
The effect of metastasis patterns on survival in male patients with different breast cancer subtypes: results from the Surveillance, Epidemiology, and End Results (SEER) database
Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death worldwide, with an estimated 2,088,849 new diagnoses and 626,679 mortalities in 2018 (1). However, male breast cancer (MBC) is uncommon. It accounts for less than 1% of all breast carcinomas worldwide (2). Although male patients account for only a small proportion of breast cancer patients, the incidence of MBC continues to increase by 1.1% annually, and the reported mortality rates are comparable to those in women (3-5). Further, the overall breast cancer mortality rate has improved over time, but less improvement has occurred for men (6).
Many previous studies have reported the clinical characteristics, hormonal conditions, optimal treatment, and prognosis of female breast cancer (FBC). However, MBC mortality has been rising, possibly due to delayed diagnosis and lack of male-specific information. The diagnosis, treatment, and prevention of MBC have not been paid as much attention as those of FBC because of the low incidence of MBC. For a long time, our understanding of MBC was based on that of FBC. However, MBC differs from FBC in some important aspects (7). Relevant studies focusing on this particular population are rare due to its smaller proportion. To date, very few studies have evaluated the effect of molecular subtypes on specific metastatic sites in male patients. In particular, the distribution of molecular subtypes at different metastatic sites has been poorly understood. Therefore, further study of the prognostic factors affecting the survival of men with breast cancer is needed.
In the present study, we used the Surveillance, Epidemiology, and End Results (SEER) registered database to analyze the relationships between MBC subtypes and distant metastasis patterns. Importantly, we analyzed prognosis in patients with the same metastatic pattern according to different subtypes, as well as the prognosis of patients with the same subtype but different metastatic patterns.
Methods
Data collection
The population-based data for this study were extracted from the SEER database established by the National Cancer Institute. Since SEER is a publicly available database with anonymized data, no ethical review was required. We obtained data from SEER collected between 2010 and 2013, as Her2 status and the sites of distant metastases were collected by SEER starting in 2010. We put no restriction on age or race. The exclusion criteria were as follows: patients diagnosed with breast cancer at death or autopsy; primary malignant tumors in other organs; benign or borderline tumors; unknown age, cause of death, or survival time; unavailable or incomplete information on surgery or radiation therapy; and loss to follow-up. Finally, 2983 patients were included.
We extracted multiple variables from the selected object of study. Demographic characteristics consisted of age at diagnosis (20–29, 30–39, 40–49, 50–59, 60–69, 70–79, or ≥80 years) and race (white, black, or other). Pathological characteristics included T-stage (T1, T2, T3, or T4), N stage (N0, N1, N2, or N3), M stage (M0 or M1 stage), tumor grade, laterality (right, left, or bilateral), hormone receptor status, subtype, and distant metastatic sites. Treatment characteristics included surgery (yes or no). Breast tumors were classified into the following subtypes: Her2−/HR+, Her2+/HR+, Her2+/HR−, and triple negative (TN). The primary endpoints of this study were breast cancer-specific survival (BCSS) and overall survival (OS).
Statistical analysis
The univariate and multivariate logistic regression analysis were used to analyze the association between the BCS and the specific metastatic pattern. we used Cox proportional hazard regression analysis to estimate the hazard ratio (HR) and 95% confidence interval (CI). Kaplan-Meier analysis was used to generate survival curves, and the log-rank test was applied to analyze the differences among the curves. All statistical tests were two-sided, and a P<0.05 was considered statistically significant. The statistical software SPSS 22.0 (IBM Corporation, Armonk, NY, USA) and GraphPad Prism version 6 (GraphPad Software Inc., La Jolla, CA, USA) were used for all data analysis.
Results
Patient demographics
There were 2,983 MBC patients reported in the SEER database from 2010 to 2013. The clinical characteristics and pathological features of all the patients are summarized in Table 1. Most patients were diagnosed at >50 years old (91.7%). Most patients were white (80.6%). Almost half (47.9%) of the patients were diagnosed with grade II disease, and 31.4% of patients were diagnosed with grade III disease. In addition, the proportions of patients with ER-positive, PR-positive, and HER2-negative tumors were 96.5%, 90.0%, and 87.4%, respectively. Interestingly, 85.4% of patients had the HER2−/HR+ subtype. The bone was the most common metastatic site (5.7%), and the brain was the least common metastatic site (0.7%).
