Associations between polymorphisms in genes of base excision repair pathway and lung cancer risk
Introduction
Lung cancer (LC) is the most prevalent cancer and the main cause of cancer-specific death around the world, with a poor prognosis and a high mortality, there are about 228,150 new cases and 142,670 deaths of LC around the USA in 2019 (1). Non-small cell lung cancer (NSCLC) comprises 85% of all lung cancer, while small cell lung cancer (SCLC) accounts for 15–17% (2). The underlying mechanisms of LC remain unclear, however, a serious of studies indicated that tobacco smoking has been a high-risk factor (3-5). At the first years of this century, most evidence supported the notion that exposure to environmental carcinogens (6-9), including cigarette and electronic cigarette (10,11), result in alterations to the structural integrity of DNA and DNA lesions that may lead to mutations in oncogenes and tumor suppressor genes, thus initiating tumorigenesis (12-17).
The correlation between at-risk polymorphisms in genes of DNA repair pathways and LC risk was newly considered, reported from environmentally exposed workers or smokers (18-21). DNA repair pathway is a complex molecular network, which could continuously monitor and correct incorrect nucleotides after exposure to carcinogens, such as ultraviolet ray and benzene-based pollutants (22-24). There are several DNA repair pathways, which could minimize the mutant and toxic DNA sequence, including nucleotide excision repair (NER) pathway, base excision repair (BER) pathway, homologous recombination (HR) pathway, mismatch repair (MMR) pathway, as well as non-homologous end-joining (NHEJ) pathway. Among them, the BER is an essential pathway involved in genome stability maintaining and thus in human diseases’ prevention, ensuring to correct the abnormal DNA base modifications and base loss [such as apurinic/apyrimidinic (AP) sites] (25-27).
Recently, increasing studies indicated that DNA repair capacity could be influenced by genetic polymorphism in the BER pathway genes, which might also alter protein function that subsequently contributes to the unstable of gene sequence and cancer risk (28,29). Till now, numerous studies have focused on the potential relationship between genetic variants in BER pathway gene and LC risk, however, the results are discordant. In addition, many studies only focused on a few polymorphisms or neglected non-coding region genes, while other studies performed on a small number of cases. After all, we exhaustively extracted all eligible studies reported on genetic variations of BER pathway gene related to LC risk, and performing the current systematic review and meta-analysis to illustrated the overall relationship.
Methods
Obtain BER pathway gene set from KEGG
In order to obtain the whole gene set of BER pathway, we searched it on Kyoto Encyclopedia of Genes and Genomes (KEGG) website. Thirty-five genes in BER pathway were provided from online KEGG signaling database (http://software.broadinstitute.org/gsea/msigdb/geneset_page.jsp?geneSet Name=KEGG_BASE_EXCISION_REPAIR&keywords=BASE%20EXCISION%20REPAIR).
Study description
The resent study was conducted to reveal the correlation between genetic variants in BER pathway and LC risk. In current work, PubMed, Google Scholar, Medicine, EMbase and Web of Science databases were used to comprehensively enrolled and recorded all eligible publications. The retrieve formula was: (‘gene name’ OR ‘abbreviation of gene name’) AND (‘cancer’ OR ‘tumor’ OR ‘carcinoma’ OR ‘neoplasms’) AND (‘polymorphism’ OR ‘mutation’ OR ‘variant’ OR ‘SNP’ OR ‘genotype’). We also reviewed each reference of eligible articles, avoiding to missing any additional conform-to-criteria study. The entire retrieval was finished on October 5th, 2019. All enrolled studies were published in primary literature without any replication one. In addition, for these polymorphisms, whose eligible case-control studies are less than three will be excluded.
Enrolled criteria and exclusion criteria
There are several criteria which should be conformed are: (I) assessing whether the gene polymorphisms of BER pathway affect LC risk; (II) studies with specific case group and control group; and (III) genotype frequencies could be obtained directly or after calculating. Meanwhile, some other criteria should not be touched: (I) lacking control group, such as case-only study or review and (II) lacking sufficient genotype data.
Extraction of basic data
The ground on the enrollment standard mentioned above, all the basic data was extracted by two independent reviewers, accompany with an argument, discussion and reach an agreement. In each publication, several items were recorded, including the name of the first author, year of publication, ethnicity, source of control, number of each genotype group, and so on. Finally, we also estimated the quality of each enrolled study with the help of Newcastle-Ottawa Scale (NOS).
Statistical analysis
Hardy-Weinberg equilibrium (HWE) in the control group was tested, and P>0.05 means that the study does not deviate from HWE (30). Strength of the links between polymorphisms in BER pathway gene and LC risk was evaluated through calculating ORs and 95% CIs in five genetic models (W present for wild type allele; M present for mutant allele): allele contrast model (M vs. W), dominant contrast model (MM + MW vs. WW), recessive contrast model (MM vs. MW + WW), homozygous contrast model (MM vs. WW), and heterozygous contrast model (MW vs. WW). After that, subgroup analysis stratified by different items were also conducted. I2 statistics were used to evaluate the heterogeneity assumption between studies in each calculating group, aim to obtain the quantified inconsistency caused by heterogeneity (31). Among these studies, I2 value was regarded as a significant heterogeneity if it is higher than 50% (32), and random-effect model was performed the calculated the pooled OR and 95% CI; on the contrast, fixed-effect model will be hireling (33). To confirm the veracity of result, we use sensitivity analysis to assess the stability of results, use Begg’s funnel plot and Egger’s test to appraise any publication bias (34). We use STATA (version 12.0; STATA Corp.) to calculate all the results, and P<0.05 was regarded as statistically significant.
Results
The studies and meta-analysis data pool
After searching in diverse databases, we retrieved 116 publications comprising 202 case-control studies that met inclusion and exclusion criteria (at least three eligible case-control studies should be enrolled for each polymorphism). These publications concerned about five BER pathway gene, including X-Ray Repair Cross Complementing 1 (XRCC1), Apurinic/Apyrimidinic Endodeoxyribonuclease 1 (APEX1), DNA Ligase 1 (LIG1), 8-Oxoguanine DNA Glycosylase (OGG1) and MutY DNA Glycosylase (MUTYH) gene. In Table 1, characteristics and genotype frequency distributions of all enrolled studies for BER pathway gene were showed, including XRCC1-rs1799782/rs25487 (35-59), rs25489/rs3213245 (60-84), rs3547/rs915927 (85-90), PARP1-rs1136410 (87,91-94), APEX1-rs1130409/rs1760944/rs2307486 (42,43,47,74,76,79,80,89,92,95-101), LIG1-rs156641/rs20579/rs20581/rs3730931/rs439132 (64,71,102,103), OGG1-rs1052133 (43,47,49,70,72,74,84,85,89,92,104-126) and MUTYH-rs3219489 (104,115,118,127) polymorphisms, and the selection process of current work was described in Figure 1. For this study, we performed each process along with PRISMA 2009 checklist (Table 2), and with the aid of NOS, we also assessed each enrolled study, most of the enrolled study is higher than 7 star, which represented the good quality (129).
Table 1
| Gene-polymorphism | First author | Year | Ethnicity | Source of control | Case | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WW | MW | MM | WW | MW | MM | Y (HWE) | ||||||
| XRCC1-rs1799782 | David-Beabes et al. | 2001 | African | P-B | 142 | 10 | 2 | 205 | 36 | 2 | Y | |
| David-Beabes et al. | 2001 | Caucasian | P-B | 158 | 22 | 0 | 407 | 54 | 0 | Y | ||
