
Modulation of intraperitoneal (IP) chemotherapy in ovarian cancer
Rationale for IP therapy and recent Gynecologic Oncology Group (GOG) trials
Advanced epithelial ovarian cancer spreads predominantly within the peritoneal cavity, and this has prompted investigations into the delivery of chemotherapy directly into the space lined by visceral and parietal peritoneum. Intraperitoneal (IP) radionuclides, other radioimmunoconjugates, and bolus cytotoxic drugs administered in the presence of ascites were tested since the 1950s but seldom subject to rigorous clinical study. In 1978, Dedrick et al. developed the pharmacologic rationale for IP drug delivery: peripheral surfaces of small tumors within the peritoneal cavity could be exposed to higher cytotoxic drug concentrations for longer durations of time than could be safely attained with systemic drug administration (1). Further, the central portions of the tumor continue to be exposed to drug via systemic absorption from the peritoneal cavity—thus, the subsequent designation in the literature of two-way chemotherapy.
The magnitude of the pharmacologic advantage achieved with IP therapy is defined by the ratio of drug concentrations over time in the peritoneal and plasma compartments: the area under the concentration times curves, or the AUC ratio. For cisplatin and carboplatin, the AUC ratios for IP over IV delivery are in the range of 15-20, while for drugs such as paclitaxel, the AUC ratio approaches 1,000. The ideal drugs for IP delivery are those that maintain a long residence time in the peritoneal cavity and then are cleared rapidly once they are absorbed into systemic circulation across the visceral peritoneum. This limits the exposure to normal tissues and subsequent toxicities. The ideal dosing for IP drugs achieves a high AUC ratio without compromising plasma drug concentrations that can be achieved with IV delivery.
The clinical evidence to support the efficacy of the IP approach in ovarian cancer is now well established (Table 1). The GOG 104 trial looked at IV cyclophosphamide (600 mg/m2) with either IV or IP cisplatin (100 mg/m2) administered every 3 weeks for six cycles in women with small-volume disease after surgical cytoreduction (4). The largest residual tumor nodules of <2 cm in diameter (which defined optimal cytoreduction in GOG 104) account in large part for the worse outcome observed relative to subsequent trials that selected patients for study with <1 cm of residual disease following surgical cytoreduction. The IP group had a median overall survival (OS) of 49 months compared with 41 months in the IV group (P=0.02). Despite the favorable results of the IP arm of this trial, it was theorized that such advantage might not apply if one substituted IV paclitaxel for cyclophosphamide. This led to the GOG 114 trial in which patients with residual ovarian cancer (<1 cm in diameter of remaining tumor) were randomized to either IV paclitaxel (135 mg/m2) and IV cisplatin (75 mg/m2) every three weeks for six cycles, or the experimental arm consisting of ‘chemical debulking’ of IV carboplatin (AUC =9) every 28 days for two cycles, followed by IV 24-hour infusion of paclitaxel (135 mg/m2) and IP cisplatin (100 mg/m2) every 3 weeks for six cycles (3). The IP arm demonstrated improvement in both progression-free survival (PFS) (median of 28 vs. 22 months; HR, 0.78; P=0.01) and OS (median of 63 vs. 52 months; HR, 0.81; P=0.051). However, the large doses of carboplatin administered resulted in bone marrow suppression and thrombocytopenia, and subsequently 19% of patients in this arm of the study received two or fewer courses of the planned IP regimen.
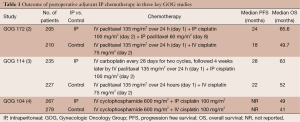
Full table
The most quoted survival data for IP therapy comes from the GOG 172 trial (2). In this study, the control arm was IV paclitaxel (135 mg/m2) followed by IV cisplatin (75 mg/m2) every 3 weeks for six cycles, and the experimental arm was IV paclitaxel (135 mg/m2) on day 1 followed by IP cisplatin (100 mg/m2) on day 2 plus IP paclitaxel (60 mg/m2) on day 8. The women in this study were also optimally cytoreduced to <1 cm, including more than 10% having bowel resections. Although the IP therapy arm was associated with considerably more toxicity (myelosuppression, abdominal pain, neuropathy), treatment with this regimen still resulted in a statistically significant improvement in both PFS (median of 24 vs. 18 months; P=0.027) and OS (median of 65.6 vs. 49.7 months; P=0.017). Only 42% of patients received all six cycles of treatment in the IP arm and many of these were shifted to receive some IV carboplatin; an analysis of quality-of-life found no differences between the two treatment groups at 12 months follow-up.
The results of these three randomized trials have demonstrated that IP administration of up to six cycles of a cisplatin-based chemotherapy regimen influences survival in women with advanced ovarian cancer and minimal residual disease. A Cochrane review of IP chemotherapy in women undergoing treatment for initial management of advanced ovarian cancer reported a 21% decrease in the risk of death (HR, 0.79; 95% CI: 0.70-0.90) in patients undergoing combined IP and IV therapy vs. those undergoing IV therapy alone (5). In 2006, based on the GOG 172 trial as well as a meta-analysis of IP vs. IV randomized trials in ovarian cancer, the U.S. National Cancer Institute (NCI) issued an announcement: “On the basis of the results of these randomized phase III clinical trials, a combination of IV and IP administration of chemotherapy conveys a significant survival benefit among women with optimally debulked ovarian cancer, compared to IV administration alone” (6).