Table 1
| Characteristics | Number (%) |
|---|---|
| Age at diagnosis (year) | |
| Mean [range] | 67.4 [22–105] |
| 20–29 years | 3 (0.1) |
| 30–39 years | 34 (1.1) |
| 40–49 years | 211 (7.1) |
| 50–59 years | 533 (17.9) |
| 60–69 years | 877 (29.4) |
| 70–79 years | 777 (26.0) |
| ≥80 years | 548 (18.4) |
| Race | |
| White | 2,386 (80.6) |
| Black | 430 (14.5) |
| Other | 146 (4.9) |
| Laterality | |
| Right | 1,364 (45.8) |
| Left | 1,575 (52.9) |
| Bilateral | 40 (1.3) |
| T stage | |
| T1 | 1,258 (45.4) |
| T2 | 1,172 (42.3) |
| T3 | 93 (3.3) |
| T4 | 248 (9.0) |
| N stage | |
| N0 | 1,644 (57.4) |
| N1 | 858 (30.0) |
| N2 | 228 (8.0) |
| N3 | 134 (4.7) |
| M stage | |
| M0 | 2,723 (91.6) |
| M1 | 250 (8.4) |
| AJCC stage | |
| I | 968 (32.5) |
| II | 1,156 (38.8) |
| III | 453 (15.2) |
| IV | 250 (8.4) |
| Unknown | 156 (5.2) |
| Tumor grade | |
| I | 338 (11.3) |
| II | 1,429 (47.9) |
| III | 938 (31.4) |
| IV | 11 (0.4) |
| Unknown | 267 (9.0) |
| ER status | |
| Positive | 2,697 (96.5) |
| Negative | 98 (3.5) |
| PR status | |
| Positive | 2,500 (90.0) |
| Negative | 279 (10.0) |
| Her2 status | |
| Positive | 331 (12.6) |
| Negative | 2,305 (87.4) |
| Breast cancer subtypes | |
| Her2−/HR+ | 2,243 (85.4) |
| Her2+/HR+ | 300 (11.4) |
| Her2+/HR− | 28 (1.1) |
| Triple negative | 54 (2.1) |
| Bone metastasis | |
| No | 2,718 (91.1) |
| Yes | 171 (5.7) |
| Unknown | 94 (3.2) |
| Brain metastasis | |
| No | 2,866 (96.1) |
| Yes | 20 (0.7) |
| Unknown | 97 (3.3) |
| Liver metastasis | |
| No | 2,852 (95.6) |
| Yes | 37 (1.2) |
| Unknown | 94 (3.2) |
| Lung metastasis | |
| No | 2,787 (93.4) |
| Yes | 98 (3.3) |
| Unknown | 98 (3.3) |
| Marital status | |
| Single | 453 (15.2) |
| Married | 2,333 (78.2) |
| Unknown | 197 (6.6) |
| Surgery | |
| No | 347 (11.6) |
| Yes | 2,588 (86.8) |
| Unknown | 48 (1.6) |
Table S1 shows the clinicopathological data of the patients with a single metastatic site. Patients ≥80 years accounted for 30.0% of the bone metastasis group, but only 18.3% of the control group (M0 group). Patients with brain or liver metastasis had a higher T stage and N stage than patients in the control group, with T3–4 stage accounting for 38.8% and 44.4% of patients with brain and liver metastasis, respectively, and N3 stage accounting for 10% and 18.5%, respectively (each P<0.001). Patients with M1 were more likely to be ER-negative and PR-negative, regardless of metastatic site (each P<0.05). As expected, patients with distant metastasis were less likely to undergo surgery than those in the control group and therefore had higher mortality rates (each P<0.05). Table S2 shows the clinicopathological data of the patients with multiple metastatic sites. Patients <60 years accounted for 42.9% of patients with three metastatic sites, suggesting that younger patients were more likely to have multiple metastases. Further, ER-negative, PR-negative, and Her2-positive tumors were extremely common in the multiple metastases group (each P<0.05). Patients with multiple metastases were not significantly less likely to receive surgery or have a higher mortality rate than those with a single metastasis.
Patterns of metastasis based on subtype
The distant metastatic sites assessed were the bone, brain, liver, and lung. The bone was the most common metastatic site and the brain was the least common metastatic site, regardless of subtype (Figure 1A). Patients with the Her2−/HR+ subtype were most likely to have bone metastasis (40.0%), followed by lung metastasis (21.6%), liver metastasis (3.6%), and brain metastasis (2.8%). Patients with the Her2+/HR− had low percentages of brain metastasis (1.6%), lung metastasis (1.2%), liver metastasis (0.8%), and brain metastasis (0.4%). Patients with the Her2+/HR+ subtype experienced lung metastasis (6.4%), liver metastasis (2.8%), and brain metastasis (2.0%), as did patients with the TN subtype (3.2%, 1.6%, and 1.6%, respectively).