| Chen et al. | 2002 | Asian | P-B | 48 | 44 | 11 | 57 | 40 | 5 | Y | ||
| Ratnasinghe et al. | 2003 | Asian | P-B | 52 | 47 | 9 | 85 | 104 | 21 | Y | ||
| Shen et al. | 2005 | Asian | P-B | 65 | 41 | 12 | 64 | 40 | 8 | Y | ||
| Chan et al. | 2005 | Asian | H-B | 50 | 22 | 3 | 79 | 67 | 16 | Y | ||
| Schneider et al. | 2005 | Caucasian | H-B | 389 | 53 | 4 | 544 | 75 | 3 | Y | ||
| Hung et al. | 2005 | Caucasian | H-B | 1878 | 259 | 10 | 1828 | 292 | 12 | Y | ||
| Hu et al. | 2005 | Asian | H-B | 335 | 311 | 64 | 339 | 308 | 63 | Y | ||
| Zienolddiny et al. | 2006 | Caucasian | P-B | 309 | 26 | 1 | 368 | 35 | 2 | Y | ||
| Landi et al. | 2006 | Caucasian | H-B | 263 | 32 | 1 | 262 | 53 | 1 | Y | ||
| Matullo et al. | 2006 | Caucasian | Mixed | 98 | 16 | 2 | 951 | 141 | 2 | Y | ||
| Hao et al. | 2006 | Asian | P-B | 524 | 409 | 91 | 572 | 459 | 87 | Y | ||
| De Ruyck et al. | 2007 | Caucasian | H-B | 101 | 8 | 1 | 93 | 17 | 0 | Y | ||
| Pachouri et al. | 2007 | Caucasian | P-B | 40 | 39 | 24 | 52 | 47 | 23 | N | ||
| Improta et al. | 2008 | Caucasian | P-B | 78 | 9 | 7 | 104 | 17 | 0 | Y | ||
| Yin et al. | 2008 | Asian | H-B | 120 | 98 | 23 | 119 | 109 | 21 | Y | ||
| Li et al. | 2008 | Asian | H-B | 184 | 136 | 30 | 196 | 133 | 21 | Y | ||
| Chang et al. | 2009 | African | P-B | 221 | 34 | 0 | 248 | 31 | 1 | Y | ||
| Yin et al. | 2009 | Asian | H-B | 29 | 21 | 1 | 28 | 38 | 8 | Y | ||
| Chang et al. | 2009 | Caucasian | P-B | 89 | 23 | 1 | 223 | 66 | 10 | Y | ||
| Tanaka et al. | 2010 | Asian | H-B | 28 | 15 | 7 | 25 | 23 | 2 | Y | ||
| Buch et al. | 2011 | Caucasian | H-B | 682 | 36 | 2 | 839 | 83 | 6 | N | ||
| Mei et al. | 2013 | Asian | P-B | 138 | 90 | 23 | 155 | 119 | 27 | Y | ||
| Du et al. | 2014 | Asian | P-B | 68 | 33 | 19 | 88 | 21 | 11 | N | ||
| Yoo et al. | 2014 | Asian | P-B | 281 | 249 | 67 | 268 | 255 | 54 | Y | ||
| Cătană et al. | 2015 | Caucasian | P-B | 89 | 3 | 10 | 197 | 22 | 3 | N | ||
| Han et al. | 2015 | Asian | P-B | 99 | 90 | 21 | 106 | 87 | 17 | Y | ||
| Zhu et al. | 2015 | Asian | P-B | 180 | 137 | 3 | 111 | 206 | 29 | N | ||
| Singh et al. | 2016 | Caucasian | P-B | 256 | 72 | 2 | 267 | 55 | 3 | Y | ||
| XRCC1-rs25487 | Divine et al. | 2001 | Caucasian | H-B | 82 | 61 | 29 | 65 | 64 | 14 | Y | |
| David-Beabes et al. | 2001 | African | P-B | 105 | 46 | 3 | 164 | 70 | 9 | Y | ||
| Ratnasinghe et al. | 2001 | Asian | P-B | 59 | 40 | 8 | 117 | 80 | 11 | Y | ||
| David-Beabes et al. | 2001 | Caucasian | P-B | 87 | 76 | 17 | 186 | 217 | 58 | Y | ||
| Chen et al. | 2002 | Asian | P-B | 55 | 43 | 5 | 52 | 40 | 7 | Y | ||
| Park et al. | 2002 | Asian | P-B | 100 | 75 | 17 | 81 | 48 | 6 | Y | ||
| Misra et al. | 2003 | Caucasian | P-B | 151 | 140 | 24 | 154 | 130 | 29 | Y | ||
| Zhou et al. | 2003 | Caucasian | P-B | 467 | 468 | 156 | 551 | 545 | 143 | Y | ||
| Harms et al. | 2004 | Caucasian | H-B | 59 | 42 | 9 | 56 | 55 | 8 | Y | ||
| Vogel et al. | 2004 | Caucasian | H-B | 117 | 104 | 35 | 108 | 121 | 40 | Y | ||
| Ito et al. | 2004 | Asian | H-B | 98 | 66 | 14 | 253 | 169 | 26 | Y | ||
| Popanda et al. | 2004 | Caucasian | H-B | 186 | 214 | 63 | 171 | 222 | 67 | Y | ||
| Liu et al. | 2004 | Caucasian | H-B | 400 | 397 | 138 | 551 | 539 | 143 | Y | ||
| Li et al. | 2005 | Asian | H-B | 22 | 20 | 8 | 27 | 21 | 2 | Y | ||
| Shen et al. | 2005 | Asian | P-B | 72 | 40 | 4 | 54 | 51 | 4 | Y | ||
| Chan et al. | 2005 | Asian | H-B | 40 | 31 | 4 | 90 | 61 | 11 | Y | ||
| Schneider et al. | 2005 | Caucasian | H-B | 199 | 198 | 49 | 264 | 280 | 78 | Y | ||
| Hu et al. | 2005 | Asian | H-B | 378 | 284 | 48 | 370 | 282 | 58 | Y | ||
| Zhang et al. | 2005 | Asian | H-B | 535 | 363 | 102 | 531 | 380 | 89 | Y | ||
| Hung et al. | 2005 | Caucasian | H-B | 844 | 951 | 254 | 874 | 881 | 260 | Y | ||
| Zienolddiny et al. | 2006 | Caucasian | P-B | 129 | 171 | 31 | 151 | 186 | 54 | Y | ||
| Hao et al. | 2006 | Asian | H-B | 566 | 376 | 82 | 585 | 432 | 101 | Y | ||
| Matullo et al. | 2006 | Caucasian | Mixed | 51 | 58 | 7 | 484 | 482 | 128 | Y | ||
| De Ruyck et al. | 2007 | Caucasian | H-B | 38 | 53 | 18 | 46 | 50 | 13 | Y | ||
| Yin et al. | 2007 | Asian | H-B | 138 | 65 | 2 | 132 | 52 | 9 | Y | ||
| Pachouri et al. | 2007 | Caucasian | P-B | 53 | 38 | 12 | 35 | 70 | 17 | Y | ||
| López-Cima et al. | 2007 | Caucasian | H-B | 222 | 219 | 75 | 217 | 234 | 82 | Y | ||
| Improta et al. | 2008 | Caucasian | P-B | 42 | 41 | 11 | 53 | 61 | 7 | N | ||
| Sreeja et al. | 2008 | Caucasian | P-B | 78 | 86 | 47 | 102 | 80 | 29 | N | ||
| Li et al. | 2008 | Asian | H-B | 168 | 139 | 43 | 201 | 123 | 26 | Y | ||
| Yin et al. | 2009 | Asian | H-B | 31 | 13 | 1 | 36 | 15 | 1 | Y | ||
| Cote et al. | 2009 | African | P-B | 86 | 23 | 6 | 88 | 28 | 5 | Y | ||
| Chang et al. | 2009 | African | P-B | 182 | 69 | 4 | 209 | 65 | 5 | Y | ||
| Chang et al. | 2009 | Caucasian | P-B | 54 | 47 | 12 | 155 | 127 | 16 | Y | ||
| Cote et al. | 2009 | Caucasian | P-B | 172 | 159 | 56 | 160 | 200 | 46 | Y | ||
| Li et al. | 2011 | Asian | H-B | 236 | 193 | 26 | 220 | 196 | 27 | Y | ||
| Kiyohara et al. | 2012 | Asian | H-B | 243 | 171 | 48 | 242 | 121 | 16 | Y | ||
| Natukula et al. | 2013 | Caucasian | P-B | 40 | 19 | 41 | 55 | 10 | 36 | N | ||
| Ouyang et al. | 2013 | Asian | P-B | 52 | 22 | 8 | 105 | 86 | 10 | Y | ||
| Mei et al. | 2013 | Asian | P-B | 142 | 95 | 14 | 145 | 126 | 30 | Y | ||
| Letkova et al. | 2013 | Caucasian | P-B | 138 | 202 | 42 | 157 | 185 | 37 | Y | ||
| Du et al. | 2014 | Asian | P-B | 81 | 16 | 23 | 95 | 15 | 10 | N | ||
| Sarlinova et al. | 2014 | Caucasian | P-B | 17 | 24 | 9 | 23 | 41 | 5 | N | ||
| Uppal et al. | 2014 | Caucasian | P-B | 18 | 32 | 50 | 12 | 65 | 23 | N | ||
| Saikia et al. | 2014 | Caucasian | P-B | 146 | 103 | 23 | 322 | 188 | 34 | Y | ||
| Yoo et al. | 2014 | Asian | P-B | 344 | 207 | 47 | 313 | 245 | 33 | Y | ||
| Han et al. | 2015 | Asian | P-B | 156 | 34 | 20 | 164 | 30 | 16 | N | ||
| Wang et al. | 2015 | Asian | P-B | 259 | 24 | 217 | 273 | 43 | 184 | N | ||
| Zhu et al. | 2015 | Asian | P-B | 221 | 80 | 19 | 269 | 72 | 5 | Y | ||
| Cătană et al. | 2015 | Caucasian | P-B | 43 | 43 | 16 | 112 | 86 | 24 | Y | ||
| Liu et al. | 2016 | Asian | P-B | 162 | 114 | 32 | 162 | 81 | 10 | Y | ||
| Singh et al. | 2016 | Caucasian | P-B | 93 | 186 | 51 | 79 | 176 | 70 | Y | ||
| XRCC1-rs25489 | Ratnasinghe et al. | 2001 | Asian | P-B | 83 | 20 | 3 | 177 | 32 | 0 | Y | |
| Misra et al. | 2003 | Caucasian | P-B | 260 | 47 | 2 | 260 | 42 | 0 | Y | ||
| Vogel et al. | 2004 | Caucasian | H-B | 229 | 26 | 1 | 241 | 28 | 0 | Y | ||
| Shen et al. | 2005 | Asian | P-B | 76 | 30 | 5 | 81 | 28 | 1 | Y | ||
| Schneider et al. | 2005 | Caucasian | H-B | 404 | 40 | 2 | 562 | 60 | 0 | Y | ||
| Hung et al. | 2005 | Caucasian | H-B | 1901 | 181 | 6 | 1896 | 190 | 6 | Y | ||
| Zienolddiny et al. | 2006 | Caucasian | P-B | 296 | 31 | 2 | 350 | 24 | 3 | N | ||
| Hao et al. | 2006 | Asian | H-B | 848 | 169 | 7 | 904 | 204 | 10 | Y | ||
| De Ruyck et al. | 2007 | Caucasian | H-B | 105 | 4 | 0 | 96 | 14 | 0 | Y | ||
| Yin et al. | 2008 | Asian | H-B | 190 | 46 | 2 | 179 | 59 | 4 | Y | ||
| Li et al. | 2008 | Asian | H-B | 266 | 79 | 5 | 74 | 72 | 4 | N | ||
| Yin et al. | 2009 | Asian | H-B | 41 | 7 | 1 | 52 | 18 | 2 | Y | ||
| Chang et al. | 2009 | Caucasian | P-B | 86 | 25 | 1 | 242 | 51 | 5 | Y | ||
| Yoo et al. | 2014 | Asian | P-B | 506 | 88 | 5 | 448 | 127 | 5 | Y | ||
| Han et al. | 2015 | Asian | P-B | 100 | 87 | 23 | 109 | 82 | 19 | Y | ||
| Singh et al. | 2016 | Caucasian | P-B | 32 | 250 | 48 | 26 | 268 | 31 | N | ||
| XRCC1-rs3213245 | Hu et al. | 2005 | Asian | H-B | 500 | 198 | 12 | 558 | 148 | 4 | Y | |
| Hao et al. | 2006 | Asian | H-B | 783 | 223 | 18 | 924 | 182 | 12 | Y | ||
| De Ruyck et al. | 2007 | Caucasian | H-B | 37 | 53 | 19 | 40 | 52 | 18 | Y | ||
| Li et al. | 2008 | Asian | H-B | 264 | 75 | 11 | 291 | 55 | 4 | Y | ||
| Hsieh et al. | 2009 | Asian | P-B | 251 | 40 | 3 | 250 | 37 | 1 | Y | ||
| Tang et al. | 2014 | Asian | P-B | 212 | 163 | 45 | 225 | 181 | 19 | N | ||
| Yoo et al. | 2015 | Asian | P-B | 494 | 104 | 4 | 462 | 111 | 4 | Y | ||
