
Dynamic monitoring of serum soluble programmed cell death ligand 1 as a response predictor to chemotherapy in metastatic or recurrent gastrointestinal cancer
Introduction
Gastric cancer (GC) and colorectal cancer (CRC) are very common malignant tumors and leading causes of cancer-related death worldwide (1,2). Despite the development of multimodality therapies such as surgery, radiation therapy, chemotherapy and introduction of molecular targeted drugs in the past decades, the mortality rates from metastatic gastrointestinal cancer remained dismal (3). The poor prognosis highlights the urgent need for novel therapeutic approaches. Recently, breakthroughs in immune checkpoint blockade have offered new therapeutic options for many malignancies (4-7). Immunotherapy targeting the checkpoint programmed cell death protein 1 (PD-1) or programmed cell death ligand 1 (PD-L1) has been shown to be effective in the management of refractory metastatic GC (mGC) and microsatellite instability high (MSI-H) metastatic CRC (mCRC) (8,9).
PD-1 is a negative co-stimulatory receptor expressed mainly on activated T cells, which downregulates excessive immune responses by binding to its ligands, PD-L1 and PD-L2 (10,11). PD-L1 is constitutively expressed in various tissues and in different tumor types including gastrointestinal cancer (12). PD-L1 expression in tumor tissue was related to anti-PD-1/PD-L1 response (12-14). Based on the results of KEYNOTE 059 study (15), Food and Drug Administration (FDA) granted accelerated approval to Pembrolizumab (Merck & Co., Inc., USA) for patients with unresected advanced/metastatic gastric or gastroesophageal junction adenocarcinoma whose tumor express PD-L1 as determined by an FDA-approved test.
Molecular analyses are typically performed on tissues at initial diagnosis (16). However, in some cases, metastatic tumors have different molecular alterations from primary tumors (17,18). PD-L1 expression level in tumor tissue is also affected by the timing of biopsy, composition of tumor tissues, cancer treatment or host immune response (19-21). Hence, a dynamic reassessment of molecular alteration might help to optimize treatment. However, serial biopsies are not practical in practical clinical activity. Soluble PD-L1 (sPDL1) is thought to be a circulating biologically active protein which is released from PD-L1-positive tumor cells or immune cells, and binds to PD-1 receptor which contributes to systemic immunosuppression (22,23). The sPDL1 expression status has been reported to be an independent prognostic factor in various malignant tumors (24-29). In these circumstances, dynamic assessment of sPDL1 expression of metastatic gastrointestinal cancer is considered as a potential strategy with more precise clinical application.
However, the prognostic value of baseline serum sPD-L1 level in gastrointestinal cancer patients remained debate (28,30). The relationship between dynamic change of serum sPD-L1 level and treatment response to chemotherapy has not been investigated. Thus, a prospective cohort study was conducted to investigate the prognostic or predictable value of serum sPD-L1 baseline level and its dynamic change for metastatic gastrointestinal cancer patients.
Methods
Patient
Patients with histologically diagnosed gastrointestinal adenocarcinoma and radiologic confirmation of metastatic or recurrent lesions in Sun Yat-sen university cancer center were enrolled.
The inclusion criteria were as follows: (I) confirmed gastrointestinal adenocarcinoma pathologically; (II) has not received any previous systemic chemotherapy for recurrent or metastatic disease; (III) at least one computed tomography (CT) or magnetic resonance imaging (MRI) response evaluation; (IV) measurable disease based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; (V) Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) 0-1; (VI) complete follow-up medical records; (VII) available informed consent for the access to medical information and blood sample.
Baseline clinical and laboratory assessments, including age, gender, tumor stage (7th AJCC TNM stage), tumor size, tumor primary site, metastatic site, metastatic organ numbers, surgical history, cigarette smoking, drinking, HER2 status (for GC) and RAS status (for CRC) were collected from hospital database.
Treatment
Patients with HER2-negative mGC received first-line dual chemotherapeutic regimens including fluoropyrimidine (S-1, capecitabine or 5-fluorouracil) and platinum (cisplatin or oxaliplatin). For patients with HER2-positive, dual chemotherapy combined with Trastuzumab was carried out.
Patients with mCRC received first-line chemotherapeutic regimens with fluoropyrimidine (capecitabine or 5-fluorouracil) combined with oxaliplatin or irinotecan. For RAS wild type patients, chemotherapy combined with Bevacizumab or Cetuximab was recommended. For RAS mutant type patients, chemotherapy combined with Bevacizumab was recommended.
Patients continued chemotherapy until disease progression or intolerable toxicity. Response evaluation was performed every 6 or 8 weeks by contrast-enhanced CT or MRI.
Blood sample collection
Blood samples, before initiating first-line chemotherapy and within 48 h before response evaluation, was drawn into Serum Separation Tubes with polymer gel/silica activator. According to standard operating procedure, serum was prepared within 1 hour of sample collection after centrifugation (1,000 ×g) for 20 min and immediately stored at –80 °C. In our study, the blood routine, biochemical test and blood samples in peripheral vein blood were detected or collected at the same time before treatment, CT or MRI response evaluation was taken simultaneously.