However, despite the NCI endorsement and improved survival demonstrated with the IP approach, it remains the subject of scientific debate and has not been widely adopted into routine clinical practice. Concerns about toxicity, dosing, patient tolerability, and catheter-related complications (such as port infection or occlusion) have continued to impede its acceptance in the medical community. The weight of the evidence supports a favorable effect on outcome with administration of IP cisplatin; however, modifications of the dose and schedules of the two agents to improve tolerance need to be explored. Currently, IP cisplatin at 75 mg/m2 is being used in non-study patients who are optimally debulked and in whom an IP catheter has been inserted, and most have switched from 24-hour paclitaxel IV to shorter infusions and omission of the day 8 IP treatment with this agent (7).
Rationale for IP carboplatin
A major drawback of IP therapy involves the use of IP cisplatin, which is more toxic compared to carboplatin, the current standard IV chemotherapy agent. When given IV, carboplatin has demonstrated therapeutic equivalence to cisplatin and has now largely replaced cisplatin as primary systemic therapy for patients with ovarian cancer. It is important to explore whether carboplatin may replace cisplatin in IP chemotherapy. There is strong evidence that the antitumor activity of platinum drugs is related to the amount of drug reaching the tumor. A preclinical study by Jandial et al. compared the ability of cisplatin vs. carboplatin to penetrate from the peritoneum into tumor nodules in an IP human ovarian cancer xenograft model (8). Following injection of equimolar doses of the two platinum drugs, cisplatin produced a 3.4-fold higher level of concentration in tumor nodules when compared with carboplatin (P=0.02). However, when cisplatin and carboplatin were injected at doses that were equitoxic to the mice, platinum tumor concentrations were equivalent (P=0.63). Equitoxic dosing in this animal model more closely reflected dosing in patients, where usual doses are determined by maximum tolerated dose. Interestingly, platinum tumor concentrations decreased with increasing tumor size following IP cisplatin, whereas the concentration level remained nearly constant with increasing tumor size following IP carboplatin (P<0.001), suggesting that for larger tumor nodules, carboplatin may be better retained in tumors than cisplatin, and thus more efficacious.
The safety and efficacy of IP carboplatin has been established in several clinical trials, however, no trials to date have directly compared IP cisplatin vs. IP carboplatin. Fujiwara et al. retrospectively analyzed the recurrence and survival of a cohort of 174 patients with ovarian cancer who were treated with first line IP carboplatin therapy (9). The chemotherapy regimen was either carboplatin alone (n=22) or in combination with cyclophosphamide (n=116) or paclitaxel (n=27). The median number of chemotherapy cycles was six and the median number of IP cycles was five, suggesting that IP therapy was well tolerated. The response in 54 patients with measurable disease was 66.4%. The 5-year survival was 94.4% for stage I and 87.9% for stage II disease. The median survival for optimal and suboptimal stage III/IV patients was 51 months and 34 months, respectively. Of note, the median survival of patients with stage III/IV disease was 51 months with carboplatin doses of 400 mg/m2 or more, but it was only 25 months with carboplatin doses lower than 400 mg/m2.
Ongoing trials involving IP carboplatin
Currently, there are three ongoing large-scale prospective randomized trials that are examining the efficacy of carboplatin-based IP therapy. The GOG 252 trial aims to improve tolerability of IP therapy and compare PFS and OS to an IV arm (Table 2). The trial has recently completed accrual in 2011 and has three study arms: (I) IV carboplatin (AUC =6) and IV dose-dense paclitaxel (80 mg/m2 on days 1, 15); (II) IP carboplatin (AUC =6) and IV dose-dense paclitaxel (80 mg/m2 on days 1, 15); and (III) IV paclitaxel (135 mg/m2 on day 1), IP cisplatin (75 mg/m2 on day 2), and IP paclitaxel (60 mg/m2 on day 8) (regimen modified from GOG 172). All three arms also receive bevacizumab 15 mg/kg followed by an additional 18 months of maintenance. The rationale for the IV arm is based on the results of the Japanese GOG (JGOG) 3016 trial, which randomized women with stage II-IV ovarian cancer to receive paclitaxel every 3 weeks or paclitaxel weekly (with IV carboplatin administered every 3 weeks in both regimens) (10). JGOG 3016 results showed that the dose-dense arm had a significant improvement in PFS (28.0 vs. 17.2 months; HR, 0.71; 95% CI: 0.58-0.88; P=0.0015) and OS (72% vs. 65%; HR, 0.75; 95% CI: 0.57-0.98; P=0.03) compared to conventional every 3 weeks dosing at 3 years. Study results are pending for GOG 252; the primary and secondary outcome measures are PFS and OS, respectively, and will allow the first trial comparison of IP carboplatin with IP cisplatin. Second, the JGOG 3019 phase III trial is evaluating IV carboplatin (AUC =6) vs. IP carboplatin (AUC =6) with both arms receiving dose-dense weekly IV paclitaxel (80 mg/m2) (Table 2) (11).