| XRCC1-rs3547 | Yin et al. | 2008 | Asian | H-B | 183 | 43 | 1 | 191 | 49 | 2 | Y | |
| Yin et al. | 2009 | Asian | H-B | 35 | 12 | 0 | 61 | 9 | 1 | Y | ||
| Chang et al. | 2009 | Caucasian | P-B | 62 | 45 | 6 | 177 | 99 | 23 | Y | ||
| Chang et al. | 2009 | African | P-B | 114 | 104 | 37 | 126 | 122 | 32 | Y | ||
| Singh et al. | 2016 | Caucasian | P-B | 61 | 142 | 127 | 124 | 127 | 74 | N | ||
| XRCC1-rs915927 | Matullo et al. | 2006 | Caucasian | Mixed | 36 | 58 | 22 | 342 | 508 | 243 | N | |
| Yin et al. | 2008 | Asian | H-B | 169 | 68 | 2 | 203 | 43 | 0 | Y | ||
| Yin et al. | 2009 | Asian | H-B | 36 | 14 | 1 | 66 | 7 | 0 | Y | ||
| Singh et al. | 2016 | Caucasian | P-B | 134 | 164 | 32 | 147 | 139 | 39 | Y | ||
| APEX1-rs1130409 | Misra et al. | 2003 | Caucasian | P-B | 64 | 167 | 79 | 65 | 160 | 77 | Y | |
| Ito et al. | 2004 | Asian | H-B | 62 | 84 | 32 | 159 | 226 | 64 | Y | ||
| Popanda et al. | 2004 | Caucasian | H-B | 135 | 235 | 89 | 118 | 233 | 106 | Y | ||
| Shen et al. | 2005 | Asian | P-B | 30 | 61 | 26 | 37 | 61 | 15 | Y | ||
| Zienolddiny et al. | 2006 | Caucasian | P-B | 117 | 67 | 80 | 138 | 60 | 122 | N | ||
| Matullo et al. | 2006 | Caucasian | P-B | 33 | 56 | 27 | 309 | 526 | 259 | Y | ||
| De Ruyck et al. | 2007 | Caucasian | H-B | 21 | 60 | 29 | 41 | 41 | 28 | N | ||
| Agachan et al. | 2009 | Caucasian | P-B | 38 | 40 | 20 | 45 | 17 | 5 | Y | ||
| Lu et al. | 2009 | Asian | H-B | 182 | 228 | 90 | 176 | 265 | 76 | Y | ||
| Lo et al. | 2009 | Asian | H-B | 261 | 349 | 119 | 272 | 332 | 118 | Y | ||
| Deng et al. | 2010 | Asian | P-B | 123 | 143 | 49 | 97 | 159 | 58 | Y | ||
| Li et al. | 2011 | Asian | H-B | 179 | 199 | 77 | 172 | 213 | 58 | Y | ||
| Xue et al. | 2013 | Asian | H-B | 116 | 183 | 111 | 130 | 190 | 90 | Y | ||
| Pan et al. | 2013 | Asian | H-B | 48 | 273 | 498 | 25 | 247 | 531 | Y | ||
| Li et al. | 2014 | Asian | H-B | 2 | 11 | 3 | 50 | 46 | 14 | Y | ||
| Sevilya et al. | 2015 | Caucasian | H-B | 34 | 50 | 15 | 42 | 46 | 11 | Y | ||
| APEX1-rs1760944 | Lu et al. | 2009 | Asian | H-B | 184 | 241 | 75 | 170 | 238 | 109 | Y | |
| Lo et al. | 2009 | Asian | H-B | 271 | 332 | 122 | 234 | 341 | 153 | Y | ||
| Li et al. | 2011 | Asian | H-B | 162 | 227 | 66 | 143 | 206 | 94 | Y | ||
| Pan et al. | 2013 | Asian | H-B | 114 | 384 | 321 | 98 | 369 | 336 | Y | ||
| Li et al. | 2014 | Asian | H-B | 3 | 10 | 3 | 36 | 56 | 18 | Y | ||
| APEX1-rs2307486 | Zienolddiny et al. | 2006 | Caucasian | P-B | 263 | 76 | 1 | 276 | 124 | 10 | Y | |
| Lo et al. | 2009 | Asian | H-B | 669 | 59 | 0 | 659 | 64 | 2 | Y | ||
| Li et al. | 2014 | Asian | H-B | 11 | 2 | 0 | 103 | 7 | 0 | Y | ||
| OGG1-rs1052133 | Kohno et al. | 1998 | Asian | Mixed | 16 | 19 | 10 | 15 | 20 | 7 | Y | |
| Sugimura et al. | 1999 | Mixed | H-B | 85 | 115 | 41 | 63 | 107 | 27 | Y | ||
| Wikman et al. | 2000 | Caucasian | P-B | 68 | 32 | 5 | 60 | 43 | 2 | Y | ||
| Marchand et al. | 2002 | Mixed | P-B | 15 | 31 | 29 | 29 | 48 | 19 | Y | ||
| Marchand et al. | 2002 | Caucasian | P-B | 78 | 39 | 9 | 98 | 53 | 8 | Y | ||
| Sunaga et al. | 2002 | Asian | H-B | 54 | 106 | 38 | 50 | 66 | 36 | Y | ||
| Marchand et al. | 2002 | Asian | P-B | 30 | 40 | 27 | 50 | 74 | 26 | Y | ||
| Ito et al. | 2002 | Asian | H-B | 40 | 71 | 27 | 68 | 118 | 54 | Y | ||
| Lan et al. | 2004 | Asian | P-B | 37 | 61 | 20 | 51 | 43 | 15 | Y | ||
| Park et al. | 2004 | Caucasian | P-B | 88 | 60 | 12 | 255 | 87 | 8 | Y | ||
| Vogel et al. | 2004 | Caucasian | P-B | 149 | 93 | 14 | 159 | 91 | 19 | Y | ||
| Liang et al. | 2005 | Asian | H-B | 27 | 132 | 68 | 28 | 123 | 76 | N | ||
| Hung et al. | 2005 | Caucasian | H-B | 1401 | 661 | 93 | 1368 | 716 | 79 | Y | ||
| Loft et al. | 2006 | Caucasian | P-B | 144 | 93 | 14 | 154 | 88 | 19 | Y | ||
| Zienolddiny et al. | 2006 | Caucasian | P-B | 182 | 100 | 44 | 194 | 117 | 75 | N | ||
| Kohno et al. | 2006 | Asian | H-B | 285 | 544 | 268 | 123 | 190 | 81 | Y | ||
| Sorensen et al. | 2006 | Caucasian | P-B | 254 | 155 | 22 | 479 | 284 | 33 | Y | ||
| Matullo et al. | 2006 | Caucasian | P-B | 66 | 46 | 4 | 673 | 371 | 50 | Y | ||
| De Ruyck et al. | 2007 | Caucasian | H-B | 74 | 33 | 3 | 60 | 46 | 4 | Y | ||
| Hatt et al. | 2008 | Caucasian | P-B | 92 | 58 | 8 | 93 | 59 | 12 | Y | ||
| Karahalil et al. | 2008 | Caucasian | H-B | 86 | 65 | 14 | 115 | 106 | 29 | Y | ||
| Miyaishi et al. | 2009 | Asian | H-B | 27 | 55 | 26 | 39 | 54 | 28 | Y | ||
| Chang et al. | 2009 | African | P-B | 170 | 78 | 6 | 202 | 70 | 8 | Y | ||
| Chang et al. | 2009 | Caucasian | P-B | 53 | 47 | 12 | 135 | 132 | 29 | Y | ||
| Chang et al. | 2009 | Asian | P-B | 142 | 518 | 436 | 154 | 482 | 361 | Y | ||
| Okasaka et al. | 2009 | Asian | H-B | 117 | 257 | 141 | 250 | 544 | 236 | Y | ||
| Liu et al. | 2010 | Asian | H-B | 68 | 158 | 132 | 110 | 294 | 312 | N | ||
| Janik et al. | 2011 | Caucasian | H-B | 48 | 24 | 16 | 57 | 21 | 1 | Y | ||
| Li et al. | 2011 | Asian | H-B | 83 | 208 | 164 | 60 | 219 | 164 | Y | ||
| Qian et al. | 2011 | Asian | H-B | 100 | 288 | 193 | 125 | 291 | 185 | Y | ||
| Cheng et al. | 2012 | Asian | P-B | 26 | 9 | 15 | 17 | 3 | 10 | N | ||
| Ouyan et al. | 2013 | Asian | P-B | 14 | 42 | 26 | 40 | 94 | 67 | Y | ||
| Letkova et al. | 2013 | Caucasian | P-B | 244 | 119 | 19 | 250 | 110 | 18 | Y | ||
| Xue et al. | 2013 | Asian | H-B | 55 | 178 | 177 | 68 | 200 | 142 | Y | ||
| Doherty et al. | 2013 | Caucasian | P-B | 440 | 265 | 39 | 873 | 519 | 85 | Y | ||
| Wang et al. | 2015 | Asian | P-B | 77 | 182 | 241 | 80 | 165 | 25 | N | ||
| Qin et al. | 2016 | Asian | P-B | 59 | 121 | 37 | 72 | 124 | 30 | N | ||
| LIG1-rs20579 | Landi et al. | 2006 | Caucasian | Mixed | 206 | 73 | 6 | 245 | 61 | 0 | Y | |
| Chang et al. | 2008 | Caucasian | P-B | 72 | 36 | 5 | 217 | 75 | 7 | Y | ||
| Chang et al. | 2008 | African | P-B | 150 | 92 | 13 | 137 | 117 | 26 | Y | ||
| Lee et al. | 2008 | Caucasian | P-B | 294 | 118 | 11 | 586 | 187 | 7 | Y | ||
| Sakoda et al. | 2012 | Caucasian | P-B | 583 | 141 | 18 | 1126 | 312 | 36 | N | ||
| LIG1-rs3730931 | Landi et al. | 2006 | Caucasian | Mixed | 220 | 64 | 5 | 255 | 52 | 2 | Y | |
| Chang et al. | 2008 | Caucasian | P-B | 79 | 30 | 4 | 226 | 67 | 6 | Y | ||
| Chang et al. | 2008 | African | P-B | 151 | 92 | 11 | 158 | 103 | 19 | Y | ||
| Sakoda et al. | 2012 | Caucasian | P-B | 595 | 137 | 11 | 1137 | 313 | 26 | Y | ||
| LIG1-rs156641 | Chang et al. | 2008 | African | P-B | 189 | 62 | 4 | 215 | 60 | 5 | Y | |
| Chang et al. | 2008 | Caucasian | P-B | 59 | 43 | 11 | 143 | 126 | 30 | Y | ||
| Sakoda et al. | 2012 | Caucasian | P-B | 271 | 352 | 121 | 596 | 709 | 164 | N | ||
| LIG1-rs20581 | Chang et al. | 2008 | African | P-B | 176 | 73 | 6 | 199 | 68 | 13 | N | |
| Chang et al. | 2008 | Caucasian | P-B | 38 | 48 | 27 | 89 | 151 | 59 | Y | ||
| Lee et al. | 2008 | Caucasian | P-B | 78 | 148 | 86 | 142 | 346 | 155 | Y | ||
| LIG1-rs439132 | Chang et al. | 2008 | Caucasian | P-B | 108 | 5 | 0 | 269 | 29 | 1 | Y | |
| Lee et al. | 2008 | Caucasian | P-B | 326 | 39 | 6 | 585 | 54 | 2 | Y | ||
| Chang et al. | 2008 | African | P-B | 129 | 112 | 14 | 117 | 91 | 12 | Y | ||
| MUTYH-rs3219489 | Al-tassan et al. | 2003 | Caucasian | P-B | 142 | 109 | 14 | 58 | 36 | 7 | Y | |
| Miyaishi et al. | 2009 | Asian | P-B | 22 | 57 | 29 | 37 | 69 | 15 | N | ||
| Qian et al. | 2011 | Asian | P-B | 230 | 261 | 90 | 243 | 283 | 77 | Y | ||
| Doherty et al. | 2013 | Caucasian | P-B | 417 | 279 | 42 | 825 | 562 | 79 | Y | ||
| PARP1-rs1136410 | Zhang et al. | 2005 | Asian | H-B | 307 | 509 | 184 | 359 | 504 | 137 | Y | |
| Yin et al. | 2011 | Mixed | H-B | 117 | 35 | 7 | 50 | 12 | 2 | Y | ||
| Xue et al. | 2013 | Asian | H-B | 129 | 202 | 79 | 138 | 205 | 67 | Y | ||
| Yu et al. | 2014 | Asian | H-B | 46 | 164 | 163 | 34 | 164 | 162 | Y | ||
| Wang et al. | 2015 | Asian | P-B | 151 | 97 | 252 | 14 | 109 | 251 | Y | ||
M, mutant allele; W, wild type allele; P-B, population-based; H-B, hospital-based; Mixed, more than one ethnicity; N.A., not mentioned; Y, studies that conforms to HWE; N, study that deviates from HWE.
