
Pretreatment neutrophil-to-lymphocyte ratio is a predictive biomarker for EGFR TKI-treated patients with advanced EGFR-mutant Non-small cell lung cancer
Introduction
Lung cancer remains one of the most common cancers and the leading cause of cancer-related mortality in China and worldwide (1-4). About 85% of lung cancers are non-small cell lung cancer (NSCLC) and most of the newly diagnosed patients are already at advanced stages (4-6). For the patients with epidermal growth factor receptor (EGFR) mutation-positive advanced NSCLC, EGFR tyrosine kinase inhibitors (TKIs) are the first-line treatment of choice (7).
Tumor-node-metastasis (TNM) staging is a significant prognostic factor for cancer patients, but its prognostic value for advanced cancer patients is limited (8). Many studies have found that the prognosis of advanced NSCLC patients is not only related to the staging but also closely related to clinical features of individual patients (9,10). It has been demonstrated that the systemic inflammation is involved in the initiation, development, and progression of several types of tumors. For example, increasing evidence has revealed that cancer-associated inflammation plays a crucial role in the clinical outcomes in colorectal cancer and NSCLC (11,12), and the neutrophil-lymphocyte ratio (NLR) has been reported to be one of the inflammation indicators which can be a potential predictor of treatment benefit (13,14).
There were very limited studies on the correlation of NLR with the clinical outcome of advanced EGFR-mutant NSCLC treated with TKIs, and the results of them were inconsistent and contradictory (15-17). Notably, a recent study reported that NLR was not a significant prognostic factor in EGFR-mutant NSCLC patients treated with TKIs (16). The prognostic significance of NLR for these lung cancer patients remains inconclusive. Therefore, we performed this retrospective study to investigate the correlation between NLR and the outcomes of patients, aiming to clarify the prognostic value of NLR in the advanced EGFR-mutant NSCLC patients treated with EGFR-TKIs.
Methods
Patient selection and treatment protocols
This was a retrospective study on patient data retrieved from hospital medical record system, an informed consent form was not required; the patient’s personal data have been secured. Sixty-five cases were hospitalized in the Cancer Center of the First Affiliated Hospital of Guangzhou University of Chinese Medicine (a teaching hospital and clinical research base of Macau University of Science and Technology) from January 1st, 2015 to October 1st, 2017. All the included patients were definitively diagnosed with stage III/IV NSCLC via imaging and pathological examinations.
The inclusion criteria for patients were as follows: (I) pathologically confirmed NSCLC and the initial stages were IIIB, IIIC, IVA or IVB; (II) molecularly identified activating mutations in the EGFR gene by amplification refractory mutation system (ARMS)-PCR or next-generation sequencing (NGS); (III) not treated with any anti-tumor drugs before inclusion; (IV) TKIs drugs were administrated regularly except for cases with disease progression or unacceptable toxicity; (V) normal liver, kidney functions, and absent of any serious diseases, acute or chronic hematological diseases, rheumatic autoimmune diseases, chronic liver disease, chronic renal insufficiency, immune diseases, and active infections, etc.; (VI) within 1 month before admission, not treated with anti-tuberculosis medication, hormone therapy or other treatments which might affect the blood system.
All the included patients received the first-line EGFR-TKIs treatment and did not switch their medicine during the treatment unless the disease progressed. Clinical features of all the patients, including gender, age, smoking status, differentiation, tumor stage, and complete blood cell (CBC) counts, were recorded before the first cycle of EGFR-TKIs therapy. All the pretreatment CBC counts were performed within 3 days before the first treatment. NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count.
Evaluation and follow-up
Patients were followed up and evaluated after EGFR-TKIs treatment, the last follow-up was March 1, 2018. All data were collected from medical records. According to Response Evaluation Criteria in Solid Tumors (RECIST) (18), complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were the common indicators for evaluating the objective response. The primary outcomes of interest were disease control rate (DCR), progression-free survival (PFS). DCR was defined as the proportion of cases with CR or PR or SD. PFS was defined as the time from the date of commencing treatment to the date of first progression or death from any cause without progression, and calculated every month.