Serum sPD-L1 measurement
Serum sPD-L1 levels were measured by a commercially available enzyme-linked immunosorbent assay (ELISA) kit (USCN, Wuhan, China, catalogue: SEA788Hu). ELISA was conducted as follows: (I) according to the manufacturer’s instructions, all chemical agent, standard dilutions, and specimen were prepared; (II) added 100 µL of the standard and sample to each well; (III) covered the plates with a plate sealer and carefully placed at 37 °C hatched for 120 min; (IV) after this step, added 100 µL of detection reagent A (1:100) to each well, and re-sealed the plates and hatched at 37 °C for another 120 min; (V) each well was washed four times by wash buffer after aspirated; (VI) added 100 µL of detection reagent B (1:100) to each well, and re-sealed the plates were and incubated for 30 min at 37 °C; (VII) washed each well five times after aspirated, added 90 µL of substrate solution, and the plates were newly sealed and incubated in a dark room for 20 min at 37 °C; (VIII) added 50 µL of stop solution to each well, and measured absorbance at 450 nm immediately in Bio-Tek EPOCH2 Microplate Reader (Bio-Rad Laboratories, USA).
We measured sPD-L1 protein levels by using standard curves. Four parameters logistic regression (4PL) calibration models were selected to design the standard curve of sPD-L1 ELISA. The detectable dose range of sPD-L1 was 0.057 to 20 ng/mL and minimum quantitative range was 0.156 ng/mL.
Statistical analysis
Responses to treatment were divided into complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), and non-evaluable (NE), according to the RECIST criteria version 1.1.
Progression-free survival (PFS) was defined as the time from initiation of first-line treatment to disease progression or death without evidence of progression. The prognostic factors of PFS were analyzed by univariate analysis. Continuous variables were summarized using and median (range). The χ2-test was used to explore the associations between sPD-L1 expression/sPD-L1 change and clinical characteristics. Differences in the distribution of more than two variables were evaluated by the Kruskal-Wallis test Means between two unrelated groups on sPD-L1/sPD-L1 change was examined by independent samples t-test.
The cut-off point for sPD-L1 was determined by the software named Xtiles. SPSS 20.0 statistical package (SPSS Inc., Chicago, IL, USA) was used for all the statistical analyses and graphics. P<0.05 was considered statistically significant. Graphs were carrying through GraphPad Prism 5.01 (GraphPad Software Inc. La Jolla, CA, USA).
Study oversight
All procedures conducted in this study were conformity with the ethical standards of the institutional research committee and with the Helsinki Declaration and its amendments in 1960s. This research protocol was approved by the ethics committee of Sun Yat-sen University Cancer Center.
Result
Patients’ clinical-pathologic characteristics
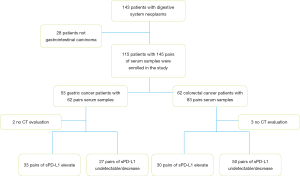
Totally 115 patients diagnosed with metastatic gastrointestinal cancer in Sun Yat-sen University Cancer Center (53 GC patients and 62 CRC patients) with detail medical records were enrolled between January 2011 to December 2017.
Table 1 summarized their clinical-pathologic characteristics. The median cycle of first-line chemotherapy was 5.5 cycles (range, 1–18). For patients with mGC,the median age was 48.6 (range, 21 to 74) years, 22 of 53 patients had proximal GC, 22 had distal GC, 9 were gastric corpus cancer. Twenty-five patients had multiple sites metastasis (≥2). For CRC, the median age was 49.6 (range, 26 to 71) years, 43 of 62 patients had left-sided CRC. Thirty-six patients had multiple sites metastasis (≥2).
Table 1
| Patients characteristics | All (N=115) | mGC (N=53) | mCRC (N=62) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| sPD-L1 ≤ cutoff, n (%) (0.9442 ng/mL) | sPD-L1 > cutoff, n (%) | P value | sPD-L1 ≤ cutoff, n (%) | sPD-L1 > cutoff, n (%) | P value | sPD-L1 ≤ cutoff, n (%) | sPD-L1 > cutoff, n (%) | P value | |||
| Gender | 0.756 | 0.340 | 0.647 | ||||||||
| Male | 35 (60.3) | 23 (39.7) | 14 (53.8) | 12 (46.2) | 21 (65.6) | 11 (35.4) | |||||
| Female | 36 (63.2) | 21 (36.8) | 18 (66.7) | 9 (33.3) | 18 (60.0) | 12 (40.0) | |||||
| Age (mean ± SD) | 50.0±1.4 | 46.0±1.6 | 0.188 | 50.9±2.3 | 45.0±2.9 | 0.465 | 50.6±1.8 | 47.9±1.7 | 0.263 | ||
| Tumor type | 0.781 | – | – | ||||||||
| CRC | 39 (62.9) | 23 (37.1) | – | – | – | – | |||||
| GC | 32 (60.4) | 21 (39.6) | – | – | – | – | |||||
| Tumor site | 0.124 | 0.024 | |||||||||
| GC | |||||||||||
| Proximal | – | – | 13 (59.1) | 9 (40.9) | – | – | |||||