Table 2
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | Page 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | Page 2–3 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | Page 4–5 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | Page 5 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | N/A |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | Study selection: page 6–7 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | Search strategy: page 5–6, |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | Search strategy: page 5 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | Figure 1 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | Data extraction and quality assessment: page 7 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | Data extraction and quality assessment: page 7 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | Statistical analysis: page 8 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | Statistical analysis: page 8 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | Statistical analysis: page 8 |
| Section/topic | |||
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | Statistical analysis: page 8 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | Statistical analysis: page 8 |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | Description of studies: page 8–9 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | Table 1–3 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | Page 10–12 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | Page 10–12 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | Page 10–12 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | Page 10–12 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16)]. | page 10 |
| Discussion | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | Page 13–15 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | Page 15 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | Page 17 |
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | Page 17 |
Adapted from ref. (128).
Meta-analysis
XRCC1 polymorphisms and LC risk
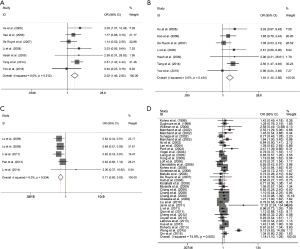
We investigated six polymorphisms in XRCC1 gene and LC risk, including rs1799782, rs25487, rs25489, rs3213245, rs3547 and rs915927 polymorphisms (Table 3). Overall, rs3213245 polymorphism was observed associated with a significantly raised susceptibility of LC in homozygote contrast model and recessive contrast model (MM vs. WW: OR 2.023, 95% CI: 1.452–2.819, P=3.124×10−5, Figure 2A; MM vs. MW + WW: OR 1.926, 95% CI: 1.396–2.656, P=6.468×10−5, Figure 2B), while for other genetic polymorphisms, overall analyses uncovered no remarkable association. In addition, for rs3213245 polymorphism, in the ethnicity subgroup analysis, a meaningful upward risk of LC for Asian population was also uncovered in homozygote and recessive models. While for the subgroup analysis by source of control subgroup, we uncovered a remarkable upgrade risk of LC for H-B groups in allelic contrast, heterogeneous and dominant models. Furthermore, for rs915927 polymorphism, we also performed the subgroup analysis in different ethnicity and source of control, and identified the raised risk for Asian, H-B group in allelic contrast model, heterozygous model, as well as dominant model. For rs25487 polymorphism, overall analysis suggested a null association. We identified that HWE (N) group was associated with LC risk in allelic, homozygote, and recessive models, suggesting potential bias existed. After removing the HWE (N) studies from the pooled analyses, and the final results also suggested a negative result for XRCC1-rs25487 polymorphism.
Table 3
| SNP | Comparison | Subgroup | N | PH | PZ | Random OR (95% CI) | Fixed OR (95% CI) |
|---|---|---|---|---|---|---|---|
| XRCC1-rs3213245 | MM vs. WW | Overall | 7 | 0.512 | 3.124*10−5 | 1.992 (1.422–2.791) | 2.023 (1.452–2.819) |
| MM vs. MW + WW | Overall | 7 | 0.434 | 6.468*10−5 | 1.894 (1.365–2.627) | 1.926 (1.396–2.656) | |
| MM vs. WW | Asian | 6 | 0.720 | 1.169*10−5 | 2.260 (1.556–3.284) | 2.285 (1.579–3.306) | |
| MM vs. MW + WW | Asian | 6 | 0.730 | 1.660*10−5 | 2.208 (1.526–3.193) | 2.231 (1.549–3.215) | |
| M vs. W | H-B | 4 | 0.406 | 1.970*10−8 | 1.433 (1.263–1.625) | 1.433 (1.264–1.625) | |
| MW vs. WW | H-B | 4 | 0.820 | 6.322*10−7 | 1.446 (1.251–1.672) | 1.446 (1.251–1.672) | |
| MW + MM vs. WW | H-B | 4 | 0.723 | 4.140*10−8 | 1.485 (1.289–1.710) | 1.485 (1.289–1.710) | |
| XRCC1-rs915927 | M vs. W | Asian | 2 | 0.180 | 9.975*10−5 | 2.292 (1.226–4.284) | 2.071 (1.435–2.988) |
| MW vs. WW | Asian | 2 | 0.234 | 2.147*10−4 | 2.252 (1.280–3.962) | 2.111 (1.421–3.136) | |
| MW + MM vs. WW | Asian | 2 | 0.203 | 9.341*10−5 | 2.395 (1.287–4.455) | 2.191 (1.478–3.247) | |
| M vs. W | H-B | 2 | 0.180 | 9.975*10−5 | 2.292 (1.226–4.284) | 2.071 (1.435–2.988) | |
| MW vs. WW | H-B | 2 | 0.234 | 2.147*10−4 | 2.252 (1.280–3.962) | 2.111 (1.421–3.136) | |
| MW + MM vs. WW | H-B | 2 | 0.203 | 9.341*10−5 | 2.395 (1.287–4.455) | 2.191 (1.478–3.247) | |
| XRCC1-rs25487 | M vs. W | N | 8 | 0.414 | 2.741*10−7 | 1.345 (1.199–1.508) | 1.343 (1.200–1.502) |
| MM vs. WW | N | 8 | 0.471 | 4.463*10−5 | 1.481 (1.223–1.793) | 1.486 (1.229–1.797) | |
| MM vs. MW + WW | N | 8 | 0.102 | 3.663*10−7 | 1.758 (1.332–2.321) | 1.592 (1.331–1.904) | |
| APEX1-rs1760944 | M vs. W | Overall | 5 | 0.530 | 7.243*10−5 | 0.851 (0.786–0.922) | 0.851 (0.786–0.921) |
| MM vs. WW | Overall | 5 | 0.534 | 3.409*10−5 | 0.705 (0.598–0.832) | 0.705 (0.598–0.832) | |
| MM vs. MW + WW | Overall | 5 | 0.315 | 1.927*10−4 | 0.770 (0.663–0.895) | 0.780 (0.684–0.889) | |
| OGG1-rs1052133 | MM vs. MW + WW | Overall | 31 | 0.106 | 2.119*10−4 | 1.143 (1.032–1.265) | 1.157 (1.071–1.249) |
| M vs. W | Asian | 13 | 0.355 | 9.988*10−5 | 1.123 (1.054–1.196) | 1.123 (1.059–1.191) | |
| MM vs. WW | Asian | 13 | 0.353 | 3.585*10−4 | 1.242 (1.090–1.414) | 1.244 (1.103–1.403) |
M, mutant allele; W, wild type allele; P-B, population-based; H-B, hospital-based; Y, studies that conforms to HWE; N, study that deviates from HWE; PH, P value of heterogeneity test; Pz, adjusted P value of Z test [P<0.05/(17 polymorphisms * 5 genetic models)].
APEX1 polymorphism and LC risk
For rs1760944 polymorphism, overall analysis suggested a sharp reduced risk of LC in allelic, homozygote and recessive models (M vs. W: OR 0.851, 95% CI: 0.786–0.922, P=7.243×10−5, Figure 2C; MM vs. WW: OR 0.705, 95% CI: 0.598–0.832, P=3.409×10−5; and MM vs. MW + WW: OR 0.780, 95% CI: 0.684–0.889, P=1.927×10−4, Table 3).
OGG1 polymorphism and LC risk
For OGG1-rs1052133 polymorphism, the recessive model showed an increased risk overall group (MM vs. MW + WW: OR 1.157, 95% CI: 1.071–1.249, P=2.119×10−4, Figure 2D). In addition, when the stratification analysis of Asian subgroup, we illustrated a significantly increased risk of LC in allelic contrast model and homozygote model (Table 3).
Other gene polymorphism and LC risk
While for other polymorphisms in genes the BER pathway, such as LIG1-rs156641, MUTYH-rs3219489, we failed to identify any significant association.
Evaluation of stability and publication bias
The test of the stability of results was assessed by sensitivity analysis, each time we separated one study form data pool, and reviewed whether it affects the ORs and 95% CIs. The results displayed that no substantial change for XRCC1-rs1799782/rs25487/rs25489/rs3213245/rs3547/rs915927, LIG1-rs156641/rs20579/rs20581/rs3730931/rs439132, APEX1-rs1130409/rs1760944/rs2307486, PARP1-rs1136410, OGG1-rs1052133 and MUTYH-rs3219489 polymorphisms.
