
Chemotherapy-induced thrombocytopenia and platelet transfusion in patients with diffuse large B-cell lymphoma
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a highly aggressive form of B-cell lymphoma, and DLBCL encompasses many different disease entities with distinct clinical, pathological and biological features (1,2). As the most common subtype of lymphoma, it accounts for approximately 30–35% of the non-Hodgkin lymphoma (NHL) cases worldwide (3,4).
Chemotherapy-induced thrombocytopenia (CIT), which is a dose-limiting toxicological response to chemotherapeutic drugs, is considered one of the most common complications that occur during tumor treatment. CIT can be particularly harmful as it can delay chemotherapy, decrease the dose of chemotherapy drugs, increase medical expenses, cause induced bleeding, and intensify the mental burden of patients (5-7). In short, CIT seriously reduces the efficacy of chemotherapy and the life quality of patients.
Thus far, only a few studies have investigated the relationship between the incidence of CIT and different chemotherapy drug types, while research on the severity and risk factors of myelotoxicity associated with clinical features is similarly scant. The existing studies primarily focus on solid tumors, with the incidence of CIT in hematological tumors reported to be as high as 75% (8,9). In light of this, it is particularly important to investigate the incidence of thrombocytopenia caused by different chemotherapy regimens in the field of hematological tumor studies, which can provide valuable information in facilitating the prevention and treatment of CIT. Additionally, to improve patients' quality of life, clarifying the relationship between CIT and different clinical features is of utmost importance.
Platelet transfusions are used in modern clinical practice to prevent and treat bleeding in thrombocytopenic patients with bone marrow failure secondary to chemotherapy, and a platelet-count threshold for prophylactic platelet transfusion in DLBCL recipients has yet to be determined. Giving prophylactic platelet transfusions at a lower prespecified threshold platelet count may increase the risk that bleeding will occur, which may cause morbidity and even mortality, whereas giving platelet transfusions at a higher prespecified threshold platelet count may mean that people receive unnecessary platelet transfusions (10,11).
Prompted by this dilemma, we conducted a study that retrospectively analyzed the rate of CIT in patients with diffuse large B-cell lymphoma and completed a matched case-control study between CIT and without CIT cases. Clinicopathological features were compared to shed light on the unique features of this disease. We surveyed those patients who received platelet transfusions and performed a retrospective study focusing on 10×109/L versus 20×109/L prophylactic transfusion thresholds in DLBCL patients to compare transfusion requirements and the incidence of bleeding and treatment outcomes.
Methods
Patients
The data collected from patients diagnosed with DLBCL at the Fujian Cancer Hospital from July 1, 2011, to December 31, 2013, were retrospectively analyzed. The research recruitment criteria were as follows: (I) cases were in accordance with the 2008 World Health Organization (WHO) classification criteria of hematopoietic and lymphoid tumors, and were confirmed by pathological biopsy and immunohistochemistry; (II) patients were aged ≥18 years; (III) sampling patients had received clinical adjuvant chemotherapy during the observation period; (IV) platelets, hemoglobin (Hb), and white blood cells were counted before and after each chemotherapy session, and sampling patients received at least 2 blood tests per week during the course of chemotherapy; (V) before chemotherapy, platelet count was ≥100×109/L; (VI) platelet count was confirmed by a manual slide review when values fell below 50×109/L. In total, 523 DLBCL patients were included.
Chemotherapy regimen
Patients who received 1 or more courses of a specific chemotherapy regimen in the observation period were incorporated into 1 research group, and the regimen consisted of a series of sequential chemotherapies. If a patient in the observation period received 2 or more chemotherapy regimens, the first chemotherapy regimen was selected as the primary regimen, and the patient was followed up until the end of it, thus avoiding the misclassification caused by chemotherapy exposure. Among the groups of chemotherapy regimens studied in this research, the chemotherapy regimen for the CHOP group was CHOP21 (cyclophosphamide + adriamycin + vinblastine + prednisone), RCHOP21 (rituximab + cyclophosphamide + doxorubicin + vincristine + prednisone), CEOP (cyclophosphamide + etoposide + vincristine + prednisone), and CDOP (cyclophosphamide + liposomal doxorubicin + vincristine + prednisone). The chemotherapy regimen for the ACVBP group was (cyclophosphamide + doxorubicin + vincristine + bleomycin + prednisone). The chemotherapy regimen for the EPOCH group was (cyclophosphamide + etoposide + adriamycin + vinblastine + prednisone) ± rituximab. The chemotherapy regimen for the ICE group was (isophosphamide + carboplatin + etoposide) ± rituximab. The chemotherapy regimen for the GDP group was (gemcitabine + dexamethasone + cisplatin) ± rituximab. The chemotherapy regimen for the Gemox group was (gemcitabine + oxaliplatin) ± rituximab. The main chemotherapy regimen for the DHAP group was (dexamethasone + cisplatin + cytarabine) ± rituximab.