Statistical analysis
All the data were analyzed using SPSS 23.0 software (SPSS, Chicago, IL, USA). Chi-square test was used to compare the categorical variable between the groups, and the numerical variables were analyzed by t-test or Mann-Whitney U test. The median value of NLR (2.57) was assigned to be the NLR cut-off point. The PFS curves were plotted by Kaplan-Meier analysis and log-rank test. Univariate and multivariate Cox regression analyses were used to determine the factors affecting PFS. P<0.05 was considered statistically significant.
Results
Baseline characteristics of the included patients
The deadline for inclusion was October 1, 2017. Thirty-one (47.7%) of these patients were men, forty-four (67.7%) were under the age of 65 years, and forty-seven (72.3%) patients were non-smokers. The pathological type of most patients was adenocarcinoma (n=55). The EGFR mutations identified and described in the medical records of the included patients were: an in-frame deletion in exon 19 in thirty-nine patients; an L858R point mutation in exon 21 in twenty-two patients; and no detailed description of specific EGFR mutations in the medical records of 4 patients. A total of 65 patients were included in the final analysis, and 18 patients did not show disease progression until the deadline for follow-up. The clinicopathological characteristics of the 65 patients were shown in Table 1.
Table 1
| Characteristics | N |
|---|---|
| Age (years) | |
| ≤65 | 44 (67.7%) |
| >65 | 21 (32.3%) |
| Gender | |
| Male | 31 (47.7%) |
| Female | 34 (52.3%) |
| Smoking | |
| Never-smoker | 47 (72.3%) |
| Current or ex-smoker | 18 (27.7%) |
| Neoplasm staging | |
| Stage IIIB/IIIC | 12 (18.5%)/1 (1.5%) |
| Stage IVA/IVB | 25 (38.5%)/27 (41.5%) |
| Pathological type | |
| Squamous carcinoma | 10 (15.4%) |
| Adenocarcinoma | 55 (84.6%) |
| Differentiation degree | |
| Poorly differentiated | 21 (32.3%) |
| Moderately differentiated | 32 (49.2%) |
| Well-differentiated | 12 (18.5%) |
| NLR (median/mean ± SD) | 2.57/3.87±2.92 |
| Prescribed EGFR-TKI drugs | |
| Gefitinib | 28 (43.1%) |
| Icotinib | 20 (30.8%) |
| Erlotinib | 9 (13.8%) |
| Afatinib | 5 (7.7%) |
| Osimertinib | 3 (4.6%) |
| PFS (months) (median/mean ± SD) | 10/10.4±4.3 |
EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor; NLR, neutrophil-to-lymphocyte ratio; PFS, progression-free survival; SD, standard deviation.
Correlation of pretreatment NLR with clinicopathological characteristics
The included patients were divided into different groups based on various clinicopathological characteristics: age (≤65 vs. >65 years), gender (male vs. female), smoking status (never-smoker vs. smoker), pathological type (squamous carcinoma vs. adenocarcinoma), differentiation degree (poor differentiation vs. moderate differentiation vs. well differentiation), and TNM staging (stage IIIB/IIIC vs. stage IVA/IVB). The association of NLR with clinicopathological features was analyzed. The results showed that NLR of the patients with smoking history was significantly higher than that of patients without smoking history (P=0.001); NLR of the patients with squamous cell carcinoma was significantly higher than that of patients with adenocarcinoma (P=0.029); NLR of the patients at stage IVA/IVB was significantly higher than that of the patients at stage IIIB/IIIC (P=0.007), and NLR of the patients with poorly differentiated cancer was significantly higher than that of the patients with well or moderately differentiated cancer (P=0.041, 0.048, respectively). The correlation of pretreatment NLR with various clinicopathological characteristics was presented in Tables 2 and 3. There was no significant difference in NLR between groups based on age or gender (P=0.751, 0.385, respectively).
