
miRNA-26a blocks interleukin-2-mediated migration and proliferation of non-small cell lung cancer cells via vascular cell adhesion molecule-1
Introduction
Due to increasing rate of air pollution and cigarette smoker’s worldwide it is anticipated that the lung cancer (LC) cases will be to their highest level in coming years (1,2). Studies have suggested that LC is one of the major causes accounting for cancer associated deaths globally (3). LC is divided into non-small cell lung cancers (NSCLC) and small cell lung cancers (SCLC). The NSCLC is further divided into 3 different types such as adenocarcinoma, squamous cell carcinoma and large cell carcinoma (4). It is reported that NSCLC’s account for about 90% cases of all LCs (5). Platinum derivatives-based chemotherapy has attained a dead end on account of efficacy, research’s now are being targeted in search of new treatment approaches for improving the survival rate in LC (6).
Researchers have confirmed that inflammation is critically linked to tumorigenesis (7). Interleukin-2 (IL-2) is one of the important members of cytokines; it exerts beneficial effects on immune system. IL-2 also described to be T-cell growth factor (TCGF) is reported to play important role in immunotherapy for human cancer (8). In a study earlier IL-2 was reported as an important candidate for cancer immunotherapy in treating metastatic renal cell carcinoma and metastatic melanoma (9). Several studies have come up describing role of IL-2 in immunity development and in structural biology of cytokines (10-12). In addition to this, expression of IL-2 is induced by IL-1β (13). It was found that high levels of IL-2 resulted in elimination of large local burdens of prostate cancer in rats (14). Furthermore, in a meta-analysis study, it was found that IL-2 enhanced the efficacy and overall survival when combined with chemotherapy in treatment of NSCLC patients (15). Also, a study evaluating the behavior of IL-2 in advanced NSCLC patients suggested that all the involved subjects showed higher serum levels of IL-2 compared to control (16). Nevertheless, the pathway of IL-2 mediated progression and migration of NSCLC and not been explored.
microRNA-26a (miR-26a) is a subtype belonging to has-miR-26 family, it 21–22 nucleotide in length. The sequence of miR-26a is an important region having potential binding sites to target mRNA. Studies involving microarray analysis of various human tumors have suggested variable expression of miR-26a (17,18). miR-26 has been found to be down-regulated in various tumors such as bladder cancer, irrespective of the cancer stage (19), breast cancer via the caspase-8 and 9 dependent pathway (20), oral squamous cell carcinoma (21) and anaplastic carcinomas (22). Contrary to this, the expression of miR-26a was upregulated in malignancies such as high-grade glioma via PTEN pathway (23) and glioblastoma multiforme (24). Recently, some of the researches have decoded the role of IL-2 and the possible interconnection between various diseases and miRNA (25,26). This important connection between IL-2 and miRNAs can hold key in therapy and diagnosis of diseases. Here in the present study, we evaluated whether miR-26a is involved in IL-2 induced progression and migration of NSCLC.
Methods
Collection of samples
For the study, a total of 36 patients having diagnosed for NSCLC were selected, among them 21 were male and 15 females aging between 33–78 years, the mean age was 47.12±11.24. All the patients underwent surgery at the department of surgery, Affiliated Hospital of Inner Mongolian University for The Nationalities, Tongliao, Inner Mongolia, China. The tumors were confirmed by histology; the grading was done by hematoxylin and eosin (H&E) staining in accordance to WHO guidelines for classification. All the 36 tumor specimens were re-investigated in accordance to their histological subtypes, the progression stage and differentiation status. The specimens were found to have 14 cases of squamous carcinoma and remaining 22 were reported to be adenocarcinoma. The p-TNM grading system was used for classifying tumor specimens. Among the 36 subjects about 24, i.e., 66.67% had a previous record of smoking. In addition to this 24 subjects reported for chronic obstructive pulmonary disease (COPD) were included in the study; the lung tissues of such subjects were isolated for the study. The tumor tissues were freezed at –80 °C in liquid nitrogen. All the subjects were educated about the study and written consents were obtained from them. The protocol was approved by the ethical committee of Affiliated Hospital of Inner Mongolian University for The Nationalities, Tongliao, Inner Mongolia, China (No. MU40012LC).