Patients with Her2−/HR+ tumors were most likely to develop metastasis, and patients with Her2+/HR− tumors were least likely to develop metastasis, regardless of metastatic pattern (Figures 1B). Patients with bone metastasis were most likely to have the Her2−/HR+ subtype, as were patients with lung metastasis.
Factors associated with distant metastasis in MBC
Of the 2983 MBC patients included in the analysis, 2723 (91.3%) were diagnosed with M0 stage, whereas 250 (8.4%) had M1 stage disease. Based on the results of the univariate analysis (Table 2), DM in MBC patients was associated with age, race, laterality, T stage, N stage, tumor grade, hormone receptor status, and subtype (all P<0.05). Compared with M0 stage patients, M1 stage patients had higher T status (T3 and T4 stage: 8.9% vs. 50.5%), higher N status (N1-3 stage: 39.0% vs. 71.1%), and more advanced disease (grade III and IV: 31.2% vs. 50.2%) (all P<0.001).
Table 2
| Characteristics | M0 (n=2,723), n (%) | M1 (n=250), n (%) | P value |
|---|---|---|---|
| Age at diagnosis (year) | 67.66±12.352 | 64.39±12.709 | <0.001* |
| <55 years | 445 (16.3) | 52 (20.8) | |
| ≥55 years | 2,278 (83.7) | 198 (79.2) | |
| Race | 0.008* | ||
| White | 2,219 (81.5) | 180 (72.0) | |
| Black | 373 (13.7) | 56 (22.4) | |
| Other | 131 (4.8) | 14 (5.6) | |
| Laterality | 0.019* | ||
| Right | 1,257 (46.2) | 105 (42.0) | |
| Left | 1,441 (52.9) | 130 (52.0) | |
| Bilateral | 25 (0.9) | 15 (6.0) | |
| T stage | <0.001* | ||
| T1 | 1,386 (50.9) | 21 (10.7) | |
| T2 | 1,096 (40.2) | 76 (38.8) | |
| T3 | 68 (2.5) | 24 (12.2) | |
| T4 | 173 (6.4) | 75 (38.3) | |
| N stage | <0.001* | ||
| N0 | 1,663 (61.1) | 65 (28.9) | |
| N1 | 756 (27.8) | 102 (45.3) | |
| N2 | 199 (7.3) | 29 (12.9) | |
| N3 | 105 (3.9) | 29 (12.9) | |
| Grade | <0.001* | ||
| I | 526 (19.3) | 10 (5.3) | |
| II | 1,345 (49.4) | 83 (44.4) | |
| III | 848 (31.1) | 90 (48.1) | |
| IV | 4 (0.1) | 4 (2.1) | |
| ER status | <0.001* | ||
| Positive | 2,656 (97.5) | 190 (86.0) | |
| Negative | 67 (2.5) | 31 (14.0) | |
| PR status | <0.001* | ||
| Positive | 2,497 (91.7) | 169 (76.1) | |
| Negative | 226 (8.3) | 53 (23.9) | |
| Her2 status | 0.005* | ||
| Positive | 585 (21.5) | 42 (20.1) | |
| Negative | 2,138 (78.5) | 167 (79.9) | |
| Subtypes | <0.001* | ||
| Her2−/HR+ | 2,403 (88.2) | 145 (70.0) | |
| Her2+/HR+ | 265 (9.7) | 35 (16.9) | |
| Her2+/HR− | 21 (0.8) | 7 (3.4) | |
| Triple negative | 34 (1.2) | 20 (9.7) |
*, represent the P value <0.05.
Distribution of distant metastasis sites
The distributions of distant metastasis sites are shown in Table 3. The most common metastatic site was the bone, followed by the lung, liver, and brain. Interestingly, many breast cancer patients developed metastasis at more than one site. A total of 115 (46.0%), 59 (23.6%), 21 (8.4%), and 3 (1.2%) patients had one, two, three, and four metastatic sites, respectively.