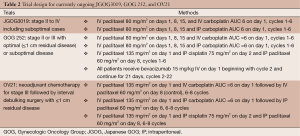
Full table
Finally, a unique trial is the OV-21/GCIG study led by the Canadian National Cancer Institute (Table 2). All patients with stage III disease will receive neoadjuvant chemotherapy. Those patients who respond to neoadjuvant therapy will undergo interval debulking surgery. If the residual disease after surgery is <1 cm, then the patient will be randomized to one of three treatment arms: (I) control arm with combination of IV paclitaxel (135 mg/m2) followed by IV carboplatin (AUC =5) on day 1 and then IV paclitaxel (60 mg/m2) on day 8; (II) Arm 2 is the same as the control, however carboplatin will be given by the IP route; and (III) Arm 3 is the modified GOG 172 arm (same as GOG 252 trial but bevacizumab will not be given). There will be a phase II run-in in this trial to narrow the selection of either IP platinum for a phase III study of IV vs. IP administration of the platinum.
Other IP doublet drug combinations
IP etoposide + carboplatin
One of the first IP doublet studies was conducted by Muggia et al. in cisplatin or carboplatin pretreated ovarian cancer patients with low-volume intra-abdominal recurrences (12). It enrolled 18 patients in a phase I study of IP carboplatin and IP etoposide. Patients received a fixed dose of carboplatin (200 mg/m2) whereas the dose of etoposide was escalated in cohorts of from patients from 50 to 75 and eventually to 100 mg/m2. As hematologic toxicities predominated, IP cisplatin at 40 mg/m2 was substituted for IP carboplatin in five patients during their last two cycles. At a median follow-up of 4 years, eight patients (out of the original 18) are alive, and four had no evidence of disease 1 to 4 years after treatment onset.
IP FUDR + platinum
A subsequent doublet study by Muggia et al. enrolled 16 patients with similar peritoneal small-volume recurrence of ovarian cancer and was a phase I/II study of IP platinums (cisplatin and/or carboplatin) and floxuridine (FUDR), a metabolite of 5-fluorouracil (5-FU) (13). In this study, patients received IP FUDR (3 mg on days 1-3) and cisplatin (60 mg/m2 on day 3), with dose adjustments or full or partial substitution of cisplatin by carboplatin for neuropathy or other major intolerance. Leucovorin was used together with FUDR at a dose of 320 mg, as previously described. Six cycles were planned. Six of 11 patients had minimal residual disease and completed six cycles of the combined treatment, and one completed five cycles. Overall, the combination of FUDR and both platinums were particularly well tolerated. The median time to progression was 15 months, with the survival being in excess of 26 months with 8 of 11 patients alive at minimum follow-up of 32 (range, 32-43) months. Another study of this doublet by Muggia extended observations of above doses of IP cisplatin and FUDR: 14 patients with stage III ovarian cancer were enrolled to receive this regimen as consolidation after induction with carboplatin and paclitaxel and a negative second-look surgical assessment (14). The mean number of cycles administered was 3.2, and the median time to recurrence was 19.4 months.
A larger study to explore the hypothesis that IP FUDR may be eradicating small foci of residual epithelial ovarian cancer beyond what may be achievable with platinum compounds would be desirable. FUDR causes DNA damage that is repaired by base excision, therefore, poly ADP ribose polymerase (PARP) inhibitors which disrupt base excision repair may sensitize ovarian cancer cells to FUDR—suggesting that this combination may have synergistic activity in this disease (15). Interestingly, PARP inhibition synergizes with FUDR but not 5-FU in ovarian cancer cells (16). Currently, there is a promising phase I trial (NCT01749397) at the Mayo Clinic looking at the combination of the PARP inhibitor ABT-888 (veliparib) with IP FUDR in ovarian, fallopian tube, and primary peritoneal cancers.
IP cyclosporine + carboplatin
Chambers et al. investigated the feasibility and pharmacokinetics of IP cyclosporine followed by a phase II dose-escalation of the combination of IP cyclosporine and carboplatin in refractory ovarian cancer patients (17). The rationale for the use of cyclosporine includes its ability to overcome classic multidrug resistance in tumors associated with resistance due to overexpression of the p-glycoprotein gene, and it may also modulate both platinum sensitivity and resistance (18,19). In this study, three patients initially received 1, 10, and 20 mg/kg of IP cyclosporine alone. A phase I trial of 35 patients was then performed in which the highest dose of IP cyclosporine delivered was 34.6 mg/kg in combination with IP carboplatin (250 or 300 mg/m2), which was not dose-escalated. This regimen was given after 1 to 9 lines of prior therapy (median, 2) and these patients had received between 1 and 4 (median, 2) prior platinum-based therapies. In this study, three objective responses (two complete and one partial) were observed for 3-11 months. At the highest dose of cyclosporine delivered (34.6 mg/kg), the mean IP cyclosporine levels of 1,110 µg/mL were overall well tolerated, and the IP fluid ratio of 1,000:1 was maintained. In addition, pharmacokinetic analysis demonstrated a significant higher exposure of the peritoneal cavity compared with the systemic circulation. Common toxicities at this dose were anemia, leukopenia, thrombocytopenia, and hypertension. The pharmacologic advantage demonstrated in this study is interesting in the support of future studies using IP cyclosporine for the modulation of platinum resistance.
