
Arsenic trioxide induces gasdermin E mediated pyroptosis in astroglioma cells
Introduction
Arsenic trioxide (ATO) has been considered as an anti-cancer activity in various malignancies with a large body of mechanisms. In acute promyelocytic leukemia (APL), ATO is an effective therapeutic intervention to improve the patients’ survival and remission (1). ATO could induce the degradation of PML-RARα to result in terminal differentiation and apoptosis which may result from the Bcl-2 downregulation and cytochrome c release (2). Recently, ATO was applied to the treatment of non-small cell lung cancer (NSCLC) by inducing cell cycle arrest (3). Moreover, ATO could trigger DNA damage and apoptosis in glioma cells (4,5).
Pyroptosis is a pro-inflammatory form of programmed cell death against pathogen infection, which is critical for the antimicrobial response (6). Prior results suggested pyroptosis was mainly observed in myeloid cells (7). Recent attention on the role of pyroptosis as a programmed cell death suggested that gasdermin E (GSDME)-mediated pyroptosis contributed to the toxicity of chemotherapy (8). Chemotherapy drugs could cause cancer cell cytotoxicity by activating caspase-3-mediated cleavage of GSMDE (9). More recently, it was reported that iron could activate reactive oxygen species (ROS) to trigger GSDME-mediated pyroptosis in melanoma cells (10).
ATO has been verified to attenuate the proliferation of glioblastoma cells through apoptosis (11). However, no evidence shows that GSDME-mediated pyroptosis is involved in ATO-induced cell death in glioblastoma cells. In the present study, we found that GSDME mediated pyroptosis took part in ATO induced cell death in astroglioma cells.
Methods
Cell culture and reagents
U251 cells were obtained from ATCC. All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and non-essential amino acids, 100 IU penicillin and 100 mg/mL streptomycin at 37 °C in a humidified incubator containing 5% CO2. GSDME antibody (ab215191) was obtained from Abcam. Caspase-3 antibody (9662S) was from Cell Signaling Technology. LaminB1 antibody (66095-1-Ig) was from Proteintech.
Cytotoxicity assay
LDH assay using CytoTox 96 Non-Radioactive Cytotoxicity Assay kit from Promega Corporation to measure cell viability and cell lysis according to the manufacturer’s instructions.
Immunoblotting
Western blotting was performed as previously described (12). Proteins were separated by SDS-PAGE and transferred to PVDF membrane. The membrane was blocked and incubated at 4 °C overnight with indicated first antibodies, followed by incubation with corresponding HRP-conjugated secondary antibodies. The protein bands were detected with an ECL. The luminescent signals of immunoblotting were analyzed by using an ImageQuan LAS 4000 Scanner (GE Healthcare).
Transmission electron microscopy
U251 cells were fixed with 2.5% (v/v) glutaraldehyde after stimulation with DMSO or ATO. The sample was prepared according to the standard protocol, and the cells’ morphology was observed with JEOL JEM21OOHC transmission electron microscopy.
Statistical analysis
All results are analyzed using the GraphPad 6.0 software and expressed as means ± standard error of the mean (SEM). The analysis was performed by two-tailed unpaired Student’s t-test and P<0.05 was considered statistically significant.
Results
GSDME was cleaved in the process of ATO treatment in U251 cells
To test whether GSDME was activated during the stimulation of ATO in U251 cells, GSDME protein level was assessed by Western blot, as it was suggested that GSDME was highly expressed in U251 cells (Figure 1). GSDME was reported to be cleaved by caspase-3 during chemotherapy drug treatment. We then tested whether GSDME in U251 cells was cleaved upon ATO treatment. As shown in Figure 1, GSDME was cleaved into two fragments in U251 cells stimulated with increasing concentrations of ATO for 24 h.
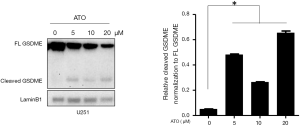
Caspase-3 was activated after ATO stimulation
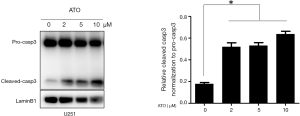
Recently, it was reported that activated caspase-3 could cleaved GSDME to execute pyroptosis. To confirm caspase-3 was activated by ATO in U251 cells, cleaved caspase-3 was detected through immunoblotting. As expected, caspase-3 was processed to an active form after ATO exposure (Figure 2). Thus, these results indicated that caspase-3 was activated during ATO-induced cell death in U251 cells.