Table 3
| Specific site of distant metastasis | n |
|---|---|
| Bone alone | 80 |
| Brain alone | 2 |
| Liver alone | 6 |
| Lung alone | 27 |
| Bone + brain | 4 |
| Bone + liver | 9 |
| Bone + lung | 44 |
| Brain + liver | 1 |
| Brain + lung | 0 |
| Liver + lung | 1 |
| Bone + brain + liver | 1 |
| Bone + brain + lung | 8 |
| Bone + liver + lung | 11 |
| Brain + liver + lung | 1 |
| Bone + brain + liver + lung | 3 |
Combination metastasis analysis based on different subtypes
The different metastasis patterns are summarized in Table 4. 21.2% of patients with the Her2−/HR+ subtype, 4.0% of patients with the Her2+/HR+ subtype, 0.4% of patients with the Her2+/HR− subtype, and 2.4% of patients with the TN subtype had only bone metastasis. Sole liver metastasis or brain metastasis were seldom seen, regardless of subtype. In patients with two sites of metastasis, the two sites were different among the subtypes. In patients with the Her2−/HR+, Her2+/HR+, and TN subtypes, the most common combination was the bone and lung (11.2%, 2.8%, and 0.8%, respectively). In patients with the Her2+/HR− subtype, the most common combination was the bone and liver (0.8%). In patients with three sites of metastasis, the most common combination was the bone, liver, and lung in all subtypes (Her2−/HR+, 1.6%; Her2+/HR+, 1.2%; and TN, 0.4%). Metastasis to four sites was rare, accounting for only 0.4% of patients across all subtypes.
Table 4
| Metastatic site | Her2-/HR +, n (%) | Her2 +/HR +, n (%) | Her2 +/HR-, n (%) | Triple negative, n (%) | P value |
|---|---|---|---|---|---|
| Only one site | 0.853 | ||||
| Bone | 53 (21.2) | 10 (4) | 1 (0.4) | 6 (2.4) | |
| Brain | 1 (0.4) | 0 | 0 | 1 (0.4) | |
| Liver | 3 (1.2) | 0 | 0 | 0 | |
| Lung | 16 (6.4) | 3 (1.2) | 1 (0.4) | 2 (0.8) | |
| Two sites | 0.094 | ||||
| Bone + brain | 3 (1.2) | 1 (0.4) | 0 | 0 | |
| Bone + liver | 2 (0.8) | 2 (0.8) | 2 (0.8) | 1 (0.4) | |
| Bone + lung | 28 (11.2) | 7 (2.8) | 0 | 2 (0.8) | |
| Brain + liver | 0 | 0 | 0 | 0 | |
| Brain + lung | 0 | 0 | 0 | 0 | |
| Liver + lung | 0 | 0 | 0 | 0 | |
| Three sites | 0.793 | ||||
| Bone + brain + liver | 0 | 1 (0.4) | 0 | 0 | |
| Bone + brain + lung | 3 (1.2) | 2 (0.8) | 0 | 2 (0.8) | |
| Bone + liver + lung | 4 (1.6) | 3 (1.2) | 0 | 1 (0.4) | |
| Brain + liver + lung | 0 | 0 | 0 | 0 | |
| Four sites | 1.0 | ||||
| Bone + brain + liver + lung | 0 | 1 (0.4) | 1 (0.4) | 1 (0.4) |
Association between subtype and site of distant metastasis
In order to further evaluate the relationship between site of metastasis and breast cancer subtype, univariate and multivariate logistic regression analyses were performed. Patients with the Her2−/HR+ subtype had a significantly higher probability of developing bone metastasis than those with other subtypes (Her2+/HR+ vs. Her2−/HR+: OR 0.958, 95% CI: 0.426–4.143; Her2+/HR− vs. Her2−/HR+: OR 0.296, 95% CI: 0.021–4.248; TN vs. Her2−/HR+: OR 0.727, 95% CI: 0.155–3.419) (Table 5). Patients with the Her2−/HR+ and TN subtypes had a significantly higher probability of lung metastasis than patients with the Her2+/HR− subtype (Her2+/HR− vs. Her2−/HR+: OR 0.860, 95% CI: 0.067–10.977; Her2+/HR− vs. TN: OR 0.415, 95% CI: 0.027–6.299). Patients with the Her2−/HR+ subtype had a significantly lower probability of liver metastasis than patients with the other three subtypes (Her2+/HR+ vs. Her2−/HR+: OR 4.035, 95% CI: 0.968–16.827; Her2+/HR− vs. Her2−/HR+: OR 7.100, 95% CI: 0.416–121.149; TN vs. Her2−/HR+: OR 5.396, 95% CI: 0.889–32.755) (Table 5). Patients with the Her2−/HR+ subtype had a significantly lower probability of brain metastasis than patients with the Her2+/HR+ and TN subtypes (Her2+/HR+ vs. Her2−/HR+: OR 9.991, 95% CI: 1.274–78.340; TN vs. Her2−/HR+: OR 12.437, 95% CI: 0.967–159.880) (Table 5).