Modification of IP drug uptake
There is strong evidence, both clinically and in model systems, that the efficacy of IP as well as IV chemotherapy is primarily limited by poor drug penetration into solid tumors (20,21). Blood flow to the tumor, capillary permeability, tumor interstitial fluid pressure, binding and inactivation of drug in the extracellular matrix, and drug diffusion coefficients all play important roles in determining the depth of drug penetration (22). With regard to IP delivery to peritoneal tumors, a preclinical xenograft model demonstrated that after IP administration of doxorubicin into tumors, the measureable drug concentrations were present only in the outermost 4-6 layers of the tumor (23). Similarly, another study found that tumor concentrations of platinum were limited to the outermost 1.5 mm following IP administration of cisplatin in a rat colon carcinoma model (20).
Strategies to improve drug delivery with anti-angiogenic therapy
Anti-angiogenic therapy has been shown to decrease tumor vessel density, diameter, and permeability (24). Based on these observations and the known determinants of capillary washout of drug after IP chemotherapy, it has been hypothesized that the addition of anti-angiogenic therapy to IP chemotherapy may increase drug penetration into tumors. Using computer simulations and animal models of ovarian cancer, Shah et al. demonstrated that anti-angiogenic therapy leads to a significant and selective increase in drug exposure to peritoneal tumors following IP chemotherapy (25). Therapeutic studies also conducted with cisplatin and topotecan showed that animals receiving a combination of IP chemotherapy and anti-angiogenic treatment had greater survival compared with animals treated with IP chemotherapy alone, IV chemotherapy with anti-angiogenic therapy, and anti-angiogenic therapy alone. Pharmacokinetic studies demonstrated that combining anti-angiogenic therapy with IP topotecan increased topotecan concentration in the peritoneal tumors by about 6.5-fold relative to the concentration observed in animals receiving IP topotecan alone (25).
The above results led to a study by the same authors, which evaluated the pharmacokinetics of bevacizumab following IP and IV administration and investigated combined bevacizumab therapy (IP or IV) with IP paclitaxel or carboplatin in a mouse model (26). Following IP administration, bevacizumab was rapidly absorbed and bioavailability was 92.8%. With the addition of IP paclitaxel, the median survival time was increased by 24% compared with 33 days for the control mice. The combination of bevacizumab and IP paclitaxel was superior to paclitaxel alone (P=0.04 for IP and P=0.01 for IV bevacizumab), and combined bevacizumab and IP carboplatin was superior to carboplatin alone (P=0.02 for IP and P=0.002 for IV bevacizumab). There was no significant difference in survival between the groups receiving bevacizumab IV or IP, either alone (P=0.586), in combination with IP paclitaxel (P=0.467) or in combination with IP carboplatin (P=0.149). Overall, following IP administration in mice, bevacizumab demonstrated rapid and near complete absorption, and there was a significant increase in animal survival following combined therapy with bevacizumab and IP paclitaxel or IP carboplatin, of which the results were not dependent on the route of bevacizumab administration (IP vs. IV).
The above study results may be viewed as strong support for the clinical evaluation of combined anti-angiogenic therapy and IP therapy in ovarian cancer. Currently, clinical trial NCT00652119 (estimated primary completion date in 2016) is a pilot study investigating the safety and tolerability of paclitaxel and carboplatin when given in combination with bevacizumab in patients with ovarian, fallopian tube, or primary peritoneal cancer. Patients are scheduled for six 3-week cycles of therapy. Cycle 1 consists of IV paclitaxel (60 mg/m2) weekly for 3 weeks and carboplatin (AUC =6) IV on day 1, and cycle 2 involves IP paclitaxel (60 mg/m2) weekly for 3 weeks, IP carboplatin (AUC =6) on day 1, and IV bevacizumab (15 mg/kg) on day 8. For cycles 3-6, the dosing regimen for paclitaxel and carboplatin is the same as in cycle 2, however, bevacizumab is administered on day 1 of the cycle instead of on day 8. As described above, the GOG 252 trial is also randomizing patients with either optimal or suboptimal disease to three arms of treatment: standard IV regimen of paclitaxel/carboplatin vs. IV paclitaxel/IP carboplatin vs. IV paclitaxel/IP paclitaxel/IP cisplatin. All arms have bevacizumab concurrent with chemotherapy and as maintenance treatment after chemotherapy completion.