Table 2
| Variables | N | Mean | SD | P value |
|---|---|---|---|---|
| Age (years) | 0.751 | |||
| ≤65 | 44 | 3.95 | 3.18 | |
| >65 | 21 | 3.7 | 2.32 | |
| Gender | 0.385 | |||
| Male | 31 | 3.54 | 2.48 | |
| Female | 34 | 4.17 | 3.27 | |
| Smoking | 0.001 | |||
| Never-smoker | 47 | 3.16 | 2.02 | |
| Current or ex-smoker | 18 | 5.74 | 3.99 | |
| Pathological type | 0.029 | |||
| Squamous carcinoma | 10 | 6.88 | 4.30 | |
| Adenocarcinoma | 55 | 3.32 | 2.24 | |
| TNM staging | 0.007 | |||
| Stage IIIB/IIIC | 13 | 2.78 | 0.73 | |
| Stage IVA/IVB | 52 | 4.14 | 3.19 |
NLR, neutrophil-to-lymphocyte ratio; SD, standard deviation.
Table 3
| Tumor differentiation | N | Mean | SD | P value |
|---|---|---|---|---|
| Type 1 | 0.048 | |||
| Poorly differentiated | 21 | 5.69 | 4.07 | |
| Moderately differentiated | 32 | 3.03 | 1.49 | |
| Type 2 | 0.041 | |||
| Poorly differentiated | 21 | 5.69 | 4.07 | |
| Well-differentiated | 12 | 2.94 | 1.95 | |
| Type 3 | 0.394 | |||
| Moderately differentiated | 32 | 3.03 | 1.49 | |
| Well-differentiated | 12 | 2.94 | 1.95 |
NLR, neutrophil-to-lymphocyte ratio; SD, standard deviation.
Correlation between pretreatment NLR and DCR of patients treated with EGFR-TKIs
Pretreatment NLR of all the included patients was 3.87±2.92 (mean ± standard deviation), and 2.57, the median value of NLR, was assigned to be the NLR cut-off point. According to the cut-off value, the patients were divided into the low NLR group (<2.57) and the high NLR group (≥2.57). There were 32 patients in the low NLR group and 33 in the high NLR group. The efficacy of EGFR-TKIs treatment was evaluated by the end of 2, 4, 6, 8 and 10 months. The results showed that the DCR of patients in the higher NLR group was notably lower than those in the low NLR group by the end of 8 and 10 months; the differences were statistically significant (P=0.014, 0.037, respectively). The difference in DCR between groups by the end of 2, 4, and 6 months was not statistically significant (P=0.492, 0.492, 0.606, respectively) (Table 4).
Table 4
| DCR | NLR <2.57 | NLR ≥2.57 | P value |
|---|---|---|---|
| 2-month DCR | 100% | 93.9% | 0.492 |
| 4-month DCR | 100% | 96.8% | 0.492 |
| 6-month DCR | 96.8% | 93.1% | 0.606 |
| 8-month DCR | 93.3% | 62.5% | 0.014 |
| 10-month DCR | 77.8% | 38.5% | 0.037 |
NLR, neutrophil-to-lymphocyte ratio; DCR, disease control rate.
Correlation between pretreatment NLR and PFS of patients treated with EGFR-TKIs
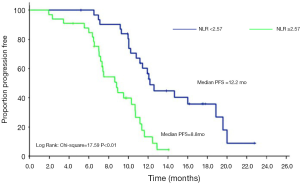
The median PFS of all the included patients was 10 months. As illustrated in Figure 1, patients with high NLR (≥2.57) had shorter PFS than those with low NLR (<2.57) after EGFR-TKI treatment (8.8 vs. 12.2 months); the difference between groups was statistically significant (P<0.01).