For behalf of evaluating potential publication bias, we use Begg’s funnel plot and Egger’s test. Significant publication bias may reflect differences in control options, age distributions and other lifestyles. Finally, the shape of Begg’s funnel plot in each polymorphism is symmetrical, while the P value of Egger’s test in each polymorphism and subgroup is higher than 0.05, indicating no evidence of publication bias was found (Table 4).
Table 4
| Gene | Polymorphism | Egger’s test (P > |t|) |
|---|---|---|
| XRCC1 | rs1799782 | 0.896 |
| rs25487 | 0.248 | |
| rs25489 | 0.99 | |
| rs3213245 | 0.497 | |
| rs3547 | 0.565 | |
| rs915927 | 0.115 | |
| LIG1 | rs156641 | 0.377 |
| rs20579 | 0.401 | |
| rs20581 | 0.388 | |
| rs3730931 | 0.127 | |
| rs439132 | 0.589 | |
| APEX1 | rs1130409 | 0.006 |
| rs1760944 | 0.312 | |
| rs2307486 | 0.38 | |
| PARP1 | rs1136410 | 0.603 |
| OGG1 | rs1052133 | 0.337 |
Discussion
The stability of the general genomic sequence is sustained by a pivotal gene family, BER signaling pathway. In human cells, the inability of remove endogenous DNA damage would link with single nucleotide polymorphisms (130-132). On the other hand, the abnormal process occurs on BER pathway or the enzymes mediate it would finally lead to the instable cell chromosomal (133). Recently, increasing evidence suggested that genetic variants in the BER pathway were associated with LC risk. However, these results were inclusive or even controversial. Therefore, we presented the comprehensively updated meta-analysis, aiming to systematically screen out the LC risk or protective factors within genes of the BER pathway.
Firstly, we investigated the XRCC1, a crucial element of the BER system, it has multiple key roles in the repair process of DNA single nucleotide polymorphism (134,135). We analyzed six commonly studied polymorphisms in XRCC1, and overall analyses suggested that MM genotype of rs3213245 (−77T > C) polymorphism was linked to a sharply enhanced risk of LC compared with WW and MW/WW genotypes, and not the rs25487 and rs1799782 polymorphisms, which were proved associated with LC risk in Chen et al.’s meta-analysis work (136). In addition, rs3213245-MM genotype was also combined with an increased hazard of LC for Asian population. For XRCC1 rs3213245 polymorphism, the affinity of XRCC1 promoter region to nuclear protein Sp1 would be enhanced by T to C mutation, caused the inhibition of its transcription (40). In our study, seven studies were focused on the correlation of rs3213245 polymorphism and LC risk, and the overall results suggested that the risk in MM genotype group was 2.023 and 1.926-fold raised than WW group and MW + WW group, respectively, almost consistent with Vineis et al.’s (137) findings.
In addition, the overall calculate illustrated a negative association between XRCC1-rs915927 and LC, but we also identified that M allele, MW and MW + MM genotypes led to an enhanced risk of LC for the Asian population. For the mechanism part, rs915927 leads to a synonymous mutation, which is a kind of mutation which may not influence the translation of amino acid product, however, this kind of mutation might change the translational efficiency of mRNA, therefore, non-synonymous mutations like XRCC1 rs1799782 (Arg194Trp) and XRCC1 rs25489 (Arg280His) might regulate LC susceptibility, affecting complex assembly or repair efficiency (138). Furthermore, for another XRCC1-rs25487 polymorphism, we observed an enhanced risk of LC in allelic, homozygote, and recessive models for HWE (N) group, which tell us that there might be some potential bias caused by HWE status. Therefore, we decided to remove these HWE (N) studies from pooled analysis, and finally negative results were obtained.
Secondly, APEX1 gene was also analyzed, which specifically activates DNA repair through the identification and cleavage of phosphodiester bonds on the 5' side of the basic site (139). APEX1 can also participate in oxidative stress, control of cell cycle, and apoptosis (140,141). Recent days, several researchers reported that APEX1 gene polymorphisms would influence the cancer risks (142-144), as well as some meta-analyses (most of them only focus on a few variants) (145). In current work, we analyzed three most commonly polymorphisms reported in APEX1 (rs1130409, rs1760944 and rs2307486) and LC risk, and we found that M allele, MM genotype at rs1760944 were associated with a reduced risk of LC relative to W allele, WW and MW+WW genotypes, respectively. While for the other two polymorphisms, we failed to identify any significant correlations.
In the progression of different types of cancers, APEX1 is another key role. For APEX1-rs1130409, Zhang et al. (146) reported that the G allele and GG/TG genotype associated with the decreased risk of ovarian carcinoma. However, Yuan et al. (147) revealed that rs1130409 do not play any role in head and neck neoplasms in Chinese, another study conducted in gastric cancer reported the same conclusion (148). In our work, we obtained the result that re1130409 is not associated with LC risks. For another role polymorphism in APEX1, Lu et al. (99) first reported the potential risk of rs1760944 in LC. In a study about Korean, rs1760944 was reported associated with the risk of gastric cancer, but another study conducted in Chinese indicated that GT or GG genotypes might have a higher survival rate (148,149). Dai et al. managed a meta-analysis, the result supported the conclusion that rs1760944 acts as a protector in cancer of Asian (150). Consistent with these data, we demonstrated that M allele and MM genotype were associated with a decreased risk of LC than W allele, WW and MW + WW genotypes.
Another BER gene we analyzed here is OGG1, which plays a key role during the repair process of oxidative DNA damage. rs1052133 polymorphism had been reported could substitution Serene to Cysteine at codon 326, and influence the function of OGG1 protein (151). As reported by Wikman et al. (122), LC susceptibility might not be impacted by the OGG1 polymorphisms in Caucasians. Hung et al. (70) and Vogel et al. (84) also observed no link between OGG1 polymorphisms and LC susceptibility. Ito et al. (107) found that OGG1-rs1052133 polymorphism had no effect on the development of adenocarcinoma or small cell carcinoma. Whereas in our work, overall results suggested a null correlation for this polymorphism and LC risk.
In this meta-analysis, we comprehensively searched all available eligible studies to obtain the precise result. Some advantages of this study should be focused on. Firstly, a wide search was conducted to identify more qualified studies for each genetic variant in BER genes, therefore these analyses were persuasive and substantive. For example, several previous meta-analyses have been published concerning XRCC1 polymorphisms and LC risk, while they only focus limited polymorphisms on LC risk, and their results were not adjusted, increasing the false-positive results rate. Secondly, we evaluated the quality of each registered research by NOS scale before calculating, and eliminated low-quality studies. and adjusted all the results according to Bonferroni corrections, making the conclusions more convincing. Thirdly, according to the subgroup, we also conducted the stratification analyses by ethnicity, source of controls, tumor type or race, in order to eliminate the influence of heterogeneity. Fourthly, the sensitivity analysis was performed to confirm the stability of the obtained results, and Egger’s test and Begg’s funnel plot were performed to draw out the potential publication bias.
Several disadvantages should also be displayed to avoid any incorrect understanding of the results. First of all, there were no sufficient samples for the analyses of some variants, and it might prove an undependable association between polymorphisms and LC. For example, there are only 3 or 4 studies in APEX1-rs2307486, LIG1-rs156641 and PARP1-rs1136410, more studies conducted in these polymorphisms are needed to reveal a more convincible result in the future. Moreover, only the articles in English were enrolled, which might miss the important result in other languages and countries. Finally, the detail information about the histological result of each LC patient was missed, so the stratification analyses based on histological type and the clinical stage could not be conducted.
Conclusions
To conclude, this meta-analysis shows that XRCC1-rs3213245 and OGG1-rs1052133 polymorphisms are risk factors for LC, while APEX1-rs1760944 polymorphism is a protective factor. Future studies with larger sample size are warranted to verify these findings.