Table 5
| Metastasis site/subtype | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Wald χ2 | P | OR (95% CI) | P | ||
| Bone | 19.788 | <0.001* | |||
| Her2+/HR+ vs. Her2−/HR+ | 0.958 (0.426–4.143) | 0.036* | |||
| Her2+/HR− vs. Her2−/HR+ | 0.296 (0.021–4.248) | 0.027* | |||
| TN vs. Her2−/HR+ | 0.727 (0.155–3.419) | 0.048* | |||
| Her2+/HR+ vs. Her2+/HR− | 4.273 (0.259–70.386) | 0.310 | |||
| Her2+/HR+ vs. TN | 1.826 (0.306–10.901) | 0.509 | |||
| Her2+/HR− vs. TN | 0.407 (0.023–7.131) | 0.538 | |||
| Lung | 9.230 | 0.002* | |||
| Her2+/HR+ vs. Her2−/HR+ | 1.626 (0.650–4.063) | 0.299 | |||
| Her2+/HR− vs. Her2−/HR+ | 0.860 (0.067–10.977) | 0.008* | |||
| TN vs. Her2−/HR+ | 2.075 (0.524–8.212) | 0.298 | |||
| Her2+/HR+ vs. Her2+/HR− | 2.114 (0.153–29.213) | 0.576 | |||
| Her2+/HR+ vs. TN | 0.784 (0.170–3.616) | 0.755 | |||
| Her2+/HR− vs. TN | 0.415 (0.027–6.299) | 0.026* | |||
| Liver | 62.243 | <0.001* | |||
| Her2+/HR+ vs. Her2−/HR+ | 4.035 (0.968–16.827) | 0.036* | |||
| Her2+/HR− vs. Her2−/HR+ | 7.100 (0.416–121.14) | 0.046* | |||
| TN vs. Her2−/HR+ | 5.396 (0.889–32.755) | 0.027* | |||
| Her2+/HR+ vs. Her2+/HR− | 0.259 (0.017–3.895) | 0.858 | |||
| Her2+/HR+ vs. TN | 0.748 (0.108–5.192) | 0.769 | |||
| Her2+/HR− vs. TN | 1.316 (0.074–23.431) | 0.852 | |||
| Brain | 59.215 | <0.001* | |||
| Her2+/HR+ vs. Her2−/HR+ | 9.991 (1.274–78.340) | 0.028* | |||
| Her2+/HR− vs. Her2−/HR+ | NI | NI | |||
| TN vs. Her2−/HR+ | 12.437 (0.967–159.880) | 0.043* | |||
| Her2+/HR+ vs. Her2+/HR− | NI | NI | |||
| Her2+/HR+ vs. TN | 0.803 (0.063–10.267) | 0.866 | |||
| Her2+/HR− vs. TN | NI | NI | |||
*, represent the P value <0.05. NI, not included in the multivariate logistic regression analysis.
Survival analysis based on metastatic pattern
The results for the univariate and multivariate analysis of BCSS are shown in Table 6. Univariate analysis showed that bone, lung, liver, and brain metastases were prognostic factors affecting BCSS in patients with the Her2−/HR+ subtype (all P<0.001). Liver metastasis was not a prognostic factor affecting BCSS in patients with the Her2+/HR+ subtype (χ2=2.380, P=0.123). Bone and lung metastases were significant factors affecting BCSS in patients with the TN subtype (χ2=17.007, P<0.001; χ2=7.426, P=0.006, respectively). However, we found no factors affecting BCSS in patients with the Her2+/HR− subtype. Further multivariate analysis showed that bone metastasis was an independent prognostic factor for BCSS (all P<0.05) (Table 6). Interestingly, lung metastasis was not an independent prognostic factor affecting BCSS in patients with the Her2+/HR+ subtype (HR 0.398, 95% CI: 0.101–1.571, P=0.188). However, it was an affecting factor in patients with the Her2+/HR− subtype (HR 0.015, 95% CI: 0.001–0.359, P=0.010).