Cell lines and culture conditions
For the study, human derived NSCLC cell lines along with A-549 and H-226 were included, the cell lines were obtained from the American Type Cell Collection (USA) and were maintained by incubating in RPMI media added with fetal bovine serum (FBS) (10%), penicillin (100 U/mL) (Merck, USA) and streptomycin (100 µg/mL) (Merck, USA) at 37 °C under 5% CO2. For conditioning the cells with IL-2, the A-549 and H-226 cell lines were treated with varied concentrations of recombinant IL-2 (20, 40, 60 and 80 ng/mL) for 12 hours. The 293T human embryonic kidney cells maintained in DMEM medium added with FBS (10%), penicillin (100 U/mL) and streptomycin (100 µg/mL).
Transfection of miR-26a mimic and miR-26a inhibitor
The cancerous NSCLC cell lines received transfection of miR-26a mimic and inhibitor as well as the negative control (miR-NC). The mimic and inhibitor (50 nM/L) were transfected using Lipofectamine-2000 transfection reagent following the supplied instructions.
Luciferase assay
The 3'-UTR region in the sequence of VCAM-1 having target sites for miR-26a were cloned into the luciferase vector of pGL3. After cloning the constructs were named as pGL3-Mut and pGL3-VCAM-1. Sequence analysis was done for confirming the constructs. The 293T cells were transferred to 96 well plates followed by transfection with pGL3-Mut or pGL3-VCAM-1 (100 ng) along with miR-26a mimic, inhibitor or NC (50 nM) with the aid of Lipofectamine-2000 reagent. The cells were collected after 24 post transfection and were subjected to lysis for performing the luciferase assay. Dual luciferase reporter assay was done for measuring firefly luciferase. Every analysis was performed in triplicate.
Cell proliferation studies
The A-549 and H-226 cell lines received transfection of miR-26a mimic, inhibitor or VCAM siRNA followed by exposure to IL-2. The cells after receiving the treatment were rinsed by phosphate buffer saline and incubated in RPMI-1640 media supplemented with FBS (10%). The cells were monitored after 24, 48, 72 and 96 hours post incubation, the extent of cell proliferation was determined by incubating the cells with Thiazolyl blue tetrazolium bromide (MTT, 5 mg/mL) for 2 hours. The samples were evaluated for optical activity at 570 nm using a plate reader.
Cell migration studies
The cell migration assay was performed by transwell chambers (Corning, USA) following the supplied instructions. Briefly, 3×105 A-549 and H-226 cells were maintained in RPMI buffer (100 mL) and transferred to the chamber in the upper portion. The lower portion of chamber was loaded with RPMI media (100 mL) supplemented with FBS (10%). The cells were incubated for 6 hours at room temperature with CO2 (5%) and the upper surface was scratched, the membranes were fixed and the migrated cells received staining of crystal violet. For the assay, the cells from 5 random fields were counted.
Transfection of siRNA
For the study, VCAM-1 siRNA, STAT3 siRNA, NF-κB (p65) siRNA or STAT3 siRNA along with respective scramble control were procured from Santa Cruz biotech, USA. The transfecting the cells with siRNA, the A-549 cells were trypsinized in suspension and 3×105 cells were transferred in 6 well plates. The cells received transfection of STAT3 siRNA and NF-κB siRNA following the manufacturers protocol, the cells were collected after defined time intervals for further study. Western blot analysis was done for confirming the transfection.
Quantitative real-time PCR (qRT-PCR)
For qRT-PCR analysis, the total RNA was isolated from the cancerous tissues or the selected cancer cell lines with the help of Trizol reagent. After extraction of RNA, 5 µg was submitted to reverse transcription in the 1st strand of cDNA. qRT-PCR analysis was done using AccuPower® DualstarTM qPCR PreMix (Bioneer, South Korea). qRT-PCR premix (5 µL), cDNA (1 µL) and primer (2 µL) (both forward and reverse) were used. The primers for the work are described in Table 1.