Acknowledgments
Funding: The study was supported by
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure from (available at http://dx.doi.org/10.21037/tcr.2020.02.44). The authors have no conflicts of interests to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Hosgood HD 3rd, Cosgrove C, Klugman M, et al. Lung cancer mortality among smokers and never-smokers in the United States. Epidemiology 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Klebe S, Leigh J, Henderson DW, et al. Asbestos, Smoking and Lung Cancer: An Update. Int J Environ Res Public Health 2019; [Crossref] [PubMed]
- Adie Y, Kats DJ, Tlimat A, et al. Neighborhood Disadvantage and Lung Cancer Incidence in Ever-Smokers at a Safety Net Health-Care System: A Retrospective Study. Chest 2019; [Epub ahead of print]. [PubMed]
- Yu S, Gong LS, Li NF, et al. Galangin (GG) combined with cisplatin (DDP) to suppress human lung cancer by inhibition of STAT3-regulated NF-kappaB and Bcl-2/Bax signaling pathways. Biomed Pharmacother 2018;97:213-24. [Crossref] [PubMed]
- Zhao J, Wen C, Li M. Association Analysis of Interleukin-17 Gene Polymorphisms with the Risk Susceptibility to Tuberculosis. Lung 2016;194:459-67. [Crossref] [PubMed]
- Wang SS, Zhu XQ, Yang SD, et al. Association of p73 G4C14-to-A4T14 polymorphism with non-small cell lung cancer risk. Oncol Lett 2015;10:995-9. [Crossref] [PubMed]
- Barnes JL, Zubair M, John K, et al. Carcinogens and DNA damage. Biochem Soc Trans 2018;46:1213-24. [Crossref] [PubMed]
- Ganapathy V, Manyanga J, Brame L, et al. Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PLoS One 2017;12:e0177780. [Crossref] [PubMed]
- Izzotti A, Balansky R, Micale RT, et al. Modulation of smoke-induced DNA and microRNA alterations in mouse lung by licofelone, a triple COX-1, COX-2 and 5-LOX inhibitor. Carcinogenesis 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Smith LE, Denissenko MF, Bennett WP, et al. Targeting of lung cancer mutational hotspots by polycyclic aromatic hydrocarbons. J Natl Cancer Inst 2000;92:803-11. [Crossref] [PubMed]
- Brancato B, Munnia A, Cellai F, et al. 8-Oxo-7,8-dihydro-2'-deoxyguanosine and other lesions along the coding strand of the exon 5 of the tumour suppressor gene P53 in a breast cancer case-control study. DNA Res 2016;23:395-402. [Crossref] [PubMed]
- Peng Z, Wang J, Shan B, et al. Genome-wide analyses of long noncoding RNA expression profiles in lung adenocarcinoma. Sci Rep 2017;7:15331. [Crossref] [PubMed]
- Gan PP, Zhou YY, Zhong MZ, et al. Endoplasmic Reticulum Stress Promotes Autophagy and Apoptosis and Reduces Chemotherapy Resistance in Mutant p53 Lung Cancer Cells. Cell Physiol Biochem 2017;44:133-51. [Crossref] [PubMed]
- Chen J, Wu L, Wang Y, et al. Effect of transporter and DNA repair gene polymorphisms to lung cancer chemotherapy toxicity. Tumour Biol 2016;37:2275-84. [Crossref] [PubMed]
- Yang B, Zhao F, Zong Z, et al. Preferences for treatment of lobectomy in Chinese lung cancer patients: video-assisted thoracoscopic surgery or open thoracotomy? Patient Prefer Adherence 2014;8:1393-7. [Crossref] [PubMed]
- Li W, Zhang M, Huang C, et al. Genetic variants of DNA repair pathway genes on lung cancer risk. Pathol Res Pract 2019;215:152548. [Crossref] [PubMed]
- Lawania S, Singh A, Sharma S, et al. The multi-faceted high order polymorphic synergistic interactions among nucleotide excision repair genes increase the risk of lung cancer in North Indians. Mutat Res 2019;816-818:111673. [Crossref] [PubMed]
- Singh A, Singh N, Behera D, et al. Role of polymorphic XRCC6 (Ku70)/XRCC7 (DNA-PKcs) genes towards susceptibility and prognosis of lung cancer patients undergoing platinum based doublet chemotherapy. Mol Biol Rep 2018;45:253-61. [Crossref] [PubMed]
- Munnia A, Giese RW, Polvani S, et al. Bulky DNA Adducts, Tobacco Smoking, Genetic Susceptibility, and Lung Cancer Risk. Adv Clin Chem 2017;81:231-77. [Crossref] [PubMed]
- Trenner A, Sartori AA. Harnessing DNA Double-Strand Break Repair for Cancer Treatment. Front Oncol 2019;9:1388. [Crossref] [PubMed]
- Lee TH, Kang TH. DNA Oxidation and Excision Repair Pathways. Int J Mol Sci 2019; [Crossref] [PubMed]
- Zhou R, Xu T, Nguyen QN, et al. Radiation Dose, Local Disease Progression, and Overall Survival in Patients With Inoperable Non-Small Cell Lung Cancer After Concurrent Chemoradiation Therapy. Int J Radiat Oncol Biol Phys 2018;100:452-61. [Crossref] [PubMed]
- Whitaker AM, Schaich MA, Smith MS, et al. Base excision repair of oxidative DNA damage: from mechanism to disease. Front Biosci (Landmark Ed) 2017;22:1493. [Crossref] [PubMed]
- Poletto M, Legrand AJ, Dianov GL. DNA base excision repair: the Achilles' heel of tumour cells and their microenvironment? Curr Pharm Des 2017;23:4758-72. [Crossref] [PubMed]
- Abbotts R. Coordination of DNA single strand break repair. Free Radic Biol Med 2017;107:228-44. [Crossref] [PubMed]
- Alberg AJ, Jorgensen TJ, Ruczinski I, et al. DNA repair gene variants in relation to overall cancer risk: a population-based study. Carcinogenesis 2013;34:86-92. [Crossref] [PubMed]
- Howard MJ, Wilson SH. DNA scanning by base excision repair enzymes and implications for pathway coordination. DNA Repair (Amst) 2018;71:101-7. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820-6. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Yong G, Pan X, Su T, et al. Glutathione S-transferase P1 Ile105Val polymorphism and colorectal cancer risk: a meta-analysis and HuGE review. Eur J Cancer 2009;45:3303-14. [Crossref] [PubMed]
- Harbord RM, Egger M, Sterne JAC. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006;25:3443-57. [Crossref] [PubMed]
- Cătană A, Pop M, Hincu BD, et al. TheXRCC1Arg194Trp polymorphism is significantly associated with lung adenocarcinoma: a case-control study in an Eastern European Caucasian group. Onco Targets Ther 2015;8:3533-8. [Crossref] [PubMed]
- Chan EC, Lam SY, Fu KH, et al. Polymorphisms of the GSTM1, GSTP1, MPO, XRCC1, and NQO1 genes in Chinese patients with non-small cell lung cancers: relationship with aberrant promoter methylation of the CDKN2A and RARB genes. Cancer Genet Cytogenet 2005;162:10. [Crossref] [PubMed]
- David-Beabes GL, London SJ. Genetic polymorphism of XRCC1 and lung cancer risk among African-Americans and Caucasians. Lung Cancer 2001;34:333. [Crossref] [PubMed]
- Divine KK, Gilliland FD, Crowell RE, et al. The XRCC1 399 glutamine allele is a risk factor for adenocarcinoma of the lung. Mutat Res 2001;461:273-8. [Crossref] [PubMed]
- Han JC, Zhang YJ, Li XD. Association between polymorphisms in the XRCC1 gene and the risk of non-small cell lung cancer. Genet Mol Res 2015;14:12888. [Crossref] [PubMed]
- Hao B, Miao X, Li Y, et al. A novel T-77C polymorphism in DNA repair gene XRCC1 contributes to diminished promoter activity and increased risk of non-small cell lung cancer. Oncogene 2006;25:3613-20. [Crossref] [PubMed]
- Harms C, Salama SA, Sierra-Torres CH, et al. Polymorphisms in DNA repair genes, chromosome aberrations, and lung cancer. Environ Mol Mutagen 2004;44:74-82. [Crossref] [PubMed]
- Ito H, Matsuo K, Hamajima N, et al. Gene-environment interactions between the smoking habit and polymorphisms in the DNA repair genes, APE1 Asp148Glu and XRCC1 Arg399Gln, in Japanese lung cancer risk. Carcinogenesis 2004;25:1395. [Crossref] [PubMed]
- K DR. Polymorphisms in base-excision repair and nucleotide-excision repair genes in relation to lung cancer risk. Mutat Res 2007;631:101-10. [Crossref] [PubMed]
- Kiyohara C, Horiuchi T, Takayama K, et al. Genetic Polymorphisms Involved in Carcinogen Metabolism and DNA Repair and Lung Cancer Risk in a Japanese Population. J Thorac Oncol 2012;7:954-62. [Crossref] [PubMed]
- Liu G, Zhou W, Park S, et al. The SOD2 Val/Val genotype enhances the risk of nonsmall cell lung carcinoma by p53 and XRCC1 polymorphisms. Cancer 2004;101:2802. [Crossref] [PubMed]
- Liu HX, Li J, Ye BG. Correlation between gene polymorphisms of CYP1A1, GSTP1, ERCC2, XRCC1, and XRCC3 and susceptibility to lung cancer. Genet Mol Res 2016;15. [PubMed]
- Matullo G, Dunning AM, Guarrera S, et al. DNA repair polymorphisms and cancer risk in non-smokers in a cohort study. Carcinogenesis 2006;27:997-1007. [Crossref] [PubMed]
- Mei C, Mei H, Guo S, et al. Polymorphisms in DNA repair genes of XRCC1, XPA, XPC, XPD and associations with lung cancer risk in Chinese people. Thoracic Cancer 2014;5:232-42. [Crossref] [PubMed]
- Ouyang FD, Yang FL, Chen HC, et al. Polymorphisms of DNA repair genes XPD, XRCC1, and OGG1, and lung adenocarcinoma susceptibility in Chinese population. Tumour Biol 2013;34:2843-8. [Crossref] [PubMed]
- Park JY, Lee SY, Jeon HS, et al. Polymorphism of the DNA repair gene XRCC1 and risk of primary lung cancer. Cancer Epidemiol Biomarkers Prev 2002;11:23-7. [PubMed]
- Ratnasinghe D, Yao SX, Tangrea JA, et al. Polymorphisms of the DNA repair gene XRCC1 and lung cancer risk. Cancer Epidemiol Biomarkers Prev 2001;10:119-23. [PubMed]
- Ratnasinghe DL, Yao SX, Forman M, et al. Gene-environment interactions between the codon 194 polymorphism of XRCC1 and antioxidants influence lung cancer risk. Anticancer Res 2003;23:627-32. [PubMed]
- Ryk C, Kumar R, Thirumaran RK, et al. Polymorphisms in the DNA repair genes XRCC1, APEX1, XRCC3 and NBS1, and the risk for lung cancer in never- and ever-smokers. Lung Cancer 2006;54:285-92. [Crossref] [PubMed]