Univariate and multivariate Cox regression analyses of potential factors affecting PFS
We performed univariate and multivariate analyses to identify the potential factors affecting PFS. The age, gender, history of smoking, pathological type, neoplasm staging, tumor differentiation, NLR level (with NLR of 2.57 as the cut-off, NLR ≥2.57 as the high NLR group, NLR <2.57 as the low NLR group) were included in the univariate analysis. The results showed that NLR level, pathological type and smoking history were related with PFS of patients (P<0.001, =0.002 and =0.011, respectively) (Table 5). High NLR, pathological type of squamous carcinoma and history of smoking are the risk factors affecting PFS of patients with advanced NSCLC. We included age, pathological type, neoplasm staging, smoking history, tumor differentiation and NLR in the COX proportional hazard model for multivariate analysis. Multivariable analysis demonstrated that higher NLR [hazard ratio (HR) 3.560, 95% confidence interval (CI): 1.736–7.301, P<0.001], squamous carcinoma (HR 0.222, 95% CI: 0.087–0.567, P=0.002) were significantly associated with shorter PFS, indicating that NLR and pathological type were independent prognostic factors for PFS (Table 6).
Table 5
| Variables | HR (95% CI) | P |
|---|---|---|
| NLR (<2.57/≥2.57) | 3.959 (2.083–7.525) | <0.001 |
| Gender (male/female) | 0.964 (0.545–1.704) | 0.898 |
| Smoking (never-smoker/current or ex-smoker) | 2.32 (1.214–4.434) | 0.011 |
| Age (≤65/>65 years) | 1.448 (0.795–2.638) | 0.226 |
| Pathological type (squamous carcinoma/adenocarcinoma) | 0.302 (0.143–0.638) | 0.002 |
| TNM staging (stage III/IV) | 1.174 (0.544–2.536) | 0.682 |
| Tumor Differentiation (well/moderately/poorly) | 0.863 (0.550–1.353) | 0.520 |
NLR, neutrophil-to-lymphocyte ratio; PFS, progression-free survival; TNM, The TNM Classification of Malignant Tumors.
Table 6
| Variables | HR (95% CI) | P |
|---|---|---|
| NLR (<2.57/≥2.57) | 3.560 (1.736–7.301) | <0.001 |
| Pathological type (squamous carcinoma/adenocarcinoma) | 0.222 (0.087–0.567) | 0.002 |
NLR, neutrophil-to-lymphocyte ratio; PFS, progression-free survival; HR, hazard ratio.
Discussion
The hypothesis of the relationship between tumor and inflammation was first proposed by Virchow (19). More intensive research is providing further evidence that encompasses these microscopic to epidemiological perspectives. Clinical studies have confirmed the inextricable relationship between inflammation and tumor, such as the correlation of viral hepatitis and hepatocellular carcinoma, or Helicobacter pylori infection and gastric cancer, etc.
Both the tumor cells and their microenvironment contribute to the formation of the inflammatory microenvironment, which conversely promotes tumor growth and development. Some inflammatory indices, including NLR, have been found to be associated with tumor sufferers’ outcomes and the efficacy of anti-tumor therapy (13,14,20). However, the correlation of NLR with the prognosis of EGFR-mutant NSCLC patients treated with TKIs remains controversial. A previous study by Sim et al. reported that NLR was not associated with the prognosis of these TKIs-treated patients (16). The results of our study clearly indicated that DCR of the higher NLR group (NLR ≥2.57) was much lower than that of the low NLR group by the end of 8 and 10 months after TKIs treatment. Furthermore, through plotting the PFS curve of the enrolled patients, we found that the patients with high NLR levels had significantly shorter PFS than those with low NLR levels. EGFR-TKIs treatment was less effective in patients with high NLR levels than those with low NLR, which was consistent with the results of some previous studies (15,17), suggesting that the elevated NLR could be a predictor of shorter survival in patients with advanced EGFR-mutant NSCLC.