Table 6
| Subtype/Metastasis site | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Log rank | P | HR (95% CI) | P | ||
| Her2−/HR+ | |||||
| Bone metastasis (no vs. yes) | 214.182 | <0.001* | 0.105 (0.062–0.178) | <0.001* | |
| Brain metastasis (no vs. yes) | 16.007 | <0.001* | 0.153 (0.052–0.452) | 0.001* | |
| Liver metastasis (no vs. yes) | 26.440 | <0.001* | 0.235 (0.093–0.596) | 0.002* | |
| Lung metastasis (no vs. yes) | 70.037 | <0.001* | 0.731 (0.368–1.451) | 0.370 | |
| Her2+/HR+ | |||||
| Bone metastasis (no vs. yes) | 18.241 | <0.001* | 0.272 (0.078–0.947) | 0.041* | |
| Brain metastasis (no vs. yes) | 5.322 | 0.021* | 0.645 (0.106–3.937) | 0.635 | |
| Liver metastasis (no vs. yes) | 2.380 | 0.123 | 2.676 (0.419–17.082) | 0.298 | |
| Lung metastasis (no vs. yes) | 15.952 | <0.001* | 0.398 (0.101–1.571) | 0.188 | |
| Her2+/HR− | |||||
| Bone metastasis (no vs. yes) | 3.342 | 0.068 | 0.030 (0.002–0.548) | 0.018* | |
| Brain metastasis (no vs. yes) | 0.170 | 0.680 | NI | ||
| Liver metastasis (no vs. yes) | 0.465 | 0.496 | 5.625 (0.234–135.439) | 0.287 | |
| Lung metastasis (no vs. yes) | 2.768 | 0.096 | 0.015 (0.001–0.359) | 0.010* | |
| Triple negative | |||||
| Bone metastasis (no vs. yes) | 17.007 | <0.001* | 0.196 (0.067–0.572) | 0.003* | |
| Brain metastasis (no vs. yes) | 2.701 | 0.100 | 1.185 (0.211–6.652) | 0.847 | |
| Liver metastasis (no vs. yes) | 3.340 | 0.068 | 1.158 (0.216–6.223) | 0.864 | |
| Lung metastasis (no vs. yes) | 7.426 | 0.006* | 0.833 (0.196–3.530) | 0.804 | |
*, represent the P value <0.05. NI, not included in the multivariate Cox regression analysis.
The results for the univariate and multivariate analyses of OS are shown in Table 7. Univariate analysis showed that bone, lung, liver and brain metastases were prognostic factors for OSS except for Her2+/HR−subtype patients (all P<0.05). Further multivariate analysis showed that bone metastasis was an independent prognostic factor for BCSS with Her2−/HR+, Her2+/HR− and TN (all P<0.05) (Table 7). Interestingly, lung metastasis was an affecting prognostic factor affecting BCSS in patients with the Her2+/HR− subtype (HR 0.015, 95% CI: 0.001–0.359, P=0.010).
Table 7
| Subtype/metastasis site | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Log rank | P | HR (95% CI) | P | ||
| Her2−/HR+ | |||||
| Bone metastasis (no vs. yes) | 123.631 | <0.001* | 0.205 (0.137–0.305) | <0.001* | |
| Brain metastasis (no vs. yes) | 15.545 | <0.001* | 2.061 (0.606–7.005) | 0.247 | |
| Liver metastasis (no vs. yes) | 20.044 | <0.001* | 0.788 (0.242–2.570) | 0.693 | |
| Lung metastasis (no vs. yes) | 46.210 | <0.001* | 0.921 (0.533–1.592) | 0.768 | |
| Her2+/HR+ | |||||
| Bone metastasis (no vs. yes) | 11.596 | <0.001* | 0.753 (0.258–2.203) | 0.605 | |
| Brain metastasis (no vs. yes) | 26.166 | <0.001* | 0.203 (0.054–0.770) | 0.019* | |
| Liver metastasis (no vs. yes) | 6.834 | 0.009* | 2.184 (0.517–9.228) | 0.288 | |
| Lung metastasis (no vs. yes) | 14.258 | <0.001* | 0.432 (0.142–1.315) | 0.140 | |
| Her2+/HR− | |||||
| Bone metastasis (no vs. yes) | 3.342 | 0.068 | 0.030 (0.002–0.548) | 0.018* | |
| Brain metastasis (no vs. yes) | 0.170 | 0.680 | NI | ||
| Liver metastasis (no vs. yes) | 0.465 | 0.496 | 5.625 (0.234–135.439) | 0.287 | |
| Lung metastasis (no vs. yes) | 2.768 | 0.096 | 0.015 (0.001–0.359) | 0.010* | |
| Triple negative | |||||
| Bone metastasis (no vs. yes) | 19.196 | <0.001* | 0.247 (0.088–0.692) | 0.008* | |
| Brain metastasis (no vs. yes) | 4.606 | 0.032* | 1.411 (0.319–6.250) | 0.650 | |
| Liver metastasis (no vs. yes) | 10.339 | 0.001* | 0.668 (0.160–2.778) | 0.579 | |
| Lung metastasis (no vs. yes) | 12.095 | 0.001* | 0.744 (0.195–2.842) | 0.665 | |
*, represent the P value <0.05. NI, not included in the multivariate survival analysis.