| Distal | – | – | 16 (72.7) | 6 (27.3) | – | – | |||||
| CRC | |||||||||||
| Left-sided | – | – | – | – | 31 (72.1) | 12 (27.9) | |||||
| Right-sided | – | – | – | – | 8 (42.1) | 11 (57.9) | |||||
| Differentiation | 0.278 | 0.581 | 0.207 | ||||||||
| Well/intermediate differentiated | 22 (55.0) | 18 (45.0) | 3 (50.0) | 3 (50.0) | 19 (55.9) | 15 (44.1) | |||||
| Poorly differentiated | 49 (65.3) | 26 (34.7) | 29 (61.7) | 18 (38.3) | 20 (71.4) | 8 (28.6) | |||||
| Clinical stage (AJCC, 7th) | 0.216 | 0.692 | 0.225 | ||||||||
| I and II | 5 | 5 | 2 | 1 | 3 | 4 | |||||
| III and IV | 63 (61.8) | 39 (38.2) | 29 (59.2) | 20 (40.8) | 33 (63.5) | 19 (36.5) | |||||
| Metastasis | |||||||||||
| Lymph node (no/yes) | 9/59 (13.2/86.8) | 12/32 (27.3/72.7) | 0.063 | 2/29 (6.5/93.5) | 2/19 (9.5/90.5) | 0.683 | 7/30 (18.9/81.1) | 10/13 (43.5/56.5) | 0.04 | ||
| Liver (no/yes) | 55/16 (77.5/22.5) | 26/18 (59.1/40.9) | 0.036 | 29/3 (90.6/9.4) | 17/4 (80.9/19.1) | 0.309 | 26/13 (66.7/33.3) | 9/14 (39.1/60.9) | 0.035 | ||
| Multi-site transfer (no/yes) | 31/37 (45.6/54.4) | 20/24 (45.5/54.5) | 0.989 | 15/15 (50/50) | 11/10 (52.4/47.6) | 0.867 | 15/22 (40.5/59.5) | 9/14 (39.1/60.9) | 0.914 | ||
| Surgery history | 0.871 | 0.997 | 0.726 | ||||||||
| None | 26 | 14 | 12 | 8 | 14 | 6 | |||||
| Radical operation | 24 | 16 | 10 | 7 | 13 | 9 | |||||
| Palliative operation | 21 | 14 | 9 | 6 | 12 | 8 | |||||
| Family tumor history (no/yes) | 45/26 (63.4/36.6) | 24/20 (54.4/45.5) | 0.347 | 22/10 (68.8/31.2) | 12/9 (57.1/42.9) | 0.389 | 23/16 (59/41) | 12/11 (52.2/47.8) | 0.602 | ||
| Smoking | 0.927 | 0.448 | 0.469 | ||||||||
| Current | 13 | 7 | 7 | 3 | 6 | 4 | |||||
| Former | 7 | 5 | 4 | 1 | 3 | 4 | |||||
| Never | 51 | 32 | 21 | 17 | 30 | 15 | |||||
| Drinking | 0.896 | 0.824 | 0.741 | ||||||||
| Current | 8 | 4 | 3 | 1 | 5 | 3 | |||||
| Former | 4 | 2 | 3 | 2 | 1 | 0 | |||||
| Never | 59 | 38 | 26 | 18 | 33 | 20 | |||||
| Treatment response (CR + PR + SD/PD) | 41/28 | 38/4 | 0.000460 | 18/13 | 17/3 | 0.043 | 23/15 | 21/1 | 0.003 | ||
| MMR | – | – | – | ||||||||
| dMMR | 3 | 2 | 3 | 1 | 1 | 0 | |||||
| pMMR | 22 | 13 | 12 | 6 | 10 | 7 | |||||
| HER2 | – | – | – | ||||||||
| Negative | – | – | 16 | 11 | – | – | |||||
| Positive | – | – | 2 | 0 | – | – | |||||
| Unknown | – | – | 13 | 10 | – | – | |||||
| RAS | – | – | – | ||||||||
| Wild | – | – | – | – | 19 | 16 | |||||
| Mutated | – | – | – | – | 6 | 4 | |||||
sPD-L1, soluble programmed cell death ligand 1; mGC, metastatic gastric cancer; mCRC, metastatic colorectal cancer; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; MMR, mismatch repair; dMMR, deficient MMR; pMMR, proficient MMR.
Among 115 patients, 89 patients had one serum sample, 23 patients had two serial serum samples, two patients had serial three samples, and one patient had serial four samples within 48 h before response evaluation.
As of the data cutoff date of December 2017, the median duration follow-up was 16.1 (range, 2 to 81) months.
Correlation of baseline sPD-L1 expression with clinical characteristics
The mean serum sPD-L1 level of the whole cohort was 0.944 (median: 0.77; range, <0.156–6.68) ng/mL (Figure 1). In 33 patients (28.7%), serum PD-L1 levels was lower than limit of the ELISA detection (0.156 ng/mL). There was no difference in sPD-L1 values between in patients with mGC (median 0.802 ng/mL; range, <0.156–6.680 ng/mL) and in patients with mCRC (median sPD-L1 level was 0.772 ng/mL; range, <0.156–4.750 ng/mL). By Xtiles software, 0.944 ng/mL was chosen as cut-off value which divided all the patients into sPD-L1 level high subgroup and sPD-L1 level low subgroup.
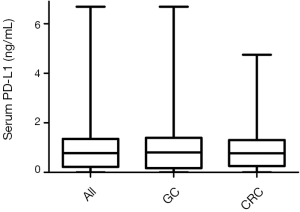
Table 1 summarized the associations between baseline serum sPD-L1 level and the clinical-pathological features. In whole study population, there was no significant difference of baseline sPD-L1 level between GC and CRC patients. No significant difference was found in the baseline characteristics of patients with high as opposed to low serum sPD-L1 levels. However, liver metastasis was more frequently observed in patients with high levels of sPD-L1 compared with those with low levels of sPD-L1 (mean in liver metastasis group and in no liver metastasis group were 0.750 and 1.048 ng/mL respectively, P=0.036). Patients with mCRC in left-sided diseases had lower baseline sPD-L1 level compared with those with right-sided (mean: 0.714 vs. 1.147 ng/mL, P=0.024). The patients with distant lymph node metastasis had lower baseline sPD-L1 level (P=0.04) and those with liver metastasis had higher baseline sPD-L1 levels (P=0.035).