Thrombocytopenia
The judgment of thrombocytopenia is based on the number of the measured platelet counts during chemotherapy. According to the Common Terminology Criteria for Adverse Events version 4.03 (CTCAE 4.03) established by the National Institute of Health (NIH), the platelet count in peripheral blood <100×109/L in the course of chemotherapy can be defined as thrombocytopenia. Thrombocytopenia can be divided into 4 grades (CTCAE 4.03): Grade I, 75×109–99×109/L; Grade II, 50×109–74×109/L; Grade III, 25×109–49×109/L; Grade IV, <25×109/L.
Thrombocytopenia during chemotherapy is often accompanied by a reduction of other blood cell counts. The definition of isolated thrombocytopenia is platelet count <100×109/L and without anemia Hb <120 g/L for males and <110 g/L for female) and leucopenia (white blood cell count <4×109/L). The lowest level of platelet count during the same chemotherapy regimen was recorded to determine whether the patient has CIT and whether there is simple thrombocytopenia.
The selection and comparison of DLBCL platelet transfusion thresholds
For the enrollment of platelet transfusion, patients were divided into 2 groups for receiving platelet transfusions depending on if their platelet counts were ≤20×109/L (lower transfusion threshold) or ≤10×109/L (lower transfusion threshold). The control procedure employed strict matching criteria: gender (male or female), age, cell origin, pretransfusion platelet count chemotherapy-induced anemia (CIA), chemotherapy-induced leucopenia (CIL), standard international prognosis index (IPI) score, lactate dehydrogenase level, and Ann Arbor stage, B symptoms were recorded before transfusion, and data types, including the occurrence of hemorrhage and infection, the morning body temperature, the number of platelets transfused, the increase platelet count posttransfusion, days in the hospital, the use of thrombopoietin, chemotherapy and some biochemical indexes after transfusion, were compared. All patients received ABO-compatible single-donor apheresis platelet products, when available, and all platelet products were leukocyte-depleted and irradiated. One therapeutic dose of the apheresis platelets contained about 2.5×1011 platelets.
Statistical analysis
All data were analyzed by IBM SPSS version 19.0. The clinical data of patients and the percentages of CIT for different chemotherapy regimens are presented by exact percentages or median values. Chi-square or t-tests were used for univariate analysis. The logistic regression forward method was applied for multivariate analysis. A P value of <0.05 was considered to indicate a statistically significant difference. GraphPad Prism 7 was used for analyzing graphs.
Results
Incidents of CIT and CIT associated with different chemotherapy regimens
A total of 523 consecutive patients with de novo DLBCL were collected in this study. CIT occurred in 227 patients (43.4%), while isolated thrombocytopenia occurred in 63 patients (12.0%). Table 1 displays the frequency of thrombocytopenia and isolated thrombocytopenia stratified by the grade of severity. The chemotherapy regimens used in DLBCL most commonly associated with thrombocytopenia included DHAP (92.3%), ICE (89.7%), GDP (89.7%), and Gemox (69%). The highest frequencies of isolated thrombocytopenia occurred in patients receiving the ACVBP (22.2%), ICE (20.7%), Gemox (20.7%), and GDP (19.4%) chemotherapy regimens (Table 2). The platelet count nadir in patients caused by different chemotherapy regimens was 24.54±44.13 for DHAP, 55.53±35.72 for ICE, 78.28±38.22 for Gemox, and 78.72±45.83 for GDP (Figure 1).
Table 1
| Grade | Frequency, n (%) | |
|---|---|---|
| Overall thrombocytopenia | Isolated thrombocytopenia | |
| Overall | 227 (43.4) | 63 (12.0) |
| I | 89 (17.0) | 34 (6.5) |
| II | 53 (10.1) | 20 (3.8) |
| III | 42 (8.0) | 9 (1.7) |
| IV | 43 (8.2) | 0 (0.0) |
I, platelets 75×109–99×109/L; the lower limit of normal was defined as 100×109/L; II, (50×109–74×109/L; III, 25×109–49×109/L; IV, <25×109/L.
Table 2
| Type of cytotoxic drug | No. of patients | Frequency of overall thrombocytopenia, n (%) | Frequency of isolated thrombocytopenia, n (%) |
|---|---|---|---|
| ACVBP | 18 | 8 (44.4) | 4 (22.2) |
| CHOP | 246 | 62 (25.2) | 22 (8.9) |
| DHAP | 13 | 12 (92.3) | 0 (0.0) |
| EPOCH | 123 | 48 (39.0) | 12 (9.8) |
| GDP | 36 | 25 (69.4) | 7 (19.4) |
| Gemox | 29 | 20 (69.0) | 6 (20.7) |
| ICE | 58 | 52 (89.7) | 12 (20.7) |
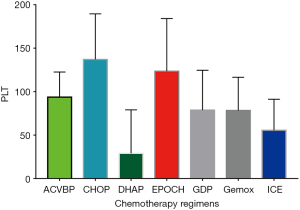
Clinical features of CIT
Patients who developed clinically significant CIT were examined for predictive factors that impacted the development of clinical complications. These factors are listed in Table 3. Overall, Ann Arbor stage was a significant predictor of the risk of CIT (P<0.001), while the level of LDH appeared to increase the risk of CIT (P<0.001), with IPI scores (P=0.015) and gender (P=0.029) also being prominent factors for CIT. Five variables were chosen to contribute to the model in the multivariate logistic regression analysis (Table 5). Results of this analysis (Table 5) revealed that chemotherapy regimens (P<0.001), LDH (P=0.004), and Ann Arbor Stage (P=0.024) were independent risk factors for thrombocytopenia.