Table 1
| Name of gene | Sequence | |
|---|---|---|
| Forward | Reverse | |
| miR-26a | CCGCCGTTCAAGTAATCCAG | CGCGGGGGCUGUUCAUAACUUACAUUGG |
| U6 | CGCTTCGGCAGCACATATAC | CAGGGGCCATGCTAATCTT |
| IL-2 | CCTGAGCAGGATGGAGAATTACA | TCCAGAACATGCCGCAGAG |
| β-Actin | CATCCTCACCCTGAAGTACCC | AGCCTGGATAGCAACGTACATG |
| STAT3 | CCGCTCGAGATGGCCCAATGGAATCAGCTAC | ATCGTTAACTCACATGGGGGAGGTAGCGC |
| VCAM-1 | AGAGGCAAGACUUCCCUGAAUGUA | GACTGTGATCGGCTTCCCAG |
Western blot study
For expression levels of proteins western blot study was done. The cancerous tissues or cell lines were homogenized followed by lysis using a lysis buffer (Sigma Aldrich, USA). The proteins were separated using SDS-PAGE (12%) followed by blotting on a PVDF membrane. The non-specific proteins were blocked by nonfat milk (5%) at 37 °C for 60 minutes. The proteins were incubated for 12 hours at controlled temperature conditions (4 °C) along with Iry antibodies (anti-VCAM-1, 1:1,000) (anti-IL-2, 1:1,000), anti- NF-κB (1:1,000), anti-STAT3 (1:1,000), anti-phosphorylated p65 (1:1,000) and anti-p-STAT3 (1:1,000). The membranes were then incubated with horseradish peroxidase-conjugated IIry antibodies for 60 minutes. All the antibodies were obtained from Santa Cruz Biotech, USA. The membranes were washed with Tris-buffered saline, the blots were visualized using enhanced chemiluminescence. Actin was used as loading control and internal standard.
Statistical analysis
All the results presented are mean ± SD. All the statistics was done using GraphPad prism software. Student’s t-test or one-way ANOVA was done for comparing the results. Pearson’s correlation was measured for establishing correlation between mRNA levels of miR-26a or between miR-26a and mRNA levels of VCAM-1. Value of P<0.05 was regarded significant.
Results
Levels of miR-26a are negatively linked to VCAM-1 and IL-2 in NSCLC
miR-26a has been reported to behave as tumor suppressor in number of cancers such as colorectal cancer (27), gastric cancer (28), hepatic carcinoma (29), nasopharyngeal cancer (30) and breast cancer (31). However, role of miR-26a under the IL-2 mediated inflammation which is the common feature in LC showing metastasis haven’t been explored. Here the study evaluated the levels of IL-2 and miR-26a in NSCLC (36 cases) and normal lung tissues (24 cases). The outcomes of qRT-PCR analysis suggested decreased levels of miR-26a (Figure 1A), whereas the levels of IL-2 were over-expressed in NSCLC (Figure 1B).
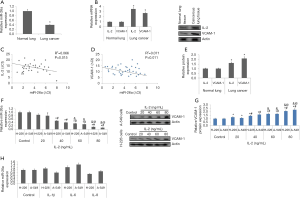
VCAM-1 is important contributing for adhesion of cancer cells. Studies involving abnormal expression of VCAM-1 associated with cancer have been previously studied (32,33). We observed that levels of VCAM-1 were upregulated significantly in NSCLC cells (Figure 1B). The findings also suggested that the levels of miR-26a were linked negatively to IL-2 and VCAM-1 (Figure 1C,D). The analysis by western blot analysis of cancer tissues for expression of VCAM-1 and IL-2 also suggested a significant elevation in NSCLC tissues compared to normal (Figure 1E).
Treatment of IL-2 decreases the expression of miR-26a but increases the levels of VCAM-1 in NSCLC cells
In direction to evaluate the effects of IL-2 on expression of miR-26a in A-549 and H-226 cells, the cells were treated overnight to varied concentrations of IL-2 (20–80 ng/mL). The outcomes suggested that treatment of IL-2 decreased the levels of miR-26a significantly when the concentrations exceeded 40 ng/mL compared to control cells (Figure 1F). Consequently, exposure of IL-2 resulted in a significant elevation in protein levels of VCAM-1 in A-549 and H-226 cells (Figure 1G), however when both the NSCLC cell lines received exposure of other members of IL family such as IL-6, IL-1β and IL-8 at concentration of 80 ng/mL. did not cause any significant change in levels of VCAM-1 (Figure 1H). These findings indicate that IL-2 suppressed the expression levels of miR-26a and upregulated the levels of VCAM-1 in the NSCLC cells.