Correlation of dynamic change sPD-L1 expression with clinical characteristics
According to the dynamic change of Serum sPD-L1 level, the patients were divided into two groups: undetected/decrease group and elevated group. The relationship between sPD-L1 level change and clinical characteristics were evaluated (Table 2). In whole study population, the patients with family history (P=0.027) or no lymph node metastasis (P=0.003) were less likely to have sPD-L1 level dynamic elevation. The sPD-L1 level elevation was more common in older CRC patients (P=0.016) and lymph node metastasis (P=0.009).
Table 2
| Patients characteristics | All (N=115) | GC (N=53) | CRC (N=62) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number (%) (n=115) | Undetectable/decrease in sPDL1 | Elevation in sPDL1 | P value | Undetectable/decrease in sPDL1 | Elevation in sPDL1 | P value | Undetectable/decrease in sPDL1 | Elevation in sPDL1 | P value | |||
| Gender (male/female) | 58/57 (50.4/49.6) | 31/32 (51.2/48.8) | 27/25 (54/46) | 0.772 | 13/13 (50/50) | 14/13 (51.9/48.1) | 0.893 | 18/20 (47.4/52.6) | 14/11 (56/44) | 0.503 | ||
| Age (young/old) | 59/56 (51.3/48.7) | 36/27 (57.1/42.9) | 23/29 (44.2/55.8) | 0.168 | 12/14 (46.2/53.8) | 15/12 (55.6/44.4) | 0.494 | 24/14 (63.2/36.8) | 8/17 (32/68) | 0.016 | ||
| Tumor type | 0.254 | |||||||||||
| CRC | 62 (53.9) | 37 (59.7) | 25 (40.3) | |||||||||
| GC | 53 (46.1) | 26 (49.0) | 27 (51.0) | |||||||||
| Tumor site | – | 0.160 | 0.135 | |||||||||
| Proximal | – | – | – | 10 | 12 | – | – | |||||
| Distal | – | – | – | 9 | 13 | – | – | |||||
| Left-sided | – | – | – | – | – | 23 | 20 | |||||
| Right-sided | – | – | – | – | – | 14 | 5 | |||||
| Differentiation | 0.225 | 0.413 | 0.282 | |||||||||
| Well/intermediate differentiated | 40 | 25 (62.5) | 15 (37.5) | 2 (33.3) | 4 (66.7) | 23 (67.6) | 11 (32.4) | |||||
| Poorly differentiated | 75 | 38 (50.7) | 37 (49.3) | 24 (51.0) | 23 (49.0) | 15 (51.7) | 14 (48.3) | |||||
| Metastasis | ||||||||||||
| Lymph nodes (yes/no) | 91/21 (81.2/18.8) | 45/18 (71.4/28.6) | 46/3 (93.9/6.1) | 0.003 | 23/3 (88.5/11.5) | 25/1 (96.2/3.8) | 0.298 | 23/15 (60.5/39.5) | 21/2 (91.3/8.7) | 0.009 | ||
| Liver (yes/no) | 34/81 | 22/41 | 12/40 | 0.166 | 4/22 | 3/24 | 0.646 | 18/19 | 9/16 (36/64) | 0.324 | ||
| Multi-site transfer (yes/no) | 61/51 (54.5/45.5) | 32/31 (50.8/49.2) | 29/20 (59.2/40.8) | 0.376 | 13/12 (52/48) | 12/14 (46.2/53.8) | 0.676 | 19/19 (50/50) | 17/6 (73.9/26.1) | 0.066 | ||
| Clinical stage (AJCC, 7th) | 0.306 | 0.513 | 0.529 | |||||||||
| I and II | 10 (8.7) | 7 (11.1) | 3 (5.8) | 2 (7.7) | 1 (3.7) | 32 (84.2) | 21 (84.0) | |||||
| III and IV | 105 (91.3) | 56 (88.9) | 49 (94.2) | 24 (92.3) | 26 (96.3) | 6 (15.8) | 4 (16.0) | |||||
| Surgery history | 0.902 | 0.566 | 0.791 | |||||||||
| None | 40 | 23 (36.5) | 17 (32.7) | 11 (42.3) | 9 (33.3) | 12 (31.6) | 8 (32.0) | |||||
| Radical operation | 40 | 21 (33.3) | 19 (36.5) | 7 (26.9) | 11 (40.7) | 15 (39.5) | 8 (32.0) | |||||
| Palliative operation | 35 | 19 (30.2) | 16 (30.8) | 8 (30.8) | 7 (25.9) | 11 (28.9) | 9 (36.0) | |||||
| Family tumor history (yes/no) | 46/69 (40/60) | 31/32 (51.2/48.8) | 15/37 (28.8/71.2) | 0.027 | 13/13 (50/50) | 6/21 (22.2/77.8) | 0.035 | 19/19 (50/50) | 9/16 (36/64) | 0.274 | ||
| Smoking | 0.965 | 0.709 | 0.598 | |||||||||
| Current | 20 | 11 (17.7) | 9 (17.3) | 4 (15.4) | 6 (22.2) | 7 (18.4) | 3 (12.0) | |||||
| Former | 12 | 7 (11.1) | 5 (9.6) | 2 (7.7) | 3 (11.1) | 5 (13.2) | 2 (8.0) | |||||
| Never | 83 | 38 (73.1) | 45 (71.4) | 20 (76.9) | 18 (66.7) | 26 (68.4) | 20 (80.0) | |||||
| Drinking | 0.906 | 0.913 | 0.598 | |||||||||
| Current | 12 | 6 (9.5) | 6 (11.5) | 2 (7.7) | 2 (7.4) | 4 (10.5) | 4 (16.0) | |||||
| Former | 6 | 3 (4.8) | 3 (5.8) | 2 (7.7) | 3 (11.1) | 1 (2.6) | 0 | |||||
| Never | 97 | 54 (85.7) | 43 (82.7) | 22 (84.6) | 22 (81.5) | 33 (86.8) | 21 (84.0) | |||||
sPD-L1, soluble programmed cell death ligand 1; GC, gastric cancer; CRC, colorectal cancer.
