
Rectal metastasis from a previously resected carcinoma of the pancreas: a case report
Introduction
Pancreatic cancer (PC) is the fifth most frequent cause of cancer death in the world (1,2), and its overall 5-year survival is less than 4% (3). At diagnosis, many cases are in well-advanced stages of metastasis and dissemination with peripheral invasion of the retroperitoneum vascular system or nerves (4-6), and the types of PC recurrence frequently involve liver metastasis (LM) and peritoneum dissemination (7-9). In contrast, rectal metastasis is extremely rare. Here, we report a case of an elderly patient with metastatic disease to the rectum who previously presented with a primary pancreatic adenocarcinoma. This case report was prepared following the CARE Guidelines (10).
Case presentation
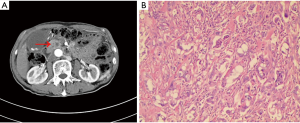
A 75-year-old man with a previous history of rectal polypectomy in 2013 underwent a pancreatoduodenectomy (PPPD) in December 2014 for pancreatic head cancer (Figure 1A). The patient didn’t have any special medical, family and psycho-social history. Histopathology showed an adenocarcinoma with perineuronal invasion (Figure 1B). Only one regional lymph node was resected, with no invasion. No cancer cells were found in the margin of the resected tissue, indicating an R0 resection. The pathologic staging of the adenocarcinoma was IIA stage (pT3N0M0) according to American Joint Committee of Cancer (AJCC) criteria, eighth edition. We didn’t collect genetic information because few mutations were found in pancreatic adenocarcinoma and there were few effective targeted therapies for pancreatic adenocarcinoma.
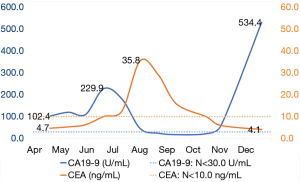
The patient received no further treatment after the surgery until April 2016, when an increase in serum carbohydrate antigen 19-9 (CA19-9), at 102.4 U/mL (N<30.0), was found during follow-up. Nine courses of chemotherapy with gemcitabine (1,000 mg/m2, days 1 and 8, 3 weeks for one cycle) and capecitabine (1,250 mg/m2, days 1 to 14, 3 weeks for one cycle) was applied, despite a lack of radiological [computed tomography (CT)] evidence of relapse or metastasis. For subsequent maintenance, the patient was treated for two courses with capecitabine (1,250 mg/m2, days 1 to 14, 3 weeks for one cycle). During chemotherapy, routine CT scans were performed every 2 months, with no sign of relapse or metastasis noted. The serum CA19-9 level had decreased to 17.2 U/mL in September 2016, but there was a sudden re-increase to 534.4 U/mL in December 2016 (Figure 2).
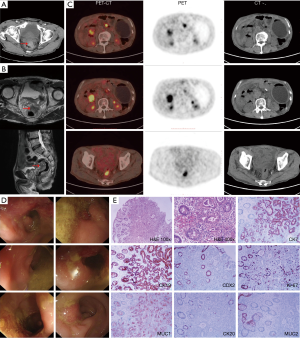
In January 2017, the patient started complaining of bloody stool with increased defecation frequency (3–4 times per day). Chest and abdomen CT on February 17 revealed a high-density shadow in the thickening wall of the cecum (Figure 3A). Magnetic resonance imaging (MRI) of the lower abdomen on February 27 revealed a mass located in the middle and lower section of the rectum, with the peritoneum refolded (Figure 3B). Multiple small lymph nodes around the tumor were noted. A soft-tissue shadow with high metabolic activity in front of the right kidney and multiple enlarged lymph nodes behind the retroperitoneum were observed by positron emission tomography-computed tomography (PET-CT) (Figure 3C). Colonoscopy revealed an irregular mass at about 5–7 cm from the anus in the rectum, occluding 50% of the lumen (Figure 3D). Histological examination (hematoxylin and eosin staining) of biopsy specimens taken from the mass revealed an adenocarcinoma. However, a further increase in serum CA19-9 to 3,426 U/mL prompted us to further explore the possibility of relapse or metastasis of the previous pancreatic adenocarcinoma. Subsequent immunohistological analysis showed the specimens to be positive for cytokeratin 7 (CK7), cytokeratin 19 (CK19), CDX2 protein, Ki-67 protein, mucoprotein 1 (MUC1), and negative for cytokeratin 20 (CK20) and mucoprotein 2 (MUC2) (Figure 3E). These results indicated that the rectum lesion might have originated from the pancreas adenocarcinoma that was previously resected.