- Saikia BJ, Phukan RK, Sharma SK, et al. Interaction of XRCC1 and XPD gene polymorphisms with lifestyle and environmental factors regarding susceptibility to lung cancer in a high incidence population in North East India. Asian Pac J Cancer Prev 2014;15:1993. [Crossref] [PubMed]
- Schneider J, Classen V, Bernges U, et al. XRCC1 polymorphism and lung cancer risk in relation to tobacco smoking. Int J Mol Med 2005;16:709. [PubMed]
- Singh A, Singh N, Behera D, et al. Association and multiple interaction analysis among five XRCC1 polymorphic variants in modulating lung cancer risk in North Indian population. Dna Repair 2016;47:30-41. [Crossref] [PubMed]
- Tang J, Zhao J, Zhao J. The relationship between genetic variants of XRCC1 gene and lung cancer susceptibility in Chinese Han population. Med Oncol 2014;31:157. [Crossref] [PubMed]
- Tecmer P, Bast R, Ruud K, et al. Polymorphisms of the DNA repair genes XRCC1 and XRCC3 and risk of lung and colorectal cancer: a case-control study in a Southern Italian population. Anticancer Res 2008;28:2941-6. [PubMed]
- Yin J, Vogel U, Ma Y, et al. The DNA repair gene XRCC1 and genetic susceptibility of lung cancer in a northeastern Chinese population. Lung Cancer 2007;56:153-60. [Crossref] [PubMed]
- Yin J, Vogel U, Ma Y, et al. Association of DNA repair gene XRCC1 and lung cancer susceptibility among nonsmoking Chinese women. Cancer Genet Cytogenet 2009;188:26-31. [Crossref] [PubMed]
- Yin J, Vogel U, Ma Y, et al. Haplotypes of nine single nucleotide polymorphisms on chromosome 19q13.2-3 associated with susceptibility of lung cancer in a Chinese population. Mutat Res 2008;641:12. [Crossref] [PubMed]
- Hu Z1. A promoter polymorphism (-77T>C) of DNA repair gene XRCC1 is associated with risk of lung cancer in relation to tobacco smoking. Pharmacogenet Genomics 2005;15:457. [Crossref] [PubMed]
- Zhu DQ, Zou Q, Hu CH, et al. XRCC1 genetic polymorphism acts a potential biomarker for lung cancer. Tumour Biol 2015;36:3745-50. [Crossref] [PubMed]
- Chang JS, Wrensch MR, Hansen HM, et al. Nucleotide excision repair genes and risk of lung cancer among San Francisco Bay Area Latinos and African Americans. Int J Cancer 2008;123:2095. [Crossref] [PubMed]
- Chen S, Tang D, Xue K, et al. DNA repair gene XRCC1 and XPD polymorphisms and risk of lung cancer in a Chinese population. Carcinogenesis 2002;23:1321. [Crossref] [PubMed]
- Buch SC, Diergaarde B, Nukui T, et al. Genetic variability in DNA repair and cell cycle control pathway genes and risk of smoking-related lung cancer. Mol Carcinog 2012;51:E11. [Crossref] [PubMed]
- Cote ML, Yoo W, Wenzlaff AS, et al. Tobacco and estrogen metabolic polymorphisms and risk of non-small cell lung cancer in women. Carcinogenesis 2009;30:626. [Crossref] [PubMed]
- Du Y, He Y, Mei Z, et al. Association between genetic polymorphisms in XPD and XRCC1 genes and risks of non-small-cell lung cancer in East Chinese Han population. Clin Respir J 2016;10:311. [Crossref] [PubMed]
- Hsieh WC, Cheng YW, Lin CJ, et al. Prognostic significance of X-ray cross-complementing group 1 T-77C polymorphism in resected non-small cell lung cancer. Jpn J Clin Oncol 2009;39:81-5. [Crossref] [PubMed]
- Hung RJ, Brennan P, Canzian F, et al. Large-scale investigation of base excision repair genetic polymorphisms and lung cancer risk in a multicenter study. J Natl Cancer Inst 2005;97:567-76. [Crossref] [PubMed]
- Landi S, Gemignani F, Canzian F, et al. DNA repair and cell cycle control genes and the risk of young-onset lung cancer. Cancer Res 2006;66:11062. [Crossref] [PubMed]
- Letkova L, Matakova T, Musak L, et al. DNA repair genes polymorphism and lung cancer risk with the emphasis to sex differences. Mol Biol Rep 2013;40:5261-73. [Crossref] [PubMed]
- Li M, Yin Z, Guan P, et al. XRCC1 polymorphisms, cooking oil fume and lung cancer in Chinese women nonsmokers. Lung Cancer 2008;62:145. [Crossref] [PubMed]
- Li Z, Guan W, Li MX, et al. Genetic Polymorphism of DNA Base-excision Repair Genes (APE1, OGG1 and XRCC1) and Their Correlation with Risk of Lung Cancer in a Chinese Population. Arch Med Res 2011;42:226. [Crossref] [PubMed]
- Lópezcima MF, Gonzálezarriaga P, Garcíacastro L, et al. Polymorphisms in XPC, XPD, XRCC1, and XRCC3 DNA repair genes and lung cancer risk in a population of Northern Spain. BMC Cancer 2007;7:162. [Crossref] [PubMed]
- Misra RR, Ratnasinghe D, Tangrea JA, et al. Polymorphisms in the DNA repair genes XPD, XRCC1, XRCC3, and APE/ref-1, and the risk of lung cancer among male smokers in Finland. Cancer Lett 2003;191:171. [Crossref] [PubMed]
- Natukula K, Jamil K, Pingali UR, et al. The codon 399 Arg/Gln XRCC1 polymorphism is associated with lung cancer in Indians. Asian Pac J Cancer Prev 2013;14:5275-9. [Crossref] [PubMed]
- Pachouri SS, Sobti RC, Kaur P, et al. Contrasting impact of DNA repair gene XRCC1 polymorphisms Arg399Gln and Arg194Trp on the risk of lung cancer in the north-Indian population. DNA Cell Biol 2007;26:186-91. [Crossref] [PubMed]
- Popanda O, Schattenberg T, Phong CT, et al. Specific combinations of DNA repair gene variants and increased risk for non-small cell lung cancer. Carcinogenesis 2004;25:2433. [Crossref] [PubMed]
- Shen M, Berndt SI, Rothman N, et al. Polymorphisms in the DNA base excision repair genes APEX1 and XRCC1 and lung cancer risk in Xuan Wei, China. Anticancer Res 2005;25:537. [PubMed]
- Sreeja L, Syamala VS, Syamala V, et al. Prognostic importance of DNA repair gene polymorphisms of XRCC1 Arg399Gln and XPD Lys751Gln in lung cancer patients from India. J Cancer Res Clin Oncol 2008;134:645-52. [Crossref] [PubMed]
- Tanaka Y, Maniwa Y, Bermudez VP, et al. Nonsynonymous single nucleotide polymorphisms in DNA damage repair pathways and lung cancer risk. Cancer 2010;116:896. [Crossref] [PubMed]
- Uppal V, Mehndiratta M, Mohapatra D, et al. XRCC-1 Gene Polymorphism (Arg399Gln) and Susceptibility to Development of Lung Cancer in Cohort of North Indian Population: A Pilot Study. J Clin Diagn Res 2014;8:17-20. [PubMed]
- Vogel U, Nexø BA, Wallin H, et al. No Association Between Base Excision Repair Gene Polymorphisms and Risk of Lung Cancer. Biochem Genet 2004;42:453-60. [Crossref] [PubMed]
- Wang X, Ma KW, Zhao YG, et al. XRCC1 rs25487 polymorphism is associated with lung cancer risk in epidemiologically susceptible Chinese people. Genet Mol Res 2015;14:15530. [Crossref] [PubMed]
- Yoo SS, Jin C, Jung DK, et al. Putative functional variants of XRCC1 identified by RegulomeDB were not associated with lung cancer risk in a Korean population. Cancer Genet 2015;208:19. [Crossref] [PubMed]
- Zhang X, Miao X, Liang G, et al. Polymorphisms in DNA base excision repair genes ADPRT and XRCC1 and risk of lung cancer. Cancer Res 2005;65:722-6. [PubMed]
- Zhou W, Liu G, Miller DP, et al. Polymorphisms in the DNA repair genes XRCC1 and ERCC2, smoking, and lung cancer risk. Cancer Epidemiol Biomarkers Prev 2003;12:359. [PubMed]
- Zienolddiny S, Campa D, Lind H, et al. Polymorphisms of DNA repair genes and risk of non-small cell lung cancer. Carcinogenesis 2006;27:560. [Crossref] [PubMed]
- Sarlinova M, Majerova L, Matakova T, et al. Polymorphisms of DNA repair genes and lung cancer in chromium exposure. Adv Exp Med Biol 2015;833:1-8. [PubMed]
- Wang HT, Gao Y, Zhao YX, et al. PARP-1 rs3219073 Polymorphism May Contribute to Susceptibility to Lung Cancer. Genet Test Mol Biomarkers 2014;18:736-40. [Crossref] [PubMed]
- Xue X, Yin Z, Lu Y, et al. The joint effect of hOGG1, APE1, and ADPRT polymorphisms and cooking oil fumes on the risk of lung adenocarcinoma in Chinese non-smoking females. PLos One 2013;8:e71157. [Crossref] [PubMed]
- Yin M, Liao Z, Liu Z, et al. Functional polymorphisms of base excision repair genes XRCC1 and APEX1 predict risk of radiation pneumonitis in patients with non-small cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys 2011;81:e67. [Crossref] [PubMed]
- Yu P, Liu YP, Zhang JD, et al. Correlation between PARP-1 Val762Ala polymorphism and the risk of lung cancer in a Chinese population. Tumour Biol 2015;36:177. [Crossref] [PubMed]
- Agaçhan B, Küçükhüseyin O, Aksoy P, et al. Apurinic/apyrimidinic endonuclease (APE1) gene polymorphisms and lung cancer risk in relation to tobacco smoking. Anticancer Res 2009;29:2417-20. [PubMed]
- Deng Q, Sheng L, Su D, et al. Genetic polymorphisms in ATM, ERCC1, APE1 and iASPP genes and lung cancer risk in a population of southeast China. Med Oncol 2011;28:667-72. [Crossref] [PubMed]
- Li H, Liu G, Xia L, et al. A polymorphism in the DNA repair domain of APEX1 is associated with the radiation-induced pneumonitis risk among lung cancer patients after radiotherapy. Br J Radiol 2014;87:20140093. [Crossref] [PubMed]
- Shannon AM, Telfer BA, Smith PD, et al. The mitogen-activated protein/extracellular signal-regulated kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) enhances the radiation responsiveness of lung and colorectal tumor xenografts. Clin Cancer Res 2009;15:6619-29. [Crossref] [PubMed]
- Lu J, Zhang SD. Functional characterization of a promoter polymorphism in APE1/Ref-1 that contributes to reduced lung cancer susceptibility. Faseb J 2009;23:3459. [Crossref] [PubMed]
- Pan H, Niu W, He L, et al. Contributory role of five common polymorphisms of RAGE and APE1 genes in lung cancer among Han Chinese. PLos One 2013;8:e69018. [Crossref] [PubMed]
- Sevilya Z, Leitnerdagan Y, Pinchev M, et al. Development of APE1 enzymatic DNA repair assays: low APE1 activity is associated with increase lung cancer risk. Carcinogenesis 2015;36:982-91. [Crossref] [PubMed]