The study by Sim et al. reported that NLR did not affect either the response rate or PFS, and was not a significant prognostic factor in EGFR-mutant NSCLC patients treated with TKIs (16), which were different from the results of our study. The differences between two studies might be related to the following reasons: (I) different methods to determine the NLR cut-off point: in Sim et al.’s study (16), receiver operating characteristic curve analysis was performed to determine the NLR cut-off, and NLR >3.0 was considered to be high, while NLR ≤3.0 was considered to be low; in our study, the median value of NLR (2.57) was assigned to be the NLR cut-off point; (II) different time spot for blood cell counts: in Sim et al.’s study, the CBC counts were performed within 31 days of commencing first-line treatment (after treatment) and at different times in different patients; in our research, all the pretreatment CBC counts were tested within 3 days before the first treatment and at almost the same time in all patients; (III) different distribution of pathological types: in Sim et al.’s study, 91.6% of patients had lung adenocarcinoma tumors; in our study, 84.6% of patients had adenocarcinoma. By comparing these two studies, we hypothesize that there are probably some factors affecting the predictive value of NLR for advanced EGFR mutation-positive NSCLC treated with TKIs, with more research needed to identify the potential influencing factors and their effects. For example, the method to determine the NLR cut-off point and the time spot for the CBC counts, etc. could be considered. A meta-analysis published in June 2019 also confirmed that the pretreatment NLR was a promising prognostic biomarker for NSCLC patients receiving targeted therapy (21), but, it is still necessary to further investigate the specific factors leading to the diversity of the results, and how to appropriately use NLR as a predictive biomarker for lung cancer patients.
Due to the short follow-up period, the overall survival (OS) of all patients was not evaluated, but the patients’ PFS could, to some extent, predict the prognosis of NSCLC patients. The results of the present study also showed that a higher NLR was significantly associated with smoking history, squamous carcinoma, stage IVA/IVB, or poor differentiation in our cohort. Age and gender were not associated with NLR levels. With the multivariate regression analysis, we found that NLR and pathological type were the independent risk factors for PFS, but the tumor stage and differentiation did not show significant effects on PFS. Possible reasoning points to our study, in which most of the included patients were at similarly advanced stages (IIIB–IVB) and most of the patients were with moderately or poorly differentiated cancer. The differences between the subgroups based on tumor stage and differentiation were not statistically significant. The relatively small sample size of our study might also affect the statistical significance of the results. More studies are needed to further investigate the effects of tumor stage and differentiation on the survival outcomes.
In our study, all the included participants were molecularly identified activating mutations in the EGFR gene. Among the 65 included patients, 55 patients were with adenocarcinoma and 10 with squamous carcinoma, 47 patients were never-smokers, 18 were current or ex-smokers. EGFR mutations are more common in lung adenocarcinoma and non-smokers. Our data show that the EGFR mutations are not rare in lung squamous carcinoma and smokers, which are consistent with some previous studies in the Chinese population. A recent study showed that EGFR with TKI-sensitive mutations in exon 19 was highly expressed and frequently detected in Chinese patients with lung squamous carcinoma (22). And according to a meta-analysis based on the data from six medical centers in mainland China, among the patients with NSCLC who received EGFR mutation analysis, patients with non-adenocarcinoma had an EGFR mutation rate of 9.2%, and the EGFR mutation rate for smokers was 15.1% (23). Another study on the rates of EGFR mutations also reported that, in current smokers and former smokers, the rates of the mutations were 6% and 15%, respectively (24).
Increased NLR means an increase in neutrophils and/or a decrease in lymphocytes. Although the neutrophils have the role of killing tumor cells, recent studies have found that the degree of neutrophil infiltration in tumor tissues was inversely related to the prognosis of the tumor. Recent evidence has shown that some populations of neutrophils, such as tumor-associated neutrophils (TANs), could enhance tumor progression and metastasis (25); TANs contribute to the tumor invasion and angiogenesis through the production of matrix metalloproteinase-9 (MMP9) and vascular endothelial growth factor (VEGF) in the primary and metastatic sites (26). Neutrophils can accelerate tumorigenesis on premalignant epithelial cells as well as initiate and engage in complex crosstalk with other immune cells throughout their development and activation (27). The tumor-associated neutrophils can stimulate T cell responses to further aggravate inflammatory responses through positive feedback in human lung cancer (28). Lymphocytes are the main immune cells of the body, with T lymphocytes and NK cells both playing an immune role in tumors. T lymphocytes in particular have an inhibitory effect on distant metastasis of tumors (29,30). As NLR is the ratio of neutrophils to lymphocytes, the increase of NLR in cancer patients suggests that the body’s anti-tumor function might decrease and tumor progression or metastasis is more likely to occur. On the contrary, the body’s anti-tumor function is elevated when NLR decreases, therefore, NLR can be used as an indicator to predict the prognosis of tumor, and its dynamic changes can also reflect the current anti-tumor status, thus providing an objective indicator for clinical evaluation. Moreover, the NLR index can be obtained by a routine blood test of peripheral blood, which is convenient, rapid, inexpensive, and repeatable. Its clinical value cannot be ignored. It can be used to evaluate the efficacy of clinical treatment, predict the prognosis of patients, and help the clinicians choose optimal treatment regimens for NSCLC.