LDH release was observed in the process of ATO-induced cell death
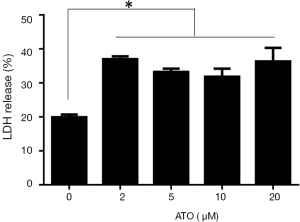
Prior reports suggested that LDH release was the specific marker for pyroptosis. To study whether LDH release occurred in the process of ATO stimulation, the level of LDH in the supernatant medium was monitored. As anticipated, we were able to observe the marked release of LDH in U251 cells after exposure to ATO treatment (Figure 3). Therefore, the administration of ATO for 24 h could initiate the significant release of LDH, indicating the integrity of the plasma membrane was disrupted and pyroptosis occurred.
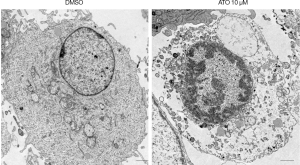
U251 treated with ATO was characterized by the rupture of the plasma membrane and cytoplasmic swelling
To further verify the cell death induced by ATO was pyroptosis, the morphologies of the ATO-induced cell death in U251 cells were analyzed through the transmission electronic microscopy (TEM). Indeed, U251 treated with ATO was characterized by the rupture of the plasma membrane and cytoplasmic swelling (Figure 4). Thus, these data confirmed that ATO stimulation can result in pyroptosis in U251 cells.
Discussion
ATO has been considered an environmental carcinogen. Until the last decade, it is explored as an anticancer drug for APL. While many research results focus on how ATO kills malignant myeloid cells through terminal differentiation and apoptosis, few reports have examined the role of other forms of cell death that are implicated in tumor cells after exposure to ATO.
In this study, we investigated ATO could induce pyroptosis in U251 cells. Although it is unknown whether pyroptosis plays a pivotal role in the treatment of APL and multiple myeloma using ATO. To better define the role of pyroptosis in ATO-induced growth inhibition in lymphoid and myeloid malignant cells, the experiment of xenograft tumor with or without GSDME expression in athymic mice needs to be established for further investigation.
Finally, our study markedly promotes the understanding of molecular mechanisms governing ATO-induced cell death. Pyroptosis may be important for developing new approaches for the combination therapy with ATO.
Acknowledgments
Funding: This work was supported by grants from
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived. This study was approved by the ethics committee of Zhongshan Hospital of Xiamen University (No. XMZSYY-AF-SC-02-03).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen GQ, Shi XG, Tang W, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood 1997;89:3345-53. [PubMed]
- Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 2008;111:2505-15. [Crossref] [PubMed]
- Zheng CY, Lam SK, Li YY, et al. Arsenic trioxide-induced cytotoxicity in small cell lung cancer via altered redox homeostasis and mitochondrial integrity. Int J Oncol 2015;46:1067-78. [PubMed]
- Cheng Y, Li Y, Ma C, et al. Arsenic trioxide inhibits glioma cell growth through induction of telomerase displacement and telomere dysfunction. Oncotarget 2016;7:12682-92. [PubMed]
- Wang J, Li Y, Jiang C. MiR-133b contributes to arsenic-induced apoptosis in U251 glioma cells by targeting the hERG channel. J Mol Neurosci 2015;55:985-94. [Crossref] [PubMed]
- Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev 2015;265:130-42. [Crossref] [PubMed]
- Robinson N, Ganesan R, Hegedus C, et al. Programmed necrotic cell death of macrophages: Focus on pyroptosis, necroptosis, and parthanatos. Redox Biol 2019;26:101239. [Crossref] [PubMed]
- Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017;547:99-103. [Crossref] [PubMed]
- Zhang CC, Li CG, Wang YF, et al. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis 2019;24:312-25. [Crossref] [PubMed]
- Zhou B, Zhang JY, Liu XS, et al. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res 2018;28:1171-85. [Crossref] [PubMed]
- Ghaffari SH, Yousefi M, Dizaji MZ, et al. Arsenic Trioxide Induces Apoptosis and Incapacitates Proliferation and Invasive Properties of U87MG Glioblastoma Cells through a Possible NF-kappaB-Mediated Mechanism. Asian Pac J Cancer Prev 2016;17:1553-64. [Crossref] [PubMed]
- Yang D, Liang Y, Zhao S, et al. ZBP1 mediates interferon-induced necroptosis. Cell Mol Immunol 2019; [Epub ahead of print]. [Crossref] [PubMed]