From March 9, 2017, palliative radiotherapy (50 Gy/25 fractions) targeting the rectum neoplasm and pelvic lymph drainage was administered; however, in consideration of the patient’s poor physical status, concurrent chemotherapy was not applied. After radiotherapy, the serum CA19-9 level had decreased significantly on April 7 to 1,255 U/mL (Figure 4A). On May 24, the patient received rectal MRI, revealing a slightly shrunken tumor (Figure 4B). On July 7, the patient was admitted to the emergency department for a severely distended abdomen. CT examination revealed an enlarged gastric cavity with retention of contents and an irregular soft tissue shadow in front of the right kidney (Figure 4C). The patient ultimately died on August 28 due to the increased tumor load (Table 1).
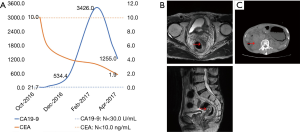
Table 1
| Time | Treatment [T]/symptoms [S]/examination [E] |
|---|---|
| 2013 | [T] Rectal polypectomy |
| Dec. 2014 | [T] PPPD |
| Apr. 2016 | [E] Elevated Serum CA19-9 at 102.4 U/mL (N<30.0) |
| Apr. 2016 | [T] Chemotherapy with gemcitabine and capecitabine |
| Oct. 2016 | [T] Chemotherapy with capecitabine for maintenance therapy |
| Sep. 2016 | [E] Serum CA19-9 level decreased to 17.2 U/mL |
| Dec. 2016 | [E] Serum CA19-9 suddenly re-increased to 534.4 U/mL |
| Jan. 2017 | [S] Bloody stool with defecation frequency increased (3–4 times per day) |
| Feb. 2017 | [E] Chest and abdomen CT revealed a high-density shadow with wall thickening of the cecum |
| [E] Magnetic resonance imaging (MRI) of the lower abdomen revealed a mass located in the middle and lower section of the rectum, with the peritoneum refolded | |
| [E] Serum CA19-9 at 3,426 U/mL | |
| [E] Immunohistological analysis indicated metastasis | |
| Mar. 9. 2017 | [T] Palliative radiotherapy started |
| Apr. 7, 2017 | [T] Palliative radiotherapy ended |
| [E] Serum CA19-9 level decreased significantly to 1,255 U/mL | |
| May 24, 2017 | [E] Rectal magnetic resonance imaging revealed that the tumor was slightly shrunken |
| Jul. 7, 2017 | [T] The patient was admitted to the emergency department |
| [E] CT examination revealed an enlarged gastric cavity with retention of contents and irregular soft tissue shadow in front of right kidney | |
| Aug. 28, 2017 | [S] The patient died |
PPPD, pancreatoduodenectomy; CA19-9, carbohydrate antigen 19-9; CT, computed tomography.
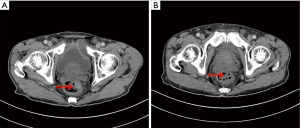
A recheck of the previous CT images from November 2017 showed a small, high-density shadow inside the rectal wall (Figure 5A) that was not observed in the images from September 2017 (Figure 5B). Because of the patient’s previous history of rectal polypectomy, the shadow was neglected by both the radiologist and the oncologist.
Discussion
PC is one of the leading causes of cancer mortality and one of the most lethal malignant neoplasms worldwide (11). Approximately 60–70% of PCs arise in the head of the pancreas, whereas 20–25% arise in the body and the tail (12). The two main pathological types of PC are adenocarcinoma (approximately 85% of cases) and pancreatic endocrine tumors (less than 5% of all cases) (13). Despite the use of multiagent chemotherapy and radiotherapy, PC treatment has remained a great challenge because few patients are eligible for resection and the median survival is only 6 to 12 months for those with metastatic diseases. Pancreatic adenocarcinoma usually metastasizes to regional lymph nodes, the liver, adjacent organs and the lung (14,15). However, metastasis to the rectum is so rare that no such case has been reported to date. In this case, the mass found on the rectum would have been misdiagnosed as primary rectal cancer if immunohistological analysis had not been performed, as H&E staining only suggested an adenocarcinoma. Therefore, it is crucial to conduct a comprehensive analysis of medical history, tumor biomarkers, and a series of thorough examinations, including immunohistochemistry if necessary, to diagnose gastrointestinal adenocarcinomas in patients with a previous history of pancreatic adenocarcinoma.