There are some limitations in our study: it is a retrospective cohort study which analyzes the pre-existing data, and is subject to some biases; the sample size included in our study is relatively small (n=65); most of the included patients were at similarly advanced stages (stage IIIB–IVB); the duration of follow-up was relatively short, etc.
In the included studies, the detection of NLR was performed before TKIs treatment. Many patients did not have regular re-examination of the laboratory parameters, including routine blood tests, thus resulting in the inability to analyze the dynamic change of NLR after TKIs treatment. Therefore, in the future study, the trend of NLR should be observed to dynamically evaluate the correlation of NLR with the outcomes of the patients.
Conclusions
In conclusion, the results of our study reveal that elevated NLR is significantly related with lower DCR, shorter PFS, and might act as a meaningful biomarker for predicting the efficacy of TKIs treatment and the prognosis of the advanced EGFR-mutant NSCLC. The findings could help doctors to identify the specific subsets of patients who may most likely benefit from EGFR TKI-targeted therapy. However, given the nature of the retrospective study, the limited sample size and relatively short follow-up period, additional large, high-quality prospective studies with longer follow-up are still needed to further confirm our findings.
Acknowledgments
We would like to thank Professor Xiongwen Wang, from the Cancer Center of the First Affiliated Hospital of Guangzhou University of Chinese Medicine, for his untiring support and overall coordination. We are grateful to Ms. Judy Kodela, from The Acupuncture Office, PLLC, NY, USA, for proofreading our manuscript.
Funding: This work was funded by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.28). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine, China (No. Y[2019]168). This is a retrospective study on patient data retrieved from hospital medical record system, an informed consent form is not required; the authors declare that the patient’s personal data have been secured. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in China, 2011. Chin J Cancer Res 2015;27:2-12. [Crossref] [PubMed]
- Wu X, Wu QB, Zhou XQ, et al. SphK1 functions downstream of IGF-1 to modulate IGF-1-induced EMT, migration and paclitaxel resistance of A549 cells: A preliminary in vitro study. J Cancer 2019;10:4264-9. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Wang X, Liu Z, Sui XB, et al. Elemene injection as adjunctive treatment to platinum-based chemotherapy in patients with stage III/IV non-small cell lung cancer: a meta-analysis following the PRISMA guidelines. Phytomedicine 2019;59:152787. [Crossref] [PubMed]
- Wang J, Li GC, Yu LL, et al. Aidi injection plus platinum-based chemotherapy for stage IIIB/IV non-small cell lung cancer: A meta-analysis of 42 RCTs following the PRISMA guidelines. J Ethnopharmacol 2018;221:137-50. [Crossref] [PubMed]
- Toh CK, Ong WS, Tan DS, et al. Improved Survival of Advanced Lung Cancer in Singapore Over the Past Decade. Ann Acad Med Singapore 2017;46:333-8. [PubMed]
- Tiefenbacher A, Pirker R. EGFR tyrosine kinase inhibitors as first-line therapy in advanced EGFR mutation-positive non-small cell lung cancer: strategies to improve clinical outcome. J Thorac Dis 2017;9:4208-11. [Crossref] [PubMed]
- Lababede O, Meziane MA. The Eighth Edition of TNM Staging of Lung Cancer: Reference Chart and Diagrams. Oncologist 2018;23:844-8.