Table 3
| Variables | Patients with CIT (n=227) | Patients without CIT (n=296) | c(t) | P* |
|---|---|---|---|---|
| Gender, n (%) | 4.75 | 0.029* | ||
| Male | 142 (62.6) | 157 (53.0) | ||
| Female | 85 (37.4) | 139 (47.0) | ||
| Age, mean ± SD, years | 51.36±14.22 | 51.40±14.09 | 0.03 | 0.976 |
| Cell origin, n (%) | 1.238 | 0.539 | ||
| GCB | 61 (26.9) | 75 (25.3) | ||
| Non-GCB | 120 (52.9) | 170 (57.4) | ||
| No test | 46 (20.3) | 51 (17.2) | ||
| HBsAg, n (%) | 0.024 | 0.876 | ||
| Positive | 72 (31.7) | 92 (31.1) | ||
| Negative | 155 (68.3) | 204 (68.9) | ||
| B symptoms, n (%) | 0.712 | 0.399 | ||
| A | 192 (84.6) | 258 (87.2) | ||
| B | 35 (15.4) | 38 (12.8) | ||
| LDH, n (%) | 20.744 | <0.001* | ||
| <190 | 111 (48.9) | 203 (68.6) | ||
| ≥190 | 116 (51.1) | 93 (31.4) | ||
| Ki67, n (%) | 0.109 | 0.947 | ||
| <80% | 93 (41.0) | 123 (41.6) | ||
| ≥80% | 105 (46.3) | 138 (46.6) | ||
| No test | 29 (12.8) | 35 (11.8) | ||
| IPI scores, n (%) | 5.928 | 0.015* | ||
| 0/1/2 | 178 (78.4) | 256 (86.5) | ||
| 3/4/5 | 49 (21.6) | 40 (13.5) | ||
| Ann Arbor stage, n (%) | 20.166 | <0.001* | ||
| I/II | 27 (11.9) | 83 (28.0) | ||
| III/IV | 200 (88.1) | 213 (72.0) |
Data were analyzed using Pearson’s χ2 test for categorical variables and t-tests for continuous variables. *P<0.05 indicates a statistically significant difference when comparing values at the same measuring points. LDH, lactic dehydrogenase.
Table 5
| Variables | SE | OR | 95% CI | P |
|---|---|---|---|---|
| Gender | 0.200 | 1.359 | 0.535-1.172 | 0.244 |
| LDH | 0.202 | 8.265 | 0.376-0.831 | 0.004* |
| Ann Arbor stage | 0.265 | 5.064 | 0.328-0.926 | 0.024* |
| Chemotherapy regimens | 0.059 | 59.549 | 0.563-0.711 | <0.001* |
Univariate comparisons were performed according to the Log-Rank Test. *P<0.05.
Platelet transfusion
Of the 227 patients with thrombocytopenia, 40 (17.6%) were administered platelet transfusions. Figure 2 shows the comparison of platelet counts before and after transfusion. The median (range) pre-transfusion platelet count was 11×109/L (3×109–23×109/L) and increased significantly post-transfusion to 29×109/L (6×109–53×109/L) (P<0.001). The pre-transfusion preclinical characteristics of the patients are shown in Table 4. The 2 groups were similar, with only the pretransfusion platelet count being different (7.53±2.03 vs. 14.00±3.00) (P<0.001). The difference in the numbers of patients with platelet transfusions per patient in the 2 groups was statistically significant (P=0.047), while the other clinical characteristic and endpoints after transfusion showed no significant difference between the two groups.