Correlation of sPD-L1 expression with response to chemotherapy
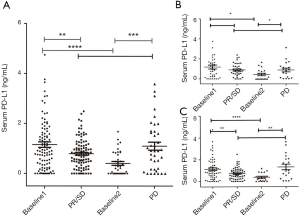
As shown in Table 1, the baseline sPD-L1 expression significantly correlated with treatment response (P=0.00046) in whole study population. Patients with serum sPD-L1 >0.944 ng/mL had better tumor response than patients with sPD-L1 <0.944 ng/mL. There was higher baseline expression sPD-L1 in patients with PR/SD, compared with patients with PD (1.153 vs. 0.406 ng/mL, P<0.0001). The same phenomenon was observed in GC and CRC subgroups (P=0.043 and P=0.003, respectively).
In this study, we collected 145 pairs data of serum sPD-L1 pre- and post-chemotherapy or between each course of treatment, and 140 cases’ response evaluation (five patients with no evaluation). According to the counterpart chemotherapy treatment responses, the pairs of serum samples were separated into Baseline1-PR/SD group and Baseline2-PD group. Thirty-three pairs of sPD-L1 elevated out of 62 pairs GC patients and 30 pairs increased in 83 pairs of patients with CRC (Figure 2). The mean sPD-L1 levels in PR/SD group decreased from pre-treatment baseline 1.153±1.115 ng/mL to post-treatment 0.791±0.574 ng/mL (P=0.004), while the mean sPD-L1 levels in PD group increased from pre-treatment baseline 0.406±0.466 ng/mL to post-treatment 1.097±0.984 ng/mL (P=0.00026) (Figure 3A). In GC subgroup, the mean sPD-L1 levels decreased from 1.185±1.269 to 0.850±0.616 ng/mL in PR/SD group and increased from 0.439±0.541 to 0.891±0.814 ng/mL in PD group (Figure 3B). In CRC subgroup, the mean sPD-L1 levels decreased from 1.132±1.010 to 0.720±0.532 ng/mL in PR/SD group and increased from 0.372±0.390 to 1.303±1.113 ng/mL in PD group, P=0.0023 (Figure 3C). Compared with baseline level, sPD-L1dynamically decreased in the PR/SD group and dynamically increased in the PD group.
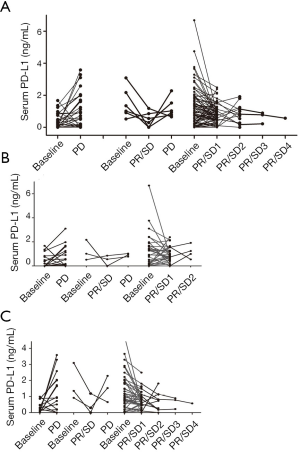
Correlation between the changes of sPD-L1 during chemotherapy and treatment response was assessed in the 145 pairs data (Figure 4). Sixty-six pairs of sPD-L1 value were elevated during chemotherapy. Corresponding evaluation of curative effect, PD was found in 29/66 cases, and PR or SD in 34/66 cases. While among 79 pairs with declining sPD-L1 during anticancer treatment, PD was observed in 9/79 cases, and PR or SD in 68/79 cases (P=0.000005). In the follow-up courses, serum sPD-L1 values of patients with PR or SD were constantly decreasing compared with increasing in patients with PD.
Correlation of serum sPD-L1 level with prognosis
Median PFS for the whole study population was 11.1 (range, 1–72) months, with a median follow-up of 16.1 (range, 2–81) months. In cox proportional hazards model, variables showing tendencies for positive association with PFS in univariate analysis were selected. As shown in Table 3, the patients with advanced tumor stage, tumor diameter >5 cm or with multiple metastases (≥2) had shorter PFS.