- Kawai H, Saito Y, Suzuki Y. Gender differences in the correlation between prognosis and postoperative weight loss in patients with non-small cell lung cancer. Interact Cardiovasc Thorac Surg 2017;25:272-7. [Crossref] [PubMed]
- Wang X, Cao L, Li S, et al. Combination of PD-L1 expression and NLR as prognostic marker in patients with surgically resected non-small cell lung cancer. J Cancer 2019;10:6703-10. [Crossref] [PubMed]
- Kumano Y, Hasegawa Y, Kawahara T, et al. Pretreatment Neutrophil to Lymphocyte Ratio (NLR) Predicts Prognosis for Castration Resistant Prostate Cancer Patients Underwent Enzalutamide. Biomed Res In 2019;9450838.
- Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol 2017;23:6261-72. [Crossref] [PubMed]
- Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176-81. [Crossref] [PubMed]
- Zer A, Sung MR, Walia P, et al. Correlation of Neutrophil to Lymphocyte Ratio and Absolute Neutrophil Count With Outcomes With PD-1 Axis Inhibitors in Patients With Advanced Non-Small-Cell Lung Cancer. Clin Lung Cancer 2018;19:426-434.e1. [Crossref] [PubMed]
- Minami S, Ogata Y, Ihara S, et al. Neutrophil-to-Lymphocyte Ratio Predicts Overall Survival of Advanced Non-Small Cell Lung Cancer Harboring Mutant Epidermal Growth Factor Receptor. World J Oncol 2017;8:180-7. [Crossref] [PubMed]
- Sim SH, Beom SH, Ahn YO, et al. Pretreatment neutrophil-lymphocyte ratio is not a significant prognostic factor in epidermal growth factor receptor-mutant non-small cell lung cancer patients treated with tyrosine kinase inhibitors. Thorac Cancer 2016;7:161-6. [Crossref] [PubMed]
- Zhang Y, Feng YC, Zhu HG, et al. The peripheral blood neutrophil-to-lymphocyte ratio is a prognostic predictor for survival of EGFR-mutant nonsmall cell lung cancer patients treated with EGFR-TKIs. Medicine (Baltimore) 2018;97:e11648. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539-45. [Crossref] [PubMed]
- Lohinai Z, Bonanno L, Aksarin A, et al. Neutrophil-lymphocyte ratio is prognostic in early stage resected small-cell lung cancer. PeerJ 2019;7:e7232. [Crossref] [PubMed]
- Wang Z, Zhan P, Lv YL, et al. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio in non-small cell lung cancer patients treated with systemic therapy: a meta-analysis. Transl Lung Cancer Res 2019;8:214-26. [Crossref] [PubMed]
- Memon AA, Zhang H, Gu Y, et al. EGFR with TKI-sensitive mutations in exon 19 is highly expressed and frequently detected in Chinese patients with lung squamous carcinoma. Onco Targets Ther 2017;10:4607. [Crossref] [PubMed]
- Wu YL, Zhong WZ, Li LY, et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: a meta-analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol 2007;2:430-9. [Crossref] [PubMed]
- D'Angelo SP, Pietanza MC, Johnson ML, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol 2011;29:2066. [Crossref] [PubMed]
- Kim J, Bae JS. Tumor-Associated Macrophages and Neutrophils in Tumor Microenvironment. Mediators Inflamm 2016;2016:6058147. [Crossref] [PubMed]
- Mizuno R, Kawada K, Itatani Y, et al. The Role of Tumor-Associated Neutrophils in Colorectal Cancer. Int J Mol Sci 2019; [Crossref] [PubMed]
- Liang W, Ferrara N. The Complex Role of Neutrophils in Tumor Angiogenesis and Metastasis. Cancer Immunol Res 2016;4:83-91. [Crossref] [PubMed]
- Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest 2014;124:5466-80. [Crossref] [PubMed]
- Montoro J, Pinana JL, Sanz J, et al. T lymphocytes as therapeutic arsenal for patients with hematological malignancies. Curr Opin Oncol 2018;30:425-34. [PubMed]
- Raverdeau M, Cunningham SP, Harmon C, et al. gamma delta T cells in cancer: a small population of lymphocytes with big implications. Clin Transl Immunology 2019;8:e01080. [Crossref] [PubMed]