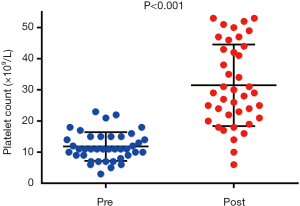
Table 4
| Variables | Lower transfusion threshold (n=15) | Higher transfusion threshold (n=25) | c(t) | P* |
|---|---|---|---|---|
| Pretransfusion | ||||
| Gender | 0.541 | 0.462 | ||
| Male | 9 (60.0) | 12 (48.0) | ||
| Female | 6 (40.0) | 13 (52.0) | ||
| Age, years | 47.33±12.37 | 45.08±14.73 | 0.496 | 0.623 |
| Cell origin | 0.711 | 0.701 | ||
| GCB | 2 (13.3) | 6 (24.0) | ||
| Non-GCB | 10 (66.7) | 14 (56.0) | ||
| No test | 3 (20.0) | 5 (20.0) | ||
| Pretransfusion platelet count (×109/L) | 7.53±2.03 | 14.00±3.00 | 7.377 | <0.001* |
| CIA | 1.946 | 0.163 | ||
| Yes | 15 (100.0) | 22 (88.0) | ||
| No | 0 (0) | 3 (12.0) | ||
| CIL | 0.889 | 0.346 | ||
| Yes | 10 (66.7) | 20 (80.0) | ||
| No | 5 (33.3) | 5 (20.0) | ||
| B symptoms | 1.615 | 0.204 | ||
| A | 10 (66.7) | 21 (84.0) | ||
| B | 5 (33.3) | 4 (16.0) | ||
| LDH | 2.416 | 0.12 | ||
| <190 | 2 (13.3) | 9 (36.0) | ||
| ≥190 | 13 (86.7) | 16 (64.0) | ||
| IPI scores | 0.239 | 0.625 | ||
| 2000/1/2 | 11 (73.3) | 20 (80.0) | ||
| 2003/4/5 | 4 (26.7) | 5 (20.0) | ||
| Ann Arbor stage | 0.667 | 0.414 | ||
| I/II | 2 (13.3) | 6 (24.0) | ||
| III/IV | 13 (86.7) | 19 (76.0) | ||
| Posttransfusion | ||||
| Bleeding | 0.615 | 0.433 | ||
| Yes | 6 (40.0) | 7 (28.0) | ||
| No | 9 (60.0) | 18 (72.0) | ||
| Infection | 0.171 | 0.68 | ||
| Yes | 7 (46.7) | 10 (40.0) | ||
| No | 8 (53.3) | 15 (60.0) | ||
| Fever | 0.96 | 0.327 | ||
| Yes | 9 (60.0) | 11 (44.0) | ||
| No | 6 (40.0) | 14 (56.0) | ||
| Thrombopoietin | 1.742 | 0.187 | ||
| Yes | 13 (86.7) | 17 (68) | ||
| No | 2 (13.3) | 8 (32) | ||
| Chemotherapy | 0.296 | 0.586 | ||
| CR/PR | 13 (86.7) | 23 (92.0) | ||
| SD/PD | 2 (13.3) | 2 (8.0) | ||
| Platelet transfusions per patient | 2.05±1.13 | 1.44±0.77 | 2.05 | 0.047* |
| Days in hospital | 34.13±19.71 | 37.04±27.48 | 0.357 | 0.723 |
| The increase platelet count of posttransfusion (×109/L) | 17.93±12.31 | 20.68±11.68 | 0.696 | 0.492 |
| Bilirubin (0–22 μmol/L) | 18.99±24.79 | 8.62±4.18 | 1.606 | 0.13 |
| Albumin (35–54 g/L) | 31.55±3.94 | 33.40±6.44 | 1.128 | 0.267 |
| ALP (34–104 IU/L) | 138.93±119.81 | 107.08±40.38 | 1.227 | 0.227 |
| LDH (80–190 IU/L) | 342.80±465.96 | 207.52±144.02 | 1.094 | 0.291 |
| CR (60–130 μmol/L) | 77.53±31.65 | 64.28±15.84 | 1.512 | 0.148 |
| BUN (3.1–7.4 mmol/L) | 4.43±3.44 | 4.01±1.46 | 0.543 | 0.59 |
Data are reported as number (%) or mean ± SD. Data were analyzed using Pearson’s χ2 test for categorical variables and the t-tests for continuous variables. *P<0.05 indicates a statistically significant difference when comparing values at the same measuring points. ALP, alkaline phosphatase; LDH, lactic dehydrogenase; GCB, germinal center b; cell-like; CR, creatinine; BUN, blood urea nitrogen.
Discussion
DLBCL, the most common subtype of NHL, is an invasive clinical process and a highly heterogeneous malignant tumor in morphology, immunology, molecular genetic abnormality, and clinical biology (2,12). Approximately 60% of DLBCL patients can reach clinical recovery, but the other 40% may suffer recurrence or treatment failures at an early stage (13). These treatment-related complications have gained increasing clinical attention, and CIT has long been regarded as a major complication of cancer treatment. CIT can lead to a dose reduction of chemotherapeutic drugs, cause delays in chemotherapy and bleeding cases, and even endanger the patient’s life.
According to the relevant literature, the incidence of CIT among DLBCL patients is 43.4%, and the incidence of isolated thrombocytopenia is 12%. Clinical studies on the incidence of CIT are limited in number, and there are even fewer reports about CIT in specific diseases. As reported in a study on different solid tumors conducted by Ten Berg et al., the CIT incidence of the solid tumor was 21.8%, and the incidence of isolated thrombocytopenia was 6.2% (14). The incidence of CIT and isolated thrombocytopenia among DLBCL patients was about 2 times that of solid tumors. In the case of hematological diseases, 1 report on the incidence of thrombocytopenia in myelodysplastic syndromes found an incidence rate of about 40–65%, which is similar to the incidence of CIT in DLBCL patients. It can be seen in Table 1 that, among CIT patients, the incidence of thrombocytopenia from Grade III to Grade IV was 37.4%. This might be a significant factor in increasing the risk of clinical bleeding (7,15,16).
