggested that ALDH1+ BCSCs may be more important in the occurrence, development, and metastasis of breast cancer, and that it is crucial to study the underlying molecular mechanism and develop corresponding drugs to improve the survival of breast cancer patients
This study has some limitations. Only four fresh breast cancer tissue samples, including Luminal A, Luminal B, HER2 overexpression, and triple-negative subtypes, could be obtained. The self-renewal and tumorigenic abilities of BCSCs of different molecular subtypes were preliminarily explored; However, the results still need to be verified by conducting further studies with a larger sample size.
Conclusions
This study demonstrated that ALDH1+ BCSC had better self-renewal and tumorigenic abilities than CD44+/CD24–/low BCSC in the four molecular subtypes of breast cancer.
Acknowledgments
Funding: This study was funded by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.01.53). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Sichuan Provincial People’s Hospital in China, human samples were collected after obtaining informed written consent from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100:3983-8. [Crossref] [PubMed]
- Ponti D, Costa A, Zaffaroni N, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells withstem/progenitor cell properties. Cancer Res 2005;65:5506-11. [Crossref] [PubMed]
- Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A 2009;106:13820-5. [Crossref] [PubMed]
- Liu R, Wang X, Chen GY, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med 2007;356:217-26. [Crossref] [PubMed]
- Croker AK, Goodale D, Chu J, et al. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J Cell Mol Med 2009;13:2236-52. [Crossref] [PubMed]
- Tanei T, Morimoto K, Shimazu K, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res 2009;15:4234-41. [Crossref] [PubMed]
- Hwang-Verslues WW, Kuo WH, Chang PH, et al. Multiple lineages of human breast cancer stem/progenitor cells identified by profiling with stem cell markers. PLoS One 2009;4:e8377. [Crossref] [PubMed]
- Ma I, Allan AL. The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell Rev Rep 2011;7:292-306. [Crossref] [PubMed]
- Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007;1:555-67. [Crossref] [PubMed]
- Krause U, Ryan DM, Clough BH, et al. An unexpected role for a Wnt-inhibitor: Dickkopf-1 triggers a novel cancer survival mechanism through modulation of aldehyde-dehydrogenase-1 activity. Cell Death Dis 2014;5:e1093. [Crossref] [PubMed]
- Lohberger B, Stuendl N, Wolf E, et al. The novel myxofibrosarcoma cell line MUG-Myx1 expresses a tumourigenic stem-like cell population with high aldehyde dehydrogenase 1 activity. BMC Cancer 2013;13:563. [Crossref] [PubMed]
- Wegman-Points LJ, Teoh-Fitzgerald ML, Mao G, et al. Retroviral-infection increases tumorigenic potential of MDA-MB-231 breast carcinoma cells by expanding an aldehyde dehydrogenase (ALDH1) positive stem-cell like population. Redox Biol 2014;2:847-54. [Crossref] [PubMed]
- Kim YS, Jung MJ, Ryu DW, et al. Clinicopathologic characteristics of breast cancer stem cells identified on thebasis of aldehyde dehydrogenase 1 expression. J Breast Cancer 2014;17:121-8. [Crossref] [PubMed]
- Charafe-Jauffret E, Ginestier C, Iovino F, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res 2009;69:1302-13. [Crossref] [PubMed]
- Morimoto K, Kim SJ, Tanei T, et al. Stem cell marker aldehyde dehydrogenase 1-positive breast cancers are characterized by negative estrogen receptor, positive human epidermal growthfactor receptor type 2, and high Ki67 expression. Cancer Sci 2009;100:1062-8. [Crossref] [PubMed]
- Aktas B, Tewes M, Fehm T, et al. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res 2009;11:R46. [Crossref] [PubMed]
- Charafe-Jauffret E, Ginestier C, Bertucci F, et al. ALDH1-positive cancer stem cells predict engraftment of primary breast tumors and are governed by a common stem cell program. Cancer Res 2013;73:7290-300. [Crossref] [PubMed]
- Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [Crossref] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61-70. [Crossref] [PubMed]
- Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105-11. [Crossref] [PubMed]
- Sheridan C, Kishimoto H, Fuchs RK, et al. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res 2006;8:R59. [Crossref] [PubMed]