Strategies to improve platinum drug delivery by targeting its receptor
The copper transporter 1 (CTR1) is a major influx transporter for platinum-based drugs such as cisplatin and carboplatin (27). Several studies have shown that the accumulation of platinum in ovarian cancer cells is limited by the rapid down-regulation of CTR1 that is triggered upon exposure to platinum agents, thereby limiting any additional platinum uptake. Once inside the cell, CTR1 is degraded in the proteasome (28). This degradation of CTR1 can be attenuated by proteasome inhibition with bortezomib in a dose-dependent fashion (29). With maintenance of CTR1 expression following bortezomib pretreatment, platinum concentrations in both ovarian cancer cells in vitro and peritoneal tumors in an ovarian cancer IP model were significantly increased [up to 2.4-fold in cells, and 33% in tumors (P=0.006)]. This study provided a novel basis on which to develop a clinical strategy that addresses the challenge of improving the efficacy of IP platinum therapy and overcoming platinum resistance by pretreating patients with IP bortezomib. It further provided preclinical animal data supporting the delivery of bortezomib via the IP route, demonstrating a pharmacologic advantage with a peritoneal:plasma AUC ratio of 252. The phase I clinical trial NCT01074411 combines IP bortezomib with IP carboplatin in patients with persistent or recurrent ovarian cancer, fallopian tube cancer, or primary peritoneal cancer, and published results are expected shortly.
Proteasome inhibition affects a myriad of cellular functions in addition to modulation of CTR1 expression. The effects of bortezomib on drug penetration in multicellular cell culture as well as animal models have been investigated. Interestingly, in a hepatocellular carcinoma model, pretreatment with bortezomib was found to enhance chemotherapeutic penetration and cytotoxicity of doxorubicin, gemcitabine and 5-FU by 1.7- to 3-fold through its effects on cellular adhesion and packing density. Bortezomib pretreatment also allowed for improved tumor distribution of doxorubicin (P=0.024 and P=0.053), however, the trend toward slowing overall tumor growth did not reach statistical significance (30). This provides further rationale that bortezomib by the IP route may work well in combination with other IP chemotherapeutic agents in order to pharmacologically enhance the delivery and efficacy of these agents.
Finally, since copper and platinum transport are both controlled by CTR1, another pharmacologic strategy uses copper chelators to modulate CTR1 expression and therefore, platinum influx into tumors. Copper chelation results in up-regulation of CTR1 and subsequently enhances platinum accumulation (31-33). A recent pilot study combining the IV use of copper chelator trientine in addition to IV carboplatin (AUC =6) in platinum-resistant patients with advanced tumors showed that this regimen was well tolerated. Of 55 patients, one patient had a partial response (1.8%) and an additional eight patients (14.5%) had stable disease lasting >6 months. While the number of patients achieving a response was low and duration was short, these results remain encouraging, considering that the study population was heavily pretreated (median of 4 prior regimens) and that platinum-resistant or refractory patients in general have such poor prognosis (34). Further investigation of this combination, perhaps incorporating IP carboplatin, is warranted.
Strategies to improve drug delivery with targeted delivery to tumors
Delivery of therapeutic agents selectively to tumor tissue, which is referred as “targeted delivery”, is one of the most ardently pursued goals of cancer therapy. Recent advances in nanotechnology enable numerous types of nanoparticles (NPs) whose properties can be designed for targeted delivery to tumors. In spite of promising early results, the delivery and therapeutic efficacy of the majority of NPs are still quite limited, and research is primarily limited to preclinical stages at this time. Challenges are mainly attributed to the limitation of currently available tumor models to test these NPs to systematically study the effects of complex transport and pathophysiological barriers around the tumors. This area of study, including how most effectively to target NPs to the tumors with targeting ligands, remains a fascinating new area of study with great potential to be able to modulate IP therapy.
In a similar approach, a recent preclinical study using a lipidoid IP delivery system to deliver small interfering RNA (siRNA) to PARP1 in a BRCA1 deficient murine ovarian cancer model showed that PARP inhibition inhibited cell growth in vitro and extended survival of mice bearing BRCA1 deficient tumors (35). Another preclinical study used a multifunctional receptor targeted DNA nanocassettes to deliver siRNA in a breast xenograft model (36). The further development of such delivery systems and introduction into clinical trials is a highly promising method to deliver siRNAs which can target a host of anticancer targets, and potentially modulate IP therapy.