The incidence and severity of CIT vary according to the type of chemotherapy regimen applied. Our research showed that the thrombocytopenia incidences of the DHAP and the ICE were 92.3% and 89.7%, respectively, and the average platelet count after chemotherapy decreased to (24.54±44.13)×109/L and (55.53±35.72)×109/L. Furthermore, the CIT incidences of the GDP regimen and the Gemox regimen were 69.4% and 69.0%, respectively. Clinically, there are few reports on the thrombocytopenia caused by different lymphoma chemotherapy regimens, and the existing studies have largely focused on solid tumors. In 2011, 1 study involving a total of 614 solid tumor patients found that the CIT incidences of therapeutic alliance with carboplatin or gemcitabine were 58.2% and 64.4%, respectively; meanwhile, the therapeutic alliance of gemcitabine + carboplatin and that of gemcitabine + cisplatin had higher CIT incidences of 85.7% and 54.8%, respectively (14). In their research on solid tumor chemotherapy regimens, Wu et al. revealed that the incidences of Grade III and Grade IV thrombocytopenia of platinum-based chemotherapy regimens were 6.5% and 4.1%, respectively, while the incidences of thrombocytopenia in gemcitabine-based chemotherapy regimens were 7.8% for the Grade III and 3.4% for Grade IV types. Accordingly, it can be inferred that platinum-based and gemcitabine-based drugs are the ones most likely to cause thrombocytopenia (17). Weycker et al. retrospectively examined a cohort comprising adults who received selected myelosuppressive chemotherapy regimens for solid tumors or non-Hodgkin’s lymphoma. They found the CIT incidence ranged from 6.1% (5.9–6.3%) for regimens containing cyclophosphamide to 13.5% (12.7–14.3%) for regimens containing gemcitabine (18). In our study, the DHAP, ICE, GDP, and Gemox regimens all contained platinum-based drugs, and the GDP and Gemox regimens contained gemcitabine-based drugs. The BC Cancer Agency pointed out that thrombocytopenia induced by the application of cytarabine chemotherapy was frequent (19) and that the incidence of thrombocytopenia induced by etoposide reached 22–41% (20). The mechanism of thrombocytopenia varies according to therapeutic regimens; for example, alkylating chemotherapeutic drugs mainly affect multipotent stem cells (21,22); cyclophosphamide mainly affects megakaryocyte progenitor cells (23); bortezomib causes thrombocytopenia by inhibiting nuclear transcription factor κB (24), etoposide stimulates platelet apoptosis by reducing the activity of Bcl-x (L) (25), and gemcitabine and mitomycin C induce thrombocytopenia by mediating endothelial cell injuries (26). Ultimately, the fundamental cause of thrombocytopenia induced by chemotherapeutic drugs in normal doses is the underdevelopment of bone marrow megakaryocyte (27). In addition to bone marrow suppression, thrombocytopenia is also partially attributable to immune-mediated factors (14,28). In regards to isolated thrombocytopenia, we acknowledge that it may be caused by the effect of drugs being inhibited by megakaryocytopoiesis, but it may also be a specific clinical manifestation of immune-mediated thrombocytopenia (29,30). In the present study, it was observed that isolated thrombocytopenia was commonly caused by the ACVBP (22.2%), ICE (20.7%), Gemox (20.7%), and GDP (19.4%) chemotherapy regimens. Considering the limited number of specimens in the current research, we recommend a larger-scale study that includes more participants and medical centers are conducted in order to confirm the incidence of isolated thrombocytopenia caused by different chemotherapy regimens and to further establish the existence of immune-mediated thrombocytopenia by measuring drug-related antibodies.
CIT, as predicted in this research, can also be attributable to other risk factors. Therefore, our study also collected patients with clinical characteristics and baseline data before they received chemotherapy in order to determine other risk factors that may affect the incidence of thrombocytopenia. Table 3 shows that gender (P=0.029), LDH (P<0.001), IPI score (P=0.015), and Ann Arbor stage (P<0.001) may be factors that can potentially influence the patient’s thrombocytopenia after chemotherapy. CIT may be more directly affected by choice of chemotherapy regimen; thus, the validity of the clinical data collected in this research might have been significantly influenced by the chemotherapy regimen type. Therefore, we conducted a multivariate analysis in order to identify other risk factors that may affect CIT by reducing the effects of the chemotherapy regimen. Table 5 shows that in addition to chemotherapy regimens, LDH (P=0.004) and Ann Arbor stage (P=0.024) were possible risk factors influencing CIT. LDH is a glycolytic enzyme that exists in the cytoplasm of all body tissues. The kidney, in particular, has a higher level of LDH. The level of LDH contained in serum increases in hematological tumors, such as in Hodgkin’s lymphoma tumors, NHL, and multiple myeloma, etc. (31). LDH has been identified as a significant risk factor in the IPI (32). An elevated level of LDH in serum suggests a poor prognostic condition, as it is indicative of NHL tumor proliferation activity and tumor burden. Although no correlation between CIT and LDH or Ann Arbor staging has been identified in this research, statistically speaking, increases in serum LDH and Ann Arbor stage III/IV were associated with a higher incidence of thrombocytopenia among DLBCL patients. Kim et al. (33) revealed that an increase of serum LDH and a decrease of platelets could increase the risk of bleeding for acute promyelocytic leukemia patients; based on these findings, we conclude that an elevated level of LDH and a reduced level of platelets are closely associated, and these 2 factors are responsible for an increased risk of bleeding. We appeal for the completion of more prospective studies that can provide additional empirical data to analyze further the impact of LDH and Ann Arbor staging on platelets.