Table 3
| Variables | All | Stomach | Intestinal | |||
|---|---|---|---|---|---|---|
| P value (log-rank) | HR (95% CI) | P value (log-rank) | P value (log-rank) | |||
| Gender (male/female) | 0.408 | 1.215 (0.754–1.956) | 0.182 | 0.952 | ||
| Age (<50/≥50) | 0.177 | 1.368 (0.853–2.194) | 0.467 | 0.241 | ||
| Family tumor history (yes/no) | 0.118 | 0.69 (0.426–1.118) | 0.032 | 0.797 | ||
| Smoking (yes/no/yet) | 0.485 | 0.998 (0.824–1.21) | 0.65 | 0.597 | ||
| Drinking (yes/no/yet) | 0.858 | 1.055 (0.836–1.333) | 0.677 | 0.045 | ||
| Tumor type (GC/CRC) | 0.751 | 0.955 (0.592–1.54) | ||||
| Tumor site | 0.852 | 0.649 | 0.533 | |||
| Proximal | 0.794 (0.396–1.589) | |||||
| Distal | 0.884 (0.541–1.444) | |||||
| Left-sided | 1.233 (0.756–2.01) | |||||
| Right-sided | 0.994 (0.668–1.48) | |||||
| Clinical stage (AJCC, 7th) | 0.035 | 0.482 (0.179–1.299) | 0.025 | 0.67 | ||
| I and II | ||||||
| III and IV | ||||||
| Bulky disease (yes/no) | 0.047 | 1.376 (0.983–1.928) | 0.153 | 0.31 | ||
| Metastasis | ||||||
| Lymph nodes (yes/no) | 0.464 | 1.283 (0.645–2.552) | 0.951 | 0.254 | ||
| Liver (yes/no) | 0.084 | 0.601 (0.330–1.093) | ||||
| Multi-site transfer (yes/no) | 0.016 | 1.773 (1.082–2.905) | 0.056 | 0.101 | ||
| Differentiation (well/intermediate vs. poor) | 0.572 | 1.251 (0.794–1.969) | 0.808 | 0.102 | ||
| Surgery history(none/radical/palliative) | 0.067 | 0.724 (0.522–1.003) | 0.004 | 0.794 | ||
| sPDL1 (high/low) | 0.016 | 0.600 (0.227–1.585) | 0.092 | 0.097 | ||
| sPD-L1 (up/down) | 0.00026 | 3.032 (1.323–6.948) | 0.029 | 0.01 | ||
sPD-L1, soluble programmed cell death ligand 1; GC, gastric cancer; CRC, colorectal cancer; PFS, progression-free survival.
PFS was significantly longer in sPD-L1 level decreasing/stable group than those in sPD-L1 level increasing group (not reached vs. 27 months; HR, 3.032; 95% CI, 1.323–6.948; P=0.00026) (Figure 5). In subgroup analysis, PFS of sPD-L1 decreasing/ stable group vs. sPD-L1 level increasing group in GC was not reached vs. 27 months; HR, 0.649; 95% CI: 0.186–2.272; P=0.029. PFS of sPD-L1 decreasing/stable group vs. sPD-L1 level increasing group in CRC was 41 vs. 28 months; HR, 2.834; 95% CI, 0.910–8.833; P=0.01.
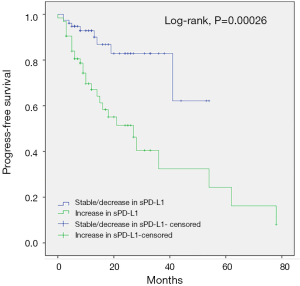
Patients with low baseline sPD-L1 level showed shorter PFS than those with high baseline sPD-L1 level (36 months vs. not reached; HR, 0.600; 95% CI, 0.227–1.584, P=0.016). In subgroup analysis, PFS of GC patients with low baseline sPD-L1 level vs. high baseline sPD-L1 level was 36 months vs. not reached; HR, 0.649; 95% CI, 0.186–2.272; P=0.092. PFS of CRC patients with low baseline sPD-L1 level vs. high baseline sPD-L1 level was 36 months vs. not reached; HR, 0.451; 95% CI, 0.091–2.230; P=0.069. We need to enlarge subgroup samples in later work to confirm this tendency.
Discussion
Biomarker-driven selection of immunotherapy responders and non-responders would minimize unnecessary exposure of patients to potentially immune-related toxicities. As anti-PD-1 pathway immunotherapies are effective in only a minority of gastric-intestinal cancer patients, there is a great need for reliable and easily available biomarkers of patient response. PD-L1 expression appeared to correlate with response to treatment from exploratory analyses of early reported trials, whether the level of expression of PD-L1 predicts response rate, PFS and OS in the context of anti-PD-1/PD-L1 therapy were explored in multiple malignant tumors with inconsistent results. sPD-L1 being released from immune cells or tumor cells can bind receptors in a similar manner as their membrane-bound counterparts and as a result may play a more widespread role in PD-1/PD-L1 axis. This study measured the baseline level of circulating sPD-L1 and dynamical change of sPD-L1serum level during treatment. No study has been reported to identify circulating sPD-L1 expression in patients with CRC until now. It is the first report to evaluate the relation between the baseline expression and dynamic change of sPD-L1 expression in metastatic gastrointestinal cancer and their prognostic or predictable value.