Strategies to improve drug delivery with hyperthermia using HIPEC
In an effort to avoid the toxicities encountered with IP chemotherapy, an innovative locoregional treatment approach that has emerged in the management of peritoneal surface malignancies is optimal cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC). HIPEC, initially developed by Paul Sugarbaker for the more chemoresistant mucinous adenocarcinomas, allowed for timed perioperative perfusion of IP chemotherapy at temperatures of 42 °C immediately following extensive surgical cytoreduction. The rationale for HIPEC includes the preferred timing of such regional treatment immediately after debulking, the individual antitumor contributions by both chemotherapy and hyperthermia, the synergistic effects of the two modalities, reversal of platinum resistance by hyperthermia, and enhanced penetration of drugs into tumors (37). Heat is known to be cytotoxic in vitro at 42-45 °C. Hyperthermia at 42 °C has been shown to enhance the antitumor effects of drugs including platinum agents by augmenting cytotoxicity and increasing the penetration of drugs into tissue (38). HIPEC has increasingly been used during initial resection of cancers presenting with peritoneal carcinomatosis including gastric, pancreatic, colorectal or appendiceal, and pseudomyxoma peritonei. This list now includes a growing experience in carcinomatosis of ovarian origin. Although encouraging data have been published for HIPEC primarily in recurrent ovarian cancer, approaches have been variable (in terms of drugs, length of hyperthermia, and clinical setting including the key platinum sensitivity of patients) and at present have not yet been evaluated in randomized studies, or compared with repeated IP or IV chemotherapy.
Role for IP consolidation therapy after neoadjuvant therapy?
Currently, the standard treatment for advanced ovarian cancer is primary cytoreductive surgery followed by adjuvant chemotherapy. Recently, there has been emerging interest in whether neoadjuvant chemotherapy followed by interval debulking surgery and additional adjuvant chemotherapy is beneficial for a select group of patients. The feasibility of intraoperative IP chemotherapy with cisplatin in ovarian cancer was assessed in a retrospective study that looked at ten patients who were diagnosed with primary epithelial ovarian cancer and underwent an optimal staging procedure (39). IP chemotherapy was administered through open or laparoscopic procedures according to the surgical approach, and a cisplatin dose of 70 mg/m2 in 1 L normal saline was administered into the abdominal cavity for 24 hours postoperatively. Adjuvant chemotherapy was started 2-4 weeks after surgery. Perioperative toxicity was found to be acceptable and majority of adverse events included grade 1 and 2 toxicity (nausea, vomiting, ileus, elevated liver enzymes and creatinine), which were self-limiting or resolved with medical treatment. One patient experienced disease recurrence in the liver 16 months after surgery. The remaining nine patients have been well controlled by chemotherapy and/or observation during the follow-up period of 4 to 39 months after surgery.
Vergote et al. performed a prospective randomized trial which compared treatment efficacy and quality of life after primary debulking surgery (PDS) plus adjuvant chemotherapy vs. neoadjuvant chemotherapy followed by interval debulking surgery (IDS) and additional adjuvant chemotherapy in 632 patients with stage IIIC or IV disease (40). The largest residual tumor was ≤1 cm in diameter in 41.6% of patients after PDS and 80.6% of patients after IDS. Postoperative adverse events and mortality were higher after primary debulking than after interval debulking. The HR for death in the group assigned to neoadjuvant chemotherapy followed by IDS when compared with the group that received PDS and adjuvant chemotherapy was 0.98 (90% CI: 0.84-1.13; P=0.01 for noninferiority). The strongest independent variable in predicting OS was complete resection of all macroscopic disease at either primary or interval surgery. The study authors found that neoadjuvant chemotherapy followed by IDS was not inferior to PDS followed by chemotherapy for patients with bulky stage IIIC or IV disease
A phase I study led by Muggia evaluated the pharmacology, tolerability, and therapeutic potential of IP topotecan alone and with IP cisplatin as potential for consolidation in ovarian cancer in 16 patients (41). IP topotecan (1.25 mg on days 1-3) with IP cisplatin (50 mg/m2) was tolerable, with occasional dose limiting myelosuppression. Patients with minimal [6] or no residual disease [3] after platinum-based induction had a median PFS of 13 months. A phase II study looked at the role of IP topotecan as consolidation chemotherapy in patients with stage II/IV ovarian cancer or primary peritoneal cancer with clinical complete response after surgical cytoreduction and IV carboplatin/paclitaxel chemotherapy and had minimal residual disease (≤1 cm diameter) at second look surgery (42). Twenty patients were enrolled, of which 18 (90%) has ovarian cancer. IP topotecan (20 mg/m2) was infused once every 21 days for 4 to 6 cycles. The mean delivered dose was 18 mg/m2. Totally 16 patients received four cycles, three patients received six cycles, and one patient withdrew after one cycle. Grade 3 and 4 toxicities included neutropenia and thrombocytopenia (45% for both). Median PFS was 24 months from second-look surgery. OS estimated at 3 years from initial diagnosis was 84% (95% CI: 68-100%). Consolidation IP topotecan may therefore be a feasible option for women with advanced ovarian cancer.