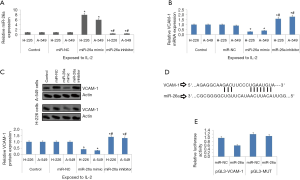
miR-26a modulated the levels of VCAM-1 in NSCLC cells
To evaluate the involvement of miR-26a in the expression of VCAM-1, both the NSCLC cell lines i.e., A-549 and H-226 cells were transfected with miR-26a mimic and inhibitor prior to exposing them to IL-2 (80 ng/mL). The outcomes of experiment demonstrated that transfection of miR-26a mimic upregulated the expression of miR-26a whereas inhibitor down-regulated it (Figure 2A). It was also found that the upregulated miR-26a inhibited the expression of VCAM-1 in both the NSCLC cell lines (Figure 2B,C). These outcomes demonstrate involvement of miR-26a in regulation of VCAM-1 via IL-2. Simultaneously, we performed bio-informatics analysis by searching the database of TragetScan, we found that the seed sequence of VCAM-1 had potential binding site for miR-26a (Figure 2D). For confirming the outcomes of bio-informatics analysis, we constructed plasmid having VCAM-1 3'-UTR or the mutant named pGL3-VCAM-1 or pGL3-Mutant respectively (Figure 2D). The 293T cells received transfection of miR-26a mimic and plasmids. The outcomes demonstrated that miR-26a halted the luciferase activity in the NSCLC cells transfected with pGL3-VCAM-1 and failed to do the same in cells transfected with pGL-3-mutant (Figure 2E). The outcomes confirmed that miR-26a modulated the expression VCAM-1 in NSCLC by binding to the 3'-UTR binding sequence.
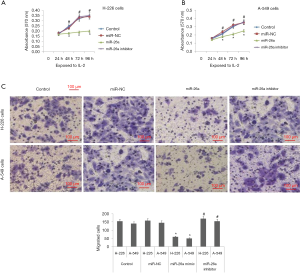
miR-26a modulates the proliferation and migration of NSCLC cell lines induced by IL-2
To evaluate the effect of miR-26a on behavior of NSCLC cells, the cells were transfected with miR-26a mimic and miR-26a inhibitor before exposing them to IL-2. The outcomes of proliferation studies suggested that miR-26a mimic halted in vitro proliferation of cells, whereas the inhibitor caused cell proliferation (Figure 3A,B). The results also suggested that miR-26a mimic caused a significant inhibition of cell migration in A-549 and H-226 cells induced by IL-2. However, it was also found that miR-26a inhibitor stimulated the cell migration (Figure 3C).
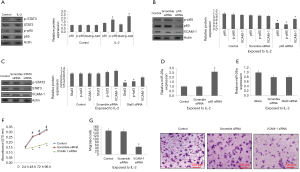
IL-2 regulates expression of miR-26a via NF-κB pathway
It was evidenced that IL-2 can lead to activation of STAT3 and NF-κB cascades (34,35). In the present work we evaluated the effects of IL-2 on STAT3 and NF-κB which are the inflammatory factors. We evidenced that IL-2 encouraged the activation of STAT3 and NF-κB in the A-549 cells (Figure 4A). In addition to this, STAT3 siRNA and p65 siRNA were transfected in the NSCLC cells for inhibiting their expression (Figure 4B,C). It was evidenced that inhibition of NF-κB blocked the expression of miR-26a (Figure 4D), whereas the inhibition of STAT3 did not influenced the expression of miR-26a (Figure 4E). It was also found that, inhibition of NF-κB decreased the levels of VCAM-1 (Figure 4B), which remained unaffected by inhibition of STAT3 in A-549 cells (Figure 4C). Also, expression of VCAM-1 in A-549 cells was blocked when transfected with VCAM-1 siRNA. Being a potential target of miR-26a, the study was directed to evaluate whether blockade of VCAM-1 showed the similar effects on A-549 cells mediated via miR-26a mimic (Figure 3). The findings demonstrated that viability of cells decreased along with their migration when VCAM-1 was blocked (Figure 4F,G). The findings suggested that NF-κB may play a potential role in expression of miR-26a via IL-2.