The mean value of sPD-L1 level was 0.937±0.945 ng/mL in GC group. Our data on GC was slightly higher than those reported in previous studies, the median sPD-L1 level was 0.704 ng/mL in Japanese GC population and 0.8928 ng/mL in northern Chinese GC population, respectively (28,30). Different study population, treatment stage of specimen collection and various test methods may contribute to the differences. Patients with liver metastasis had higher sPD-L1 level in whole study population and patients with mCRC. In the subgroup analysis, patients with mCRC whose family tumor history were more likely to have higher sPD-L1level and patients with right-sided CRC tended to have higher sPD-L1 level. It is well-known that right-sided colon with mucosal immune cells had stronger immunogenicity than left-side colon (8,31,32). There is more common MSI-H tumor in the right-sided colon. The proximal colon cancer shows worse OS than the distal CRC (33). The older CRC patients tended to have sPD-L1 level elevated in our study. Previous study reported that sPD-L1 levels increased in an age-dependent manner in health donors, which suggested that serum sPD-L1 was related to the immune state of human being (20). sPDL1 was thought to be released from PD-L1-positive tumor cells or immune cells and can functionally binds to PD-1 receptor then contributed to systemic immunosuppression (23,29). In clinical research, sPD-L1 could found functionally block the regulatory effect of membrane-bound PD-1 on T cell activation in Rheumatoid Arthritis (34). Serum sPD-L1 levels significantly increased in dying septic patients compared with the survivors (P<0.05) (26). In melanoma, both tumor PD-L1 and macrophage PD-L1 expression level after effective treatment were higher than intra-tumoral CD8+ lymphocytes and CD68+ macrophages in biopsies (35).
In our study, higher baseline sPD-L1 levels were more likely to response to chemotherapy and had better PFS. However, the clinical significance of sPD-L1 remains controversial in different tumor types. Several studies showed that high level of sPD-L1 was a negative prognosis factor, indicating lower overall response rate, shorter PFS and shorter OS (24,29,36-38). In NSCLC patients, high sPD-L1 level was significantly related to abdominal organ metastasis (P=0.004) (39). On the contrary, sPD-1/sPD-L1 levels did not appear an unfavorable outcome in advanced pancreatic cancer (40) and the author thought sPD-L1 levels may reflect inflammatory tumor type in PC patients. Zheng found that in advanced GC patients high sPD-L1 correlated with better OS (30). There were three main anticancer immunity phenotypes: the immune-desert phenotype, the immune-excluded phenotype and the inflamed phenotype. Inflamed tumors were infiltrated by a number of subtypes of immune cells. Tumor cells in inflamed tumors can express inhibitory factors, down-regulating MHC class I molecule and other pathways can de-sensitize them to anticancer immunity (31) Clinical responses to anti-PD-L1/PD-1 therapy occur most often in patients with inflamed tumors (31). Finkelmeier found that in HCC sPD-L1 levels showed correlation with C-reactive protein, a typical inflammatory maker (24). Okuma inferred that sPD-L1 levels might be determined by both tumor burden and extra-tumoral inflammatory statue (29). We hypothesis that high sPD-L1 may be released by infiltrating immune cells and tumor cells, which indicate an immune-inflammatory state with better treatment outcome.
We can infer that dynamic monitor of sPD-L1 level may be used as a response predictor of chemotherapy in metastatic or recurrent gastrointestinal cancer. It is firstly reported. In our study, the patients with dynamic increase of sPD-L1 level had markedly poorer response to chemotherapy than the patients with stable or decreasing change of sPD-L1. People who got PR/SD showed a decrease/stable trend of sPD-L1 level, however, a significant increase of sPD-L1 level was found once disease progression. sPD-L1 in blood circulation can be released by antigen-presenting cell like activated mature dendritic cells or PD-L1 positive cell lines like tumor cells and bind to PD-1 receptor. We hypothesized that sPD-L1 level in blood circulation was a dynamic balance system. In pre-treatment patients, sPD-L1 is mostly released by infiltrating immune cells reflecting an immunoinflammatory state and after chemotherapy, tumor cells could be active by gene mutation and more sPD-L1 was released to escape immune surveillance which results in T cell exhaustion and dysfunction timely to avoid excessive immune damage and hold self-balance. Once disease progressed, tumor burden increased with more sPD-L1 released by tumor cells, it becomes a vicious cycle.
Our study comes with some limitations. It was a single center study. Health controls are needed to be involved to estimate sPD-L1 levels between different populations. Individuals with different digestive cancer were involved. The follow-up during was not long enough.