Kaplan-Meier analyses were also used to analyze prognosis. The results showed that patients with the TN subtype with bone or lung metastases had the worst BCSS (bone: χ2=30.54, P<0.001; lung: χ2=10.48, P=0.0149) (Figure 2A,B,C,D).
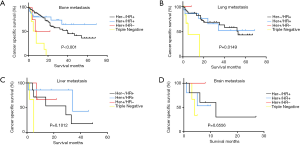
Conclusions
In this study, we investigated the characteristics of MBC patients and the effect of distant metastatic patterns of different subtypes on survival. The subtype not only affects the survival of breast cancer patients (8), but different subtypes may also be associated with different organ-specific metastases (9). Our study suggests that patients with different subtypes have different metastatic patterns; patients with all subtypes were most prone to bone metastases, and patients with the Her2−/HR+ subtype had a significantly higher probability of bone metastasis. Patients with the Her2−/HR+ subtype had a lower probability of brain or liver metastasis. Further, patients with the TN subtype primarily developed bone or lung metastasis.
The bone, liver, lung, and brain are the most common sites of distant metastasis in breast cancer (10). Some studies have shown that differences in survival in FBC may be linked to different metastatic patterns (11). However, studies on the association between different subtypes and the exact patterns of distant metastasis have been limited and inconsistent, especially for male patients.
The bone is the most common distant metastatic organ in breast cancer patients, with up to 75% of stage IV BC patients developing skeletal metastases (12-14). Bone metastasis is also the main cause of pathologic fractures and spinal cord compression, which seriously affect the quality of life of breast cancer patients (15). Our results also showed that the bone was the most common metastatic site in MBC patients. It has been reported that patients with ER-positive and PR-positive tumors have a higher risk of bone metastasis (16,17). However, a study in Korea showed that there was no significant difference in the incidence of bone metastasis among patients with different subtypes (18). In our study, patients with the Her2−/HR+ subtype had a significantly higher probability of developing bone metastases than patients with other subtypes. The study by Piggott et al. (19) suggested that breast cancer subtype could influence OS, but bone metastasis was not a factor influencing survival. Patients with bone metastasis from breast cancer often have a notably increased survival over patients with visceral or brain metastases (17,20). In agreement with these prior studies, we found that patients with bone metastasis had better BCSS only if the patients had the Her2+/HR+ or Her2−/HR+ subtypes. This can be mainly explained by the fact that patients with bone metastasis are more likely to have endocrine-responsive disease, which would lead to more lines of effective therapy, as these patients benefit from endocrine therapy. Interestingly, patients with Her2+/HR+ breast cancer have better BCSS and OS (17); this survival advantage was not affected by the receipt of Her2-targeted therapy (21). As expected, our research showed that patients with the TN subtype had the worst BCSS in all metastatic patterns. This can be mainly explained by the fact that these patients do not benefit from endocrine therapy or the targeted drug trastuzumab.
Approximately 60% of metastatic breast cancer patients develop lung or bone metastases in their lifetimes (22). Despite a variety of available treatments for lung metastasis, such as chemotherapy, radiotherapy, and targeted therapy, the survival rate of these patients remains very low. The incidence of lung metastasis can reach up to 50% in TNBC cases, compared to only 17.98% in non-TNBC cases (23), which is in accordance with our conclusion. Xiao et al. analyzed the survival of patients with lung metastasis and found that Her2+/HR− and Her2−/HR+ breast cancers had the best clinical outcome, whereas TNBCs had the worst prognosis (24). Similarly, we found that TN patients in our study had the worst prognosis, especially compared to Her2+/HR+ and Her2−/HR+ patients. Endocrine therapy may prolong the survival of HR+ patients compared to TN patients, who are not sensitive to endocrine therapy.
The survival of patients with liver metastasis is lower than that of patients with bone or lung metastasis (25). They not only bear a great burden of tumor cells but also have a progressive deterioration of liver function, which made the overall survival rates very low and most patients died within the first year after diagnosis of liver lesions (26). Breast cancer with liver metastasis has a poor outcome if left untreated, with a survival period ranging from 4 to 8 months (27). A previous study revealed that, compared with the TN subtype, the Her2+ subtype was significantly associated with liver metastasis (9). Our results showed that patients with the Her2−/HR+ subtype had a significantly lower probability of developing liver metastasis than patients with the other three subtypes. Further, Her2+ patients had a better prognosis than TN patients after 10 months. There are several treatment options for patients with liver metastasis, including surgical resection, systematic chemotherapy, and transarterial chemoembolization. The choice of different therapies may have an impact on patient survival, and our results may have incorporated some clinical confounding factors.