It is a common practice that in the course of chemotherapy, patients with lymphoma or other tumors are infused with platelets in order to decrease the risk of bleeding (34,35). The American Association of Blood Banks (AABB) found that 70% of platelet transfusion cases are prophylactic in nature (36). Clinically, the threshold value of platelet transfusion may be different according to the types of the disease, bleeding conditions, and treatment prescriptions. This research retrospectively analyzed the platelet threshold value of DLBCL patients to identify the transfusion threshold value for DLBCL patients in the course of chemotherapy. Among the 227 CIT patients, 40 had been infused with platelets (17.6%). After platelet transfusion, the median level of their platelets reached 29×109/L (Figure 2), which proved that platelet transfusion had achieved significant results. Due to this ability, platelet transfusion has become an essential means to increase the level of platelets and prevent bleeding. Based on the pre-transfusion platelet threshold values, we divided the 40 patients into two groups: a low-threshold group and a high-threshold group. All 40 patients underwent CVC prior to their chemotherapy. Comparing the basic information of these 40 patients before platelet transfusion, we found that the 2 groups had no significant statistical differences, which indicates that these 2 groups were comparable. According to the analysis of the platelet transfusion data, the 2 groups only differed in the average amount of platelet infusion (the low-threshold group, 2.05±1.13 vs. the high-threshold group, 1.44±0.77). Generally, the low-threshold group’s platelet infusion was higher than that of the high-threshold group, and the results showed no significant statistical differences in some other aspects. Given this, platelet counts ≤20×109/L may be the better choice for DLBCL patients with CVC in the course of chemotherapy. Thrombocytopenia has long been considered a serious complication caused by cancer treatment, but there is currently a lack of consensus on the optimal threshold for platelet transfusion (37-39), Some physicians tend to use the platelet counts ≤10×109/L in peripheral blood as a transfusion indicator (40-42), while others set the platelet transfusion threshold as ≤20×109/L (43,44). Aside from these, there are also experts who prefer to adopt a higher cutoff value (43,45). Although there is an extensive number of studies aimed at solving the problem of the transfusion threshold, many platelet transfusion problems still have not been solved. Currently, there are 2 relatively large prospective studies on prophylactic platelet transfusions. One is the Investigators in the Trial of Prophylactic Platelets (TOPPS) research (41), and another is a study conducted in Germany (46). Both compared the advantages and disadvantages of prophylactic platelet transfusion (platelet counts ≤10×109/L) and therapeutic platelet transfusion, reaching the conclusion that the prophylactic platelet transfusion group needed more platelets, which is consistent with our research findings. Similarly, Estcourt et al. compared a large number of empirical studies and also concluded that there were no obvious differences in platelet transfusion between the groups with platelet counts of ≤10×109/L and ≤20×109/L (34). The 40 platelet transfusion patients in this retrospective research had CVC prior to their chemotherapy. The clinical practice guideline on platelet transfusion released by the formerly known AABB in 2015 also recommends that the optimal platelet transfusion threshold for patients with CVC be ≤20×109/L (47,48), which is consistent with our research results.
Conclusions
Among the DLBCL patients who have received chemotherapy, the incidence of thrombocytopenia reached 43.4%, and severe thrombocytopenia (Grade III and Grade IV) accounted for 37.4% of CIT cases. These results suggest that thrombocytopenia is a common side-effect of chemotherapy for DLBCL patients, which, therefore, warrants more in-depth studies. In the clinical literature, there are quite a few studies examining how lymphoma chemotherapy regimens influence the incidence of thrombocytopenia, and the limited existing empirical studies mainly focus on thrombocytopenia caused by monotherapy. Our study investigated the most frequently used DLBCL chemotherapy regimens, and concluded that the DHAP, ICE, GDP, and Gemox regimens could easily lead to thrombocytopenia. In addition to this, a higher level of LDH and Ann Arbor stage III/IV are also significant risk factors for thrombocytopenia. As such, clinicians should attach great importance to the possible side-effects induced by thrombocytopenia and its risk factors so as to actively prevent and treat relevant symptoms. In terms of DLBCL prophylactic platelet transfusion, we can conclude that PLT ≤20×109/L is the most reasonable transfusion pointer for patients with CVC. Finally, since our research is primarily retrospective in nature, the research results may be subject to the influence of clinician’s medication experiences, the differences in the number of chemotherapy regimens, the choice of chemotherapy options, etc. Therefore, more prospective clinical research that includes a greater number of medical centers and larger sample sizes is warranted.