Incorporating the above treatment modalities, it is worthwhile to consider the potential role of consolidation with platinum based IP chemotherapy during IDS following neoadjuvant chemotherapy for patients with bulky stage III or IV disease. This approach offers the potential of IP chemotherapy in a regimen that is otherwise purely systemic and may lead to a decrease in disease recurrence, as approximately 70% of patients with ovarian cancer will experience recurrence. After IP consolidation therapy, monitoring with cytology (from the IP port) would be a simple and inexpensive test to predict and diagnose recurrence. A group at the University of Southern Alabama had recently reported results using peritoneal cytology to determine IP treatment results (43). They described 42 patients with ovarian cancer over a three year period with IP ports removed at completion of treatment, and 6 of the 42 (14.3%) had a positive or suspicious cytology. Although the two groups were similar in age, BMI, grade, stage, percent of optimal debulking and performance status, 5/6 (83.3%) in the positive cytology group vs. 13/36 (36.1%) in the negative group were alive at time of analysis. Median time to recurrence was 19 months in the positive group vs. 41.8 months in the negative group (P=0.0038). Median survival in the positive cytology group was 31 months vs. a median survival of 44.9 months in the negative group (P=0.0295). While this experience pertains to patients receiving first-line treatment, knowing that peritoneal cytology is positive at the time of completion of IP treatment and in the ensuing months may yield not only prognostic information, but may also guide subsequent treatment.
Summary
IP chemotherapy with platinum-based regimens can improve survival in women with small volume ovarian cancer. The challenge remains about how to improve the efficacy of this treatment modality while reducing toxicity. The efficacy of IP drug therapy can be hindered by poor penetration of drug from the surface of tumors. Currently, there is ongoing interest and active investigation into strategies which can improve drug penetration or delivery to tumors in the peritoneum.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Franco Muggia and Eleonora Teplinsky) for the series “Epithelial Ovarian Cancer Treatment: Integrating Molecular Targeting” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.12.01). The series “Epithelial Ovarian Cancer Treatment: Integrating Molecular Targeting” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dedrick RL, Myers CE, Bungay PM, et al. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep 1978;62:1-11. [PubMed]
- Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006;354:34-43. [PubMed]
- Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 2001;19:1001-7. [PubMed]
- Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 1996;335:1950-5. [PubMed]
- Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev 2011;CD005340 [PubMed]
- National Cancer Institute. NCI issues clinical announcement for preferred method of treatment for advanced ovarian cancer. 2006. Available online: http://www.cancer.gov/newscenter/pressreleases/2006/ipchemotherapyrelease
- Echarri Gonzalez MJ, Green R, Muggia FM. Intraperitoneal drug delivery for ovarian cancer: why, how, who, what, and when? Oncology (Williston Park) 2011;25:156-65. [PubMed]
- Jandial DD, Messer K, Farshchi-Heydari S, et al. Tumor platinum concentration following intraperitoneal administration of cisplatin versus carboplatin in an ovarian cancer model. Gynecol Oncol 2009;115:362-6. [PubMed]
- Fujiwara K, Sakuragi N, Suzuki S, et al. First-line intraperitoneal carboplatin-based chemotherapy for 165 patients with epithelial ovarian carcinoma: results of long-term follow-up. Gynecol Oncol 2003;90:637-43. [PubMed]
- Katsumata N, Yasuda M, Takahashi F, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 2009;374:1331-8. [PubMed]
- Fujiwara K, Aotani E, Hamano T, et al. A randomized Phase II/III trial of 3 weekly intraperitoneal versus intravenous carboplatin in combination with intravenous weekly dose-dense paclitaxel for newly diagnosed ovarian, fallopian tube and primary peritoneal cancer. Jpn J Clin Oncol 2011;41:278-82. [PubMed]
- Muggia FM, Groshen S, Russell C, et al. Intraperitoneal carboplatin and etoposide for persistent epithelial ovarian cancer: analysis of results by prior sensitivity to platinum-based regimens. Gynecol Oncol 1993;50:232-8. [PubMed]
- Muggia FM, Jeffers S, Muderspach L, et al. Phase I/II study of intraperitoneal floxuridine and platinums (cisplatin and/or carboplatin). Gynecol Oncol 1997;66:290-4. [PubMed]
- Lu MJ, Sorich J, Hazarika M, et al. Intraperitoneal therapy as consolidation for patients with ovarian cancer and negative reassessment after platinum-based chemotherapy. Hematol Oncol Clin North Am 2003;17:969-75. [PubMed]