Common routes of pancreatic adenocarcinoma metastasis include lymphatic metastasis, hematogenous metastasis and implantation metastasis. Because the pancreas and the lower part of the rectum are both extraperitoneal organs, it is anatomically possible for tumor cells to break through the capsule of the pancreas and seed directly into the serosa of the rectum. In this case, PET-CT revealed multiple abdominal metastases, especially in the retroperitoneum, possibly implicating implantation metastasis.
In this case, CA19-9 and carcinoembryonic antigen (CEA) detection showed good sensitivity and predictive value of a curative effect. In addition, according to changes in CA19-9 and CEA levels, the tumor was relatively sensitive to chemotherapy and radiotherapy. Regardless, this tumor exhibits a tendency toward widespread abdominal metastases, and its overall prognosis is very poor.
Collectively, rectal metastasis is a rare metastasis site of PC, which may cause misdiagnosis. If possible, genetic testing should be carried out to determine the molecular characteristics of the tumor, which may help up to have a deeper understanding of it.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.74). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Malvezzi M, Bertuccio P, Levi F, et al. mortality predictiEuropean cancer ons for the year 2014. Ann Oncol 2014;25:1650-6. [Crossref] [PubMed]
- Hijioka S, Shimizu Y, Mizuno N, et al. Can long-term follow-up strategies be determined using a nomogram-based prediction model of malignancy among intraductal papillary mucinous neoplasms of the pancreas? Pancreas 2014;43:367-72. [Crossref] [PubMed]
- Allendorf JD, Lauerman M, Bill A, et al. Neoadjuvant chemotherapy and radiation for patients with locally unresectable pancreatic adenocarcinoma: feasibility, efficacy, and survival. J Gastrointest Surg 2008;12:91-100. [Crossref] [PubMed]
- Rosenblatt R, Dorfman V, Epelboym I, et al. Demographic features and natural history of intermediate-risk multifocal versus unifocal intraductal papillary mucinous neoplasms. Pancreas 2015;44:478-83. [PubMed]
- Chang ST, Nguyen DC, Raptis C, et al. Natural history of preoperative subcentimeter pulmonary nodules in patients with resectable pancreatic adenocarcinoma: a retrospective cohort study. Ann Surg 2015;261:970-5. [Crossref] [PubMed]
- Poruk KE, Firpo MA, Adler DG, et al. Screening for pancreatic cancer: why, how, and who? Ann Surg 2013;257:17-26. [Crossref] [PubMed]
- Butturini G, Stocken DD, Wente MN, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg 2008;143:75-83; discussion 83. [Crossref] [PubMed]
- Kim J, Lee YS, Hwang IK, et al. Postoperative carcinoembryonic antigen as a complementary tumor marker of carbohydrate antigen 19-9 in pancreatic ductal adenocarcinoma. J Korean Med Sci 2015;30:259-63. [Crossref] [PubMed]
- Shrikhande SV, Kleeff J, Reiser C, et al. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol 2007;14:118-27. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694-705. [Crossref] [PubMed]
- Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v56-68. [Crossref] [PubMed]
- Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607-20. [Crossref] [PubMed]
- Tani M, Kawai M, Miyazawa M, et al. Liver metastasis as an initial recurrence has no impact on the survival of patients with resectable pancreatic adenocarcinoma. Langenbecks Arch Surg 2009;394:249-53. [Crossref] [PubMed]
- Voutsadakis IA, Doumas S, Tsapakidis K, et al. Bone and brain metastases from ampullary adenocarcinoma. World J Gastroenterol 2009;15:2665-8. [Crossref] [PubMed]