Acknowledgments
Funding: This study was financially sponsored by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.01.64). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethical Committee of Fujian Cancer Hospital (YKT2019-024-01) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011;117:5019-32. [Crossref] [PubMed]
- Miao Y, Medeiros LJ, Li Y, et al. Genetic alterations and their clinical implications in DLBCL. Nat Rev Clin Oncol 2019;16:634-52. [Crossref] [PubMed]
- Xie Y, Pittaluga S, Jaffe ES. The histological classification of diffuse large B-cell lymphomas. Semin Hematol 2015;52:57-66. [Crossref] [PubMed]
- Kuai Y, Gong X, Ding L, et al. Wilms' tumor 1-associating protein plays an aggressive role in diffuse large B-cell lymphoma and forms a complex with BCL6 via Hsp90. Cell Commun Signal 2018;16:50. [Crossref] [PubMed]
- Wu S, Zhang Y, Xu L, et al. Multicenter, randomized study of genetically modified recombinant human interleukin-11 to prevent chemotherapy-induced thrombocytopenia in cancer patients receiving chemotherapy. Support Care Cancer 2012;20:1875-84. [Crossref] [PubMed]
- Vamvakas EC. Allogeneic blood transfusion and cancer recurrence: 20 years later. Transfusion 2014;54:2149-53. [Crossref] [PubMed]
- Vadhan-Raj S. Management of chemotherapy-induced thrombocytopenia: current status of thrombopoietic agents. Semin Hematol 2009;46:S26-32. [Crossref] [PubMed]
- Liou SY, Stephens JM, Carpiuc KT, et al. Economic burden of haematological adverse effects in cancer patients: a systematic review. Clin Drug Investig 2007;27:381-96. [Crossref] [PubMed]
- Kantarjian H, Giles F, List A, et al. The incidence and impact of thrombocytopenia in myelodysplastic syndromes. Cancer 2007;109:1705-14. [Crossref] [PubMed]
- Estcourt LJ, Stanworth SJ, Murphy MF. Different Platelet Count Thresholds to Guide Use of Prophylactic Platelet Transfusions for Patients With Hematological Disorders After Myelosuppressive Chemotherapy or Stem Cell Transplantation. JAMA Oncol 2016;2:1091-2. [Crossref] [PubMed]
- Estcourt LJ, Malouf R, Doree C, et al. Prophylactic platelet transfusions prior to surgery for people with a low platelet count. Cochrane Database Syst Rev 2018;9:CD012779. [PubMed]
- Costa LJ, Micallef IN, Inwards DJ, et al. Time of relapse after initial therapy significantly adds to the prognostic value of the IPI-R in patients with relapsed DLBCL undergoing autologous stem cell transplantation. Bone Marrow Transplant 2008;41:715-20. [Crossref] [PubMed]
- Thieblemont C, Gisselbrecht C. Second-line treatment paradigms for diffuse large B-cell lymphomas. Curr Oncol Rep 2009;11:386-93. [Crossref] [PubMed]
- Ten Berg MJ, van den Bemt PM, Shantakumar S, et al. Thrombocytopenia in adult cancer patients receiving cytotoxic chemotherapy: results from a retrospective hospital-based cohort study. Drug Saf 2011;34:1151-60. [Crossref] [PubMed]
- Schiffer CA, Anderson KC, Bennett CL, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001;19:1519-38. [Crossref] [PubMed]
- Hitron A, Steinke D, Sutphin S, et al. Incidence and risk factors of clinically significant chemotherapy-induced thrombocytopenia in patients with solid tumors. J Oncol Pharm Pract 2011;17:312-9. [Crossref] [PubMed]
- Wu Y, Aravind S, Ranganathan G, et al. Anemia and thrombocytopenia in patients undergoing chemotherapy for solid tumors: a descriptive study of a large outpatient oncology practice database, 2000-2007. Clin Ther 2009;31:2416-32. [Crossref] [PubMed]
- Weycker D, Hatfield M, Grossman A, et al. Risk and consequences of chemotherapy-induced thrombocytopenia in US clinical practice. BMC Cancer 2019;19:151. [Crossref] [PubMed]
- Anon. BC Cancer Agency Cancer Drug Manual: Revision 1 May, 2014.Cytarabine 2014;1-10.