Brain metastasis from breast cancer is usually a catastrophic event. The incidence of brain metastasis has been continuously rising because of technological advances in earlier detection and more advanced therapy (12). It has been previously demonstrated that high-grade tumors constitute a much higher proportion of breast cancers that subsequently develop brain metastases (28). We did not see this in our data, probably because of the limited sample size. Our results are consistent with previous studies that found that the risk of brain metastasis in Her2+ patients is significantly higher than that in Her2− patients (29). Further, there have also been controversies regarding the prognostic significance of breast cancer subtypes in patients with brain metastasis in the very few previously published studies. In our study, we found that, among patients with brain metastasis, only patients with the Her2+/HR− subtype have a decent prognosis. It further proves that trastuzumab can prolong survival in breast cancer patients with brain metastases.
This study also has several limitations. First, due to the absence of information on chemotherapy, targeted therapy, and Ki-67 status in the SEER database, their effects on survival could not be evaluated. Second, this study is a non-randomized study and the sample size is relatively small, so intrinsic defects exist. Although there were some instances in our study where the P value was >0.05 and the survival curves crossed, this may be due to the limited sample size or clinical confounding factors of MBC and the different recurrence peaks in different patterns. We can still draw some conclusions from the trends of these data. Third, in the present study, only metastases to the bone, lung, liver, and brain were included. Although these are the common sites of distant metastasis in breast cancer, metastases to other sites may influence the prognosis of breast cancer patients.
In conclusion, our study further clarified the relationship between distant metastatic patterns and subtypes in MBC. These results suggest that different patterns of metastasis in male patients with different breast cancer subtypes have different impacts on clinical outcomes. Importantly, we performed a prognostic analysis for patients with different distant metastatic patterns based on subtypes, which may assist physicians in evaluating the survival potential of male patients with breast cancer.
Table S1
| Variables | Control, N=2,723 (%) | Bone, N=80 (%) | Brain, N=2 (%) | Liver, N=6 (%) | Lung, N=27 (%) |
|---|---|---|---|---|---|
| Survival (months) | 29.94±20.344 | 18.31±17.647 | 5.00±1.414 | 12.00±14.913 | 25.19±22.412 |
| Age at diagnosis, y | 0.028* | 0.598 | 0.590 | 0.786 | |
| <40 | 32 (1.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 40–59 | 684 (25.1) | 18 (22.5) | 0 (0) | 1 (16.7) | 5 (18.5) |
| 60–79 | 1,509 (55.4) | 38 (47.5) | 1 (50.0) | 5 (83.3) | 18 (66.6) |
| ≥80 | 498 (18.3) | 24 (30.0) | 1 (50.0) | 0 (0) | 4 (14.8) |
| Race | 0.706 | 0.528 | 0.274 | 0.184 | |
| White | 2,199 (80.8) | 64 (80.0) | 2 (100.0) | 6 (100.0) | 18 (66.7) |
| Black | 373 (13.7) | 15 (18.8) | 0 (0) | 0 (0) | 8 (29.6) |
| Other | 131 (4.8) | 1 (1.3) | 0 (0)) | 0 (0) | 1 (3.7) |
| Unknown | 20 (0.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Grade | 0.008* | 0.666 | 0.159 | 0.623 | |
| I | 325 (11.9) | 3 (3.8) | 0 (0) | 0 (0) | 1 (3.7) |
| II | 1,345 (49.4) | 36 (45.0) | 1 (50.0) | 2 (33.3) | 8 (29.6) |
| III | 848 (31.1) | 31 (38.8) | 1 (50.0)) | 0 (0) | 10 (37.0) |
| IV | 4 (0.1) | 0 (0) | 0 (0) | 1 (16.7) | 1 (3.7) |
| Unknown | 201 (7.4) | 10 (12.5) | 0 (0) | 3 (50.0) | 7 (25.9) |
| T stage | <0.001* | 0.756 | 0.649 | <0.001* | |
| T1 | 1,237 (45.4) | 11 (13.8) | 1 (50.0) | 1 (16.7) | 2 (7.4) |
| T2 | 1,096 (40.2) | 29 (36.3) | 1 (50.0) | 2 (33.3) | 8 (29.6) |
| T3 | 68 (2.5) | 10 (12.5) | 0 (0) | 0 (0) | 3 (11.1) |
| T4 | 173 (6.4) | 21 (26.3) | 0 (0) | 1 (16.7) | 9 (33.3) |
| Unknown | 149 (5.5) | 9 (11.3) | 0 (0) | 2 (33.3) | 5 (18.5) |
| N stage | <0.001* | – | 0.736 | <0.001* | |