Discussion
It is established that the extracellular matrix compartment and the stromal and cellular compartment of tumor is involved in metastasis of NSCLC (36). The tumor micro-environment majorly constitutes of inflammatory cells, immune cells and macrophages (37). In a study earlier involving animal model of LC showed presence of Th17 and Treg cells in the tumor tissues and also suggested there potential role in tumorigenesis (38). In the current study, expression of miR-26a was negatively associated with expression of VCAM-1 and IL-2 in the LC tumor tissues, suggesting involvement of miR-26a in the progression of NSCLC cells. We used the LC cell lines A-549 and H-226 and evidenced that IL-2 acted as modulator for the expression of miR-26a in VCAM-1. Outcomes of the study suggested about the micro environment of tumor is full of inflammatory cells may affect the expression levels of VCAM-1 and may also lead to progression and migration of NSCLC cells.
VCAM-1 has been found to be implicated in number of pathological conditions such as infections (39), auto-immune disorders (40) and cardiac diseases (41). Recently, role of VCAM-1 in development, progression and metastasis in cancer has made the researchers to focus on it. In a study reported earlier for subjects with LC demonstrated VCAM-1 can be an indicator for diagnosis after chemotherapy (42). Also it was reported that the expression of VCAM-1 in the respiration condensates of LC subjects was high compared to subjects reported for COPD or the controls (43). In addition to this, levels of VCAM-1 in tumor tissues may lead to inhibition of T-cell function, promotion of T-cell migration and confab the capacity of tumor cells expressing VCAM-1 for preventing the attack from immunity (44).
In the present research, transfection of miR-26a mimic inhibited the levels of VCAM-1 mediated by IL-2, whereas miR-26a inhibitor encouraged the expression of VCAM-1 in presence of IL-2. The outcomes of luciferase studies demonstrated that miR-26a may bind VCAM-1 on its 3'-UTR region and also suppressed the expression protein levels of VCAM-1. Our experiments also demonstrated that miR-26a mimic showed adverse effect on proliferation and migration of NSCLC. Contrary to this, miR-26a inhibitor caused proliferation and migration of NSCLC under IL-2 treatment.
We also demonstrated that IL-2 activated the expression of STAT3 and NF-κB in A-549 cells. Knockdown of expression levels of NF-κB and not of STAT3 faded the inhibition of IL-2 mediated expression of miR-26a. Our findings thus established the important role of NF-κB in regulating the expression of miR-26a via IL-2, the findings were in agreement to earlier a study which have identifiedmiR-26a mediated the regulation of IL-2 expression in avian lymphocytelines (45). We also found the link between IL-2 induced cell adhesion and inflammation shown by NSCLC. As reported earlier, NF-kB has been found to mediate modulation of miR-26a in cardiac fibrosis (46).
Conclusions
Altogether, the present study demonstrated that IL-2 modulated the levels of VCAM-1 in LC cells through miR-26a and found VCAM-1 as potential target for binding of miR-26a. miR-26a could halt the migration and proliferation of NSCLC in presence of IL-2, hence suggesting that increasing the expression of miR-26a in NSCLC may be an potential way in dealing the progression of NSCLC. More studies using in vivo models involving miR-26a can support our findings.