- Huehls AM, Wagner JM, Huntoon CJ, et al. Identification of DNA repair pathways that affect the survival of ovarian cancer cells treated with a poly(ADP-ribose) polymerase inhibitor in a novel drug combination. Mol Pharmacol 2012;82:767-76. [PubMed]
- Huehls AM, Wagner JM, Huntoon CJ, et al. Poly(ADP-Ribose) polymerase inhibition synergizes with 5-fluorodeoxyuridine but not 5-fluorouracil in ovarian cancer cells. Cancer Res 2011;71:4944-54. [PubMed]
- Chambers SK, Chambers JT, Davis CA, et al. Pharmacokinetic and phase I trial of intraperitoneal carboplatin and cyclosporine in refractory ovarian cancer patients. J Clin Oncol 1997;15:1945-52. [PubMed]
- Kashani-Sabet M, Wang W, Scanlon KJ. Cyclosporin A suppresses cisplatin-induced c-fos gene expression in ovarian carcinoma cells. J Biol Chem 1990;265:11285-8. [PubMed]
- Mutch DG, Herzog TJ, Chen CA, et al. The effects of cyclosporin A on the lysis of ovarian cancer cells by cisplatin or adriamycin. Gynecol Oncol 1992;47:28-33. [PubMed]
- Los G, Mutsaers PH, van der Vijgh WJ, et al. Direct diffusion of cis-diamminedichloroplatinum(II) in intraperitoneal rat tumors after intraperitoneal chemotherapy: a comparison with systemic chemotherapy. Cancer Res 1989;49:3380-4. [PubMed]
- Kyle AH, Huxham LA, Yeoman DM, et al. Limited tissue penetration of taxanes: a mechanism for resistance in solid tumors. Clin Cancer Res 2007;13:2804-10. [PubMed]
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer 2006;6:583-92. [PubMed]
- Ozols RF, Locker GY, Doroshow JH, et al. Pharmacokinetics of adriamycin and tissue penetration in murine ovarian cancer. Cancer Res 1979;39:3209-14. [PubMed]
- Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 2001;7:987-9. [PubMed]
- Shah DK, Shin BS, Veith J, et al. Use of an anti-vascular endothelial growth factor antibody in a pharmacokinetic strategy to increase the efficacy of intraperitoneal chemotherapy. J Pharmacol Exp Ther 2009;329:580-91. [PubMed]
- Shah DK, Veith J, Bernacki RJ, et al. Evaluation of combined bevacizumab and intraperitoneal carboplatin or paclitaxel therapy in a mouse model of ovarian cancer. Cancer Chemother Pharmacol 2011;68:951-8. [PubMed]
- Ishida S, Lee J, Thiele DJ, et al. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci U S A 2002;99:14298-302. [PubMed]
- Holzer AK, Howell SB. The internalization and degradation of human copper transporter 1 following cisplatin exposure. Cancer Res 2006;66:10944-52. [PubMed]
- Jandial DD, Farshchi-Heydari S, Larson CA, et al. Enhanced delivery of cisplatin to intraperitoneal ovarian carcinomas mediated by the effects of bortezomib on the human copper transporter 1. Clin Cancer Res 2009;15:553-60. [PubMed]
- Grantab RH, Tannock IF. Penetration of anticancer drugs through tumour tissue as a function of cellular packing density and interstitial fluid pressure and its modification by bortezomib. BMC Cancer 2012;12:214. [PubMed]
- Liang ZD, Long Y, Tsai WB, et al. Mechanistic basis for overcoming platinum resistance using copper chelating agents. Mol Cancer Ther 2012;11:2483-94. [PubMed]
- Ishida S, McCormick F, Smith-McCune K, et al. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell 2010;17:574-83. [PubMed]
- Kuo MT, Fu S, Savaraj N, et al. Role of the human high-affinity copper transporter in copper homeostasis regulation and cisplatin sensitivity in cancer chemotherapy. Cancer Res 2012;72:4616-21. [PubMed]
- Fu S, Hou MM, Wheler J, et al. Exploratory study of carboplatin plus the copper-lowering agent trientine in patients with advanced malignancies. Invest New Drugs 2014;32:465-72. [PubMed]
- Goldberg MS, Xing D, Ren Y, et al. Nanoparticle-mediated delivery of siRNA targeting Parp1 extends survival of mice bearing tumors derived from Brca1-deficient ovarian cancer cells. Proc Natl Acad Sci U S A 2011;108:745-50. [PubMed]
- Cho YS, Lee GY, Sajja HK, et al. Targeted delivery of siRNA-generating DNA nanocassettes using multifunctional nanoparticles. Small 2013;9:1964-73. [PubMed]
- Sugarbaker PH. Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapy. Int J Hyperthermia 2007;23:431-42. [PubMed]
- Elias DM, Ouellet JF. Intraperitoneal chemohyperthermia: rationale, technique, indications, and results. Surg Oncol Clin N Am 2001;10:915-33. [PubMed]
- Kim MJ, Jung YW, Seong SJ, et al. Intraoperative intraperitoneal chemotherapy with cisplatin in epithelial ovarian cancer. J Gynecol Oncol 2012;23:91-7. [PubMed]
- Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943-53. [PubMed]
- Andreopoulou E, Chen T, Liebes L, et al. Phase 1/pharmacology study of intraperitoneal topotecan alone and with cisplatin: potential for consolidation in ovarian cancer. Cancer Chemother Pharmacol 2011;68:457-63. [PubMed]
- Muntz HG, Malpass TW, McGonigle KF, et al. Phase 2 study of intraperitoneal topotecan as consolidation chemotherapy in ovarian and primary peritoneal carcinoma. Cancer 2008;113:490-6. [PubMed]
- Dang D, Long B, Sullivan P, et al. Intraperitoneal port cytology after primary chemotherapy for ovarian cancer: a simple and inexpensive test to predict recurrence and survival. Gynecol Oncol 2012;125:S3-176;abstract #240.