- Hainsworth JD, Greco FA. Etoposide: twenty years later. Ann Oncol 1995;6:325-41. [Crossref] [PubMed]
- McManus PM, Weiss L. Busulfan-induced chronic bone marrow failure: changes in cortical bone, marrow stromal cells, and adherent cell colonies. Blood 1984;64:1036-41. [Crossref] [PubMed]
- Fitchen JH, Deregnaucourt J, Cline MJ. An in vitro model of hematopoietic injury in chronic hypoplastic anemia. Cell Tissue Kinet 1981;14:8590. [PubMed]
- DeZern AE, Petri M, Drachman DB, et al. High-dose cyclophosphamide without stem cell rescue in 207 patients with aplastic anemia and other autoimmune diseases. Medicine (Baltimore) 2011;90:89-98. [Crossref] [PubMed]
- Lonial S, Waller EK, Richardson PG, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood 2005;106:3777-84. [Crossref] [PubMed]
- Zhang H, Nimmer PM, Tahir SK, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ 2007;14:943-51. [Crossref] [PubMed]
- Humphreys BD, Sharman JP, Henderson JM, et al. Gemcitabine-associated thrombotic microangiopathy. Cancer 2004;100:2664-70. [Crossref] [PubMed]
- Zeuner A, Signore M, Martinetti D, et al. Chemotherapy-induced thrombocytopenia derives from the selective death of megakaryocyte progenitors and can be rescued by stem cell factor. Cancer Res 2007;67:4767-73. [Crossref] [PubMed]
- Leach M, Parsons RM, Reilly JT, et al. Autoimmune thrombocytopenia: a complication of fludarabine therapy in lymphoproliferative disorders. Clin Lab Haematol 2000;22:175-8. [Crossref] [PubMed]
- Wazny LD, Ariano RE. Evaluation and management of drug-induced thrombocytopenia in the acutely ill patient. Pharmacotherapy 2000;20:292-307. [Crossref] [PubMed]
- Grace RF, Shimano KA, Bhat R, et al. Second-line treatments in children with immune thrombocytopenia: Effect on platelet count and patient-centered outcomes. Am J Hematol 2019;94:741-50. [PubMed]
- Jung SH, Yang DH, Ahn JS, et al. Serum lactate dehydrogenase with a systemic inflammation score is useful for predicting response and survival in patients with newly diagnosed diffuse large B-cell lymphoma. Acta Haematol 2015;133:10-7. [Crossref] [PubMed]
- Augoff K, Hryniewicz-Jankowska A, Tabola R. Lactate dehydrogenase 5: an old friend and a new hope in the war on cancer. Cancer Lett 2015;358:1-7. [Crossref] [PubMed]
- Kim DY, Lee JH, Lee JH, et al. Significance of fibrinogen, D-dimer, and LDH levels in predicting the risk of bleeding in patients with acute promyelocytic leukemia. Leuk Res 2011;35:152-8. [Crossref] [PubMed]
- Estcourt L, Stanworth S, Doree C, et al. Prophylactic platelet transfusion for prevention of bleeding in patients with haematological disorders after chemotherapy and stem cell transplantation. Cochrane Database Syst Rev 2012;CD004269. [PubMed]
- Oka S, Muroi K, Mori M, et al. Evaluation of platelet transfusion thresholds in patients with acute myeloblastic leukemia receiving induction chemotherapy. Intern Med 2007;46:1669-70. [Crossref] [PubMed]
- Strauss RG, Blanchette VS, Hume H, et al. National acceptability of American Association of Blood Banks Pediatric Hemotherapy Committee guidelines for auditing pediatric transfusion practices. Transfusion 1993;33:168-71. [Crossref] [PubMed]
- Bercovitz RS, Josephson CD. Thrombocytopenia and bleeding in pediatric oncology patients. Hematology Am Soc Hematol Educ Program 2012;2012:499-505.
- Blumberg N, Heal JM, Phillips GL, et al. Platelets--to transfuse or not to transfuse. Lancet 2012;380:1287-9. [Crossref] [PubMed]
- Estcourt LJ, Birchall J, Lowe D, et al. Platelet transfusions in haematology patients: are we using them appropriately? Vox Sang 2012;103:284-93. [Crossref] [PubMed]
- Rebulla P, Finazzi G, Marangoni F, et al. The threshold for prophylactic platelet transfusions in adults with acute myeloid leukemia. Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto. N Engl J Med 1997;337:1870-5. [Crossref] [PubMed]
- Stanworth SJ, Estcourt LJ, Powter G, et al. A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med 2013;368:1771-80. [Crossref] [PubMed]
- Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of plasma and platelets. Blood Transfus 2009;7:132-50. [PubMed]
- Qureshi H, Lowe D, Dobson P, et al. National comparative audit of the use of platelet transfusions in the UK. Transfus Clin Biol 2007;14:509-13. [Crossref] [PubMed]
- Greeno E, McCullough J, Weisdorf D. Platelet utilization and the transfusion trigger: a prospective analysis. Transfusion 2007;47:201-5. [Crossref] [PubMed]
- Lieberman L, Liu Y, Portwine C, et al. An epidemiologic cohort study reviewing the practice of blood product transfusions among a population of pediatric oncology patients. Transfusion 2014;54:2736-44. [Crossref] [PubMed]
- Wandt H, Schaefer-Eckart K, Wendelin K, et al. Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open-label, multicentre, randomised study. Lancet 2012;380:1309-16. [Crossref] [PubMed]
- Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205-13. [Crossref] [PubMed]
- Zeidler K, Arn K, Senn O, et al. Optimal preprocedural platelet transfusion threshold for central venous catheter insertions in patients with thrombocytopenia. Transfusion 2011;51:2269-76. [Crossref] [PubMed]