Acknowledgments
The authors are thankful to the Affiliated Hospital of Inner Mongolian University for The Nationalities, Tongliao, Inner Mongolia, China, for providing necessary facilities and support.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.36). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocols were sanctioned by ethical committee of Affiliated Hospital of Inner Mongolian University for The Nationalities, Tongliao, Inner Mongolia, China. The present work complies with the guidelines for human studies and animal welfare. Informed consent was obtained from the subjects.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Zhao Y, Wang S, Aunan K, et al. Air pollution and lung cancer risks in China--a meta-analysis. Sci Total Environ 2006;366:500-13. [Crossref] [PubMed]
- She J, Yang P, Hong Q, et al. Lung cancer in China: challenges and interventions. Chest 2013;143:1117-26. [Crossref] [PubMed]
- Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol 2011;12:175-80. [Crossref] [PubMed]
- Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2009;59:27-41. [Crossref] [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 1976;193:1007-8. [Crossref] [PubMed]
- Malek TR. The biology of interleukin-2. Annu Rev Immunol 2008;26:453-79. [Crossref] [PubMed]
- Wang X, Lupardus P, Laporte SL, et al. Structural biology of shared cytokine receptors. Annu Rev Immunol 2009;27:29-60. [Crossref] [PubMed]
- Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013;38:13-25. [Crossref] [PubMed]
- Skrombolas D, Frelinger JG. Challenges and developing solutions for increasing the benefits of IL-2 treatment in tumor therapy. Expert Rev Clin Immunol 2014;10:207-17. [Crossref] [PubMed]
- Ganesh BB, Bhattacharya P, Gopisetty A, et al. IL-1β promotes TGF-β1 and IL-2 dependent Foxp3 expression in regulatory T cells. PLoS One 2011;6:e21949. [Crossref] [PubMed]
- Moody DB, Robinson JC, Ewing CM, et al. Interleukin-2 transfected prostate cancer cells generate a local antitumor effect in vivo. Prostate 1994;24:244-51. [Crossref] [PubMed]
- Mi D, Ren W, Yang K. Adoptive immunotherapy with interleukin-2 & induced killer cells in non-small cell lung cancer: a systematic review & meta-analysis. Indian J Med Res 2016;143:S1-10. [Crossref] [PubMed]
- Orditura M, Romano C, De Vita F, et al. Behaviour of interleukin-2 serum levels in advanced non-small-cell lung cancer patients: relationship with response to therapy and survival. Cancer Immunol Immunother 2000;49:530-6. [Crossref] [PubMed]
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006;6:857-66. [Crossref] [PubMed]
- Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res 2005;65:7065-70. [Crossref] [PubMed]
- Wang G, Zhang H, He H, et al. Up-regulation of microRNA in bladder tumor tissue is not common. Int Urol Nephrol 2010;42:95-102. [Crossref] [PubMed]
- Maillot G, Lacroix-Triki M, Pierredon S, et al. Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res 2009;69:8332-40. [Crossref] [PubMed]
- Yu T, Wang XY, Gong RG, et al. The expression profile of microRNAs in a model of 7,12-dimethyl-benz[a]anthrance-induced oral carcinogenesis in Syrian hamster. J Exp Clin Cancer Res 2009;28:64. [Crossref] [PubMed]
- Visone R, Pallante P, Vecchione A, et al. Specific microRNAs are downregulated in human thyroid anaplastic carcinomas. Oncogene 2007;26:7590-5. [Crossref] [PubMed]
- Huse JT, Brennan C, Hambardzumyan D, et al. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev 2009;23:1327-37. [Crossref] [PubMed]
- Kim H, Huang W, Jiang X, et al. Integrative genome analysis reveals an oncomir/oncogene cluster regulating glioblastoma survivorship. Proc Natl Acad Sci U S A 2010;107:2183-8. [Crossref] [PubMed]
- Smith NL, Wissink EM, Grimson A, et al. miR-150 regulates differentiation and cytolytic effector function in CD8+ T cells. Sci Rep 2015;5:16399. [Crossref] [PubMed]