Conclusions
Despite these limitations, to our knowledge, this study is the first to explore the association between soluble PD-1 level dynamic change and treatment response to gastrointestinal cancer patients. This is the first prospective study to explore sPD-L1 level in CRC and dynamic monitor sPD-L1 level change and its clinical significance in gastrointestinal cancer. We maybe provide an effective and easy way to predict the chemotherapeutic response to chemotherapy of gastrointestinal cancer patients.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.23). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Sun Yat-Sen University Cancer Center (No. GZR2016-017). Informed consent was obtained from all individual participants included in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Song Z, Wu Y, Yang J, et al. Progress in the treatment of advanced gastric cancer. Tumour Biol 2017;39:1010428317714626. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Digklia A, Wagner AD. Advanced gastric cancer: current treatment landscape and future perspectives. World J Gastroenterol 2016;22:2403-14. [Crossref] [PubMed]
- Ivashko IN, Kolesar JM. Pembrolizumab and nivolumab: PD-1 inhibitors for advanced melanoma. Am J Health Syst Pharm 2016;73:193-201. [Crossref] [PubMed]
- Morgensztern D, Herbst RS. Nivolumab and pembrolizumab for non-small cell lung cancer. Clin Cancer Res 2016;22:3713-7. [Crossref] [PubMed]
- Webster RM. The immune checkpoint inhibitors: where are we now? Nat Rev Drug Discov 2014;13:883-4. [Crossref] [PubMed]
- Topalian SL. Targeting immune checkpoints in cancer therapy. JAMA 2017;318:1647-8. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Lote H, Cafferkey C, Chau I. PD-1 and PD-L1 blockade in gastrointestinal malignancies. Cancer Treat Rev 2015;41:893-903. [Crossref] [PubMed]
- Zhang X, Schwartz JC, Guo X, et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity 2004;20:337-47. [Crossref] [PubMed]
- Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261-8. [Crossref] [PubMed]
- Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717-26. [Crossref] [PubMed]
- Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182-91. [Crossref] [PubMed]
- Bilgin B, Sendur MA, Bülent Akıncı M, et al. Targeting the PD-1 pathway: a new hope for gastrointestinal cancers. Curr Med Res Opin 2017;33:749-59. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018;4:e180013. [Crossref] [PubMed]
- Bonotto M, Garattini SK, Basile D, et al. Immunotherapy for gastric cancers: emerging role and future perspectives. Expert Rev Clin Pharmacol 2017;10:609-19. [Crossref] [PubMed]
- Uruga H, Bozkurtlar E, Huynh TG, et al. Programmed cell death ligand (PD-L1) expression in stage II and III lung adenocarcinomas and nodal metastases. J Thorac Oncol 2017;12:458-66. [Crossref] [PubMed]
- Callea M, Albiges L, Gupta M, et al. Differential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinoma. Cancer Immunol Res 2015;3:1158-64. [Crossref] [PubMed]
- Mann SA, Lopez-Beltran A, Massari F, et al. Targeting the programmed cell death-1 pathway in genitourinary tumors: current progress and future perspectives. Curr Drug Metab 2017;18:700-11. [Crossref] [PubMed]
- Kerr KM, Tsao MS, Nicholson AG, et al. Programmed death-ligand 1 immunohistochemistry in lung cancer: in what state is this art? J Thorac Oncol 2015;10:985-9. [Crossref] [PubMed]
- Kythreotou A, Siddique A, Mauri FA, et al. PD-L1. J Clin Pathol 2018;71:189-94. [Crossref] [PubMed]
- Chen Y, Wang Q, Shi B, et al. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine 2011;56:231-8. [Crossref] [PubMed]
- Frigola X, Inman BA, Krco CJ, et al. Soluble B7-H1: differences in production between dendritic cells and T cells. Immunol Lett 2012;142:78-82. [Crossref] [PubMed]
- Finkelmeier F, Canli Ö, Tal A, et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer 2016;59:152-9. [Crossref] [PubMed]
- Fukuda T, Kamai T, Masuda A, et al. Higher preoperative serum levels of PD-L1 and B7-H4 are associated with invasive and metastatic potential and predictable for poor response to VEGF-targeted therapy and unfavorable prognosis of renal cell carcinoma. Cancer Med 2016;5:1810-20. [Crossref] [PubMed]
- Liu M, Zhang X, Chen H, et al. Serum sPD-L1, upregulated in sepsis, may reflect disease severity and clinical outcomes in septic patients. Scand J Immunol 2017;85:66-72. [Crossref] [PubMed]
- Ha H, Nam AR, Bang JH, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget 2016;7:76604-12. [Crossref] [PubMed]
- Takahashi N, Iwasa S, Sasaki Y, et al. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol 2016;142:1727-38. [Crossref] [PubMed]
- Okuma Y, Hosomi Y, Nakahara Y, et al. High plasma levels of soluble programmed cell death ligand 1 are prognostic for reduced survival in advanced lung cancer. Lung Cancer 2017;104:1-6. [Crossref] [PubMed]
- Zheng Z, Bu Z, Liu X, et al. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res 2014;26:104-11. [PubMed]
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321-30. [Crossref] [PubMed]
- Schalper KA, Velcheti V, Carvajal D, et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res 2014;20:2773-82. [Crossref] [PubMed]
- Lee GH, Malietzis G, Askari A, et al. Is right-sided colon cancer different to left-sided colorectal cancer? - a systematic review. Eur J Surg Oncol 2015;41:300-8. [Crossref] [PubMed]
- Wan B, Nie H, Liu A, et al. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol 2006;177:8844-50. [Crossref] [PubMed]
- Vilain RE, Menzies AM, Wilmott JS, et al. Dynamic changes in PD-L1 expression and immune infiltrates early during treatment predict response to PD-1 blockade in melanoma. Clin Cancer Res 2017;23:5024-33. [Crossref] [PubMed]
- Wang H, Wang L, Liu WJ, et al. High post-treatment serum levels of soluble programmed cell death ligand 1 predict early relapse and poor prognosis in extranodal NK/T cell lymphoma patients. Oncotarget 2016;7:33035-45. [PubMed]
- Wang L, Wang H, Chen H, et al. Serum levels of soluble programmed death ligand 1 predict treatment response and progression free survival in multiple myeloma. Oncotarget 2015;6:41228-36. [Crossref] [PubMed]
- Rossille D, Gressier M, Damotte D, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia 2014;28:2367-75. [Crossref] [PubMed]
- Zhang J, Gao J, Li Y, et al. Circulating PD-L1 in NSCLC patients and the correlation between the level of PD-L1 expression and the clinical characteristics. Thorac Cancer 2015;6:534-8. [Crossref] [PubMed]
- Kruger S, Legenstein ML, Rösgen V, et al. Serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death ligand 1 (sPD-L1) in advanced pancreatic cancer. Oncoimmunology 2017;6:e1310358. [Crossref] [PubMed]