- Lee YC, Morgenstern H, Greenland S, et al. A case-control study of the association of the polymorphisms and haplotypes of DNA ligase I with lung and upper-aerodigestive-tract cancers. Int J Cancer 2008;122:1630-8. [Crossref] [PubMed]
- Sakoda LC, Loomis MM, Doherty JA, et al. Germ line variation in nucleotide excision repair genes and lung cancer risk in smokers. Int J Mol Epidemiol Genet 2012;3:1. [PubMed]
- Doherty JA, Sakoda LC, Loomis MM, et al. DNA repair genotype and lung cancer risk in the beta-carotene and retinol efficacy trial. Int J Mol Epidemiol Genet 2013;4:11-34. [PubMed]
- H S. hOGG1 Ser326Cys polymorphism and lung cancer susceptibility. Cancer Epidemiol Biomarkers Prev 1999;8:669-74. [PubMed]
- Hatt L, Loft S, Risom L, et al. OGG1 expression and OGG1 Ser326Cys polymorphism and risk of lung cancer in a prospective study. Mutat Res 2008;639:45-54. [Crossref] [PubMed]
- Ito H, Hamajima N, Takezaki T, et al. A limited association of OGG1 Ser326Cys polymorphism for adenocarcinoma of the lung. J Epidemiol 2002;12:258-65. [Crossref] [PubMed]
- Janik J, Swoboda M, Janowska B, et al. 8-Oxoguanine incision activity is impaired in lung tissues of NSCLC patients with the polymorphism of OGG1 and XRCC1 genes. Mutat Res 2011;709-710:21-31. [Crossref] [PubMed]
- Kohno T, Kunitoh H, Toyama K, et al. Association of the OGG1 -Ser326Cys polymorphism with lung adenocarcinoma risk. Cancer Sci 2006;97:724-8. [Crossref] [PubMed]
- Kohno T, Shinmura K, Tosaka M, et al. Genetic polymorphisms and alternative splicing of the hOGG1 gene, that is involved in the repair of 8-hydroxyguanine in damaged DNA. Oncogene 1998;16:3219-25. [Crossref] [PubMed]
- Lan Q, Mumford JL, Shen M, et al. Oxidative damage-related genes AKR1C3 and OGG1 modulate risks for lung cancer due to exposure to PAH-rich coal combustion emissions. Carcinogenesis 2004;25:2177. [Crossref] [PubMed]
- Liang G, Pu Y, Yin L. Rapid Detection of Single Nucleotide Polymorphisms Related with Lung Cancer Susceptibility of Chinese Population. Cancer Lett 2005;223:265. [Crossref] [PubMed]
- Liu CJ, Hsia TC, Tsai RY, et al. The joint effect of hOGG1 single nucleotide polymorphism and smoking habit on lung cancer in Taiwan. Anticancer Res 2010;30:4141. [PubMed]
- Loft S, Svoboda P, Kasai H, et al. Prospective study of 8-oxo-7,8-dihydro-2'-deoxyguanosine excretion and the risk of lung cancer. Carcinogenesis 2006;27:1245-50. [Crossref] [PubMed]
- Miyaishi A, Osawa K, Osawa Y, et al. MUTYH Gln324His gene polymorphism and genetic susceptibility for lung cancer in a Japanese population. J Exp Clin Cancer Res 2009;28:10. [Crossref] [PubMed]
- Okasaka T, Matsuo K, Suzuki T, et al. hOGG1 Ser326Cys polymorphism and risk of lung cancer by histological type. J Hum Genet 2009;54:739. [Crossref] [PubMed]
- Park J, Chen L, Tockman MS, et al. The human 8-oxoguanine DNA N-glycosylase 1 (hOGG1) DNA repair enzyme and its association with lung cancer risk. Pharmacogenetics 2004;14:103. [Crossref] [PubMed]
- Qian B, Zhang H, Zhang L, et al. Association of genetic polymorphisms in DNA repair pathway genes with non-small cell lung cancer risk. Lung Cancer 2011;73:138. [Crossref] [PubMed]
- Qin H, Zhu J, Zeng Y, et al. Aberrant promoter methylation of hOGG1 may be associated with increased risk of non-small cell lung cancer. Oncotarget 2017;8:8330-41. [PubMed]
- Sørensen M, Raaschou-Nielsen O, Hansen RD, et al. Interactions between the OGG1 Ser326Cys polymorphism and intake of fruit and vegetables in relation to lung cancer. Free Radic Res 2006;164:885-91. [Crossref] [PubMed]
- Sunaga N, Kohno T, Yanagitani N, et al. Contribution of the NQO1 and GSTT1 Polymorphisms to Lung Adenocarcinoma Susceptibility. Cancer Epidemiol Biomarkers Prev 2002;11:730-8. [PubMed]
- Wikman H, Risch A, Klimek F, et al. hOGG1 polymorphism and loss of heterozygosity (LOH): significance for lung cancer susceptibility in a caucasian population. Int J Cancer 2000;88:932-7. [Crossref] [PubMed]
- Cheng Z, Wang W, Song YN, et al. hOGG1, p53 genes, and smoking interactions are associated with the development of lung cancer. Asian Pacific J Cancer Prevention 2012;13:1803-8. [Crossref] [PubMed]
- Karahalil B, Emerce E, Koçer B, et al. The association of OGG1 Ser326Cys polymorphism and urinary 8-OHdG levels with lung cancer susceptibility: a hospital-based case-control study in Turkey. Arh Hig Rada Toksikol 2008;59:241-50. [Crossref] [PubMed]
- Le ML, Donlon T, Lumjones A, et al. Association of the hOGG1 Ser326Cys polymorphism with lung cancer risk. Cancer Epidemiol Biomarkers Prev 2002;11:409. [PubMed]
- Chang CH, Hsiao CF, Chang GC, et al. Interactive effect of cigarette smoking with human 8-oxoguanine DNA N-glycosylase 1 (hOGG1) polymorphisms on the risk of lung cancer: a case-control study in Taiwan. Am J Epidemiol 2009;170:695-702. [Crossref] [PubMed]
- Al-Tassan N, Eisen T, Maynard J, et al. Inherited variants in MYH are unlikely to contribute to the risk of lung carcinoma. Hum Genet 2004;114:207-10. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Wells GA, Shea BJ, O'Connell D, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. Appl Eng Agric 2012;18:727-34.
- de Souza MR, Rohr P, Kahl VFS, et al. The influence of polymorphisms of xenobiotic-metabolizing and DNA repair genes in DNA damage, telomere length and global DNA methylation evaluated in open-cast coal mining workers. Ecotoxicol Environ Saf 2020;189:109975. [Crossref] [PubMed]
- Zhang Y, Yang L, Kucherlapati M, et al. Global impact of somatic structural variation on the DNA methylome of human cancers. Genome Biol 2019;20:209. [Crossref] [PubMed]
- Lorenzo-Gonzalez M, Ruano-Ravina A, Torres-Duran M, et al. Residential radon, genetic polymorphisms in DNA damage and repair-related. Lung Cancer 2019;135:10-5. [Crossref] [PubMed]
- Degtyareva NP, Chen L, Mieczkowski P, et al. Chronic oxidative DNA damage due to DNA repair defects causes chromosomal instability in Saccharomyces cerevisiae. Mol Cell Biol 2008;28:5432-45. [Crossref] [PubMed]
- Shakeri M, Zakeri F, Changizi V, et al. Cytogenetic effects of radiation and genetic polymorphisms of the XRCC1 and XRCC3 repair genes in industrial radiographers. Radiat Environ Biophys 2019;58:247-55. [Crossref] [PubMed]
- Polo LM, Xu Y, Hornyak P, et al. Efficient Single-Strand Break Repair Requires Binding to Both Poly(ADP-Ribose) and DNA by the Central BRCT Domain of XRCC1. Cell Rep 2019;26:573-81.e5. [Crossref] [PubMed]
- Chen L, Zhuo D, Chen J, et al. XRCC1 polymorphisms and lung cancer risk in Caucasian populations: a meta-analysis. Int J Clin Exp Med 2015;8:14969-76. [PubMed]
- Vineis P, Manuguerra M, Kavvoura FK, et al. A field synopsis on low-penetrance variants in DNA repair genes and cancer susceptibility. J Natl Cancer Inst 2009;101:24-36. [Crossref] [PubMed]
- Huang G, Cai S, Wang W, et al. Association between XRCC1 and XRCC3 Polymorphisms with Lung Cancer Risk: A Meta-Analysis from Case-Control Studies. PLos One 2013;8:e68457. [Crossref] [PubMed]
- Liu ZJ, Martinez Cuesta S, van Delft P, et al. Sequencing abasic sites in DNA at single-nucleotide resolution. Nat Chem 2019;11:629-37. [Crossref] [PubMed]
- Kim JM, Yeo MK, Lim JS, et al. APEX1 Expression as a Potential Diagnostic Biomarker of Clear Cell Renal Cell Carcinoma and Hepatobiliary Carcinomas. J Clin Med 2019; [Crossref] [PubMed]
- Lu GS, Li M, Xu CX, et al. APE1 stimulates EGFR-TKI resistance by activating Akt signaling through a redox-dependent mechanism in lung adenocarcinoma. Cell Death Dis 2018;9:1111. [Crossref] [PubMed]
- Wang T, Wang H, Yang S, et al. Association of APEX1 and OGG1 gene polymorphisms with breast cancer risk among Han women in the Gansu Province of China. BMC Med Genet 2018;19:67. [Crossref] [PubMed]
- Kim H, Seo H, Park Y, et al. APEX1 Polymorphism and Mercaptopurine-Related Early Onset Neutropenia in Pediatric Acute Lymphoblastic Leukemia. Cancer Res Treat 2018;50:823-34. [Crossref] [PubMed]
- Xiao X, Yang Y, Ren Y, et al. rs1760944 Polymorphism in the APE1 Region is Associated with Risk and Prognosis of Osteosarcoma in the Chinese Han Population. Sci Rep 2017;7:9331. [Crossref] [PubMed]
- Ding G, Chen Y, Pan H, et al. Association between apurinic/apyrimidinic endonuclease 1 rs1760944 T>G polymorphism and susceptibility of cancer: a meta-analysis involving 21764 subjects. Biosci Rep 2019;39. [PubMed]
- Zhang X, Xin X, Zhang J, et al. Apurinic/apyrimidinic endonuclease 1 polymorphisms are associated with ovarian cancer susceptibility in a Chinese population. Int J Gynecol Cancer 2013;23:1393-9. [Crossref] [PubMed]
- Yuan H, Li H, Ma H, et al. Genetic polymorphisms in key DNA repair genes and risk of head and neck cancer in a Chinese population. Exp Ther Med 2012;3:719-24. [Crossref] [PubMed]
- Jin EH, Kim J, Lee SI, et al. Association between polymorphisms in APE1 and XRCC1 and the risk of gastric cancer in Korean population. Int J Clin Exp Med 2015;8:11484. [PubMed]
- Luo D, Gao Y, Wang S, et al. Genetic variation in PLCE1 is associated with gastric cancer survival in a Chinese population. J Gastroenterol 2011;46:1260. [Crossref] [PubMed]
- Dai ZJ, Wang XJ, Kang AJ, et al. Association between APE1 Single Nucleotide Polymorphism (rs1760944) and Cancer Risk: a Meta-Analysis Based on 6,419 Cancer Cases and 6,781 Case-free Controls. J Cancer 2014;5:253-9. [Crossref] [PubMed]
- Abduljaleel Z. Structural and Functional Analysis of human lung cancer risk associated hOGG1 variant Ser326Cys in DNA repair gene by molecular dynamics simulation. Noncoding RNA Res 2019;4:109-19. [Crossref] [PubMed]