- Zhong G, Cheng X, Long H, et al. Dynamically expressed microRNA-15b modulates the activities of CD8+ T lymphocytes in mice with Lewis lung carcinoma. J Transl Med 2013;11:71. [Crossref] [PubMed]
- Li Y, Sun Z, Liu B, et al. Tumor-suppressive miR-26a and miR-26b inhibit cell aggressiveness by regulating FUT4 in colorectal cancer. Cell Death Dis 2017;8:e2892. [Crossref] [PubMed]
- Deng M, Tang HL, Lu XH, et al. miR-26a suppresses tumor growth and metastasis by targeting FGF9 in gastric cancer. PLoS One 2013;8:e72662. [Crossref] [PubMed]
- Ji J, Shi J, Budhu A, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med 2009;361:1437-47. [Crossref] [PubMed]
- Alajez NM, Shi W, Hui AB, et al. Enhancer of Zeste homolog 2 (EZH2) is overexpressed in recurrent nasopharyngeal carcinoma and is regulated by miR-26a, miR-101, and miR-98. Cell Death Dis 2010;1:e85. [Crossref] [PubMed]
- Ichikawa T, Sato F, Terasawa K, et al. Trastuzumab produces therapeutic actions by upregulating miR-26a and miR-30b in breast cancer cells. PLoS One 2012;7:e31422. [Crossref] [PubMed]
- Ding YB, Chen GY, Xia JG, et al. Association of VCAM-1 overexpression with oncogenesis, tumor angiogenesis and metastasis of gastric carcinoma. World J Gastroenterol 2003;9:1409-14. [Crossref] [PubMed]
- Chaves KC, Peron JP, Chammas R, et al. Endostatin gene therapy stimulates upregulation of ICAM-1 and VCAM-1 in a metastatic renal cell carcinoma model. Cancer Gene Ther 2012;19:558-65. [Crossref] [PubMed]
- Los M, Schenk H, Hexel K, et al. IL-2 gene expression and NF-kappa B activation through CD28 requires reactive oxygen production by 5-lipoxygenase. EMBO J 1995;14:3731-40. [Crossref] [PubMed]
- Akaishi H, Takeda K, Kaisho T, et al. Defective IL-2-mediated IL-2 receptor alpha chain expression in Stat3-deficient T lymphocytes. Int Immunol 1998;10:1747-51. [Crossref] [PubMed]
- Wood SL, Pernemalm M, Crosbie PA, et al. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev 2014;40:558-66. [Crossref] [PubMed]
- Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007;7:41-51. [Crossref] [PubMed]
- Chang SH, Mirabolfathinejad SG, Katta H, et al. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci U S A 2014;111:5664-9. [Crossref] [PubMed]
- Sa Q, Ochiai E, Sengoku T, et al. VCAM-1/α4β1 integrin interaction is crucial for prompt recruitment of immune T cells into the brain during the early stage of reactivation of chronic infection with Toxoplasma gondii to prevent toxoplasmic encephalitis. Infect Immun 2014;82:2826-39. [Crossref] [PubMed]
- Jovanova-Nesic K, Shoenfeld Y. MMP-2, VCAM-1 and NCAM-1 expression in the brain of rats with experimental autoimmune encephalomyelitis as a trigger mechanism for synaptic plasticity and pathology. J Neuroimmunol 2006;181:112-21. [Crossref] [PubMed]
- Sun C, Alkhoury K, Wang YI, et al. IRF-1 and miRNA126 modulate VCAM-1 expression in response to a high-fat meal. Circ Res 2012;111:1054-64. [Crossref] [PubMed]
- Tas F, Karabulut S, Bilgin E, et al. Serum levels of vascular cell adhesion molecule-1 (VCAM-1) may have diagnostic, predictive, and prognostic roles in patients with lung cancer treated with platinum-based chemotherapy. Tumour Biol 2014;35:7871-5. [Crossref] [PubMed]
- Zhou F, Chen J, Tao G, et al. Increased levels of exhaled sICAM1, sVCAM1, and sE-selectin in patients with non-small cell lung cancer. Respir Med 2014;108:1670-6. [Crossref] [PubMed]
- Lin KY, Lu D, Hung CF, et al. Ectopic expression of vascular cell adhesion molecule-1 as a new mechanism for tumor immune evasion. Cancer Res 2007;67:1832-41. [Crossref] [PubMed]
- Xu H, Yao Y, Smith LP, et al. MicroRNA-26a-mediated regulation of interleukin-2 expression in transformed avian lymphocyte lines. Cancer Cell Int 2010;10:15. [Crossref] [PubMed]
- Wei C, Kim IK, Kumar S, et al. NF-κB mediated miR-26a regulation in cardiac fibrosis. J Cell Physiol 2013;228:1433-42. [Crossref] [PubMed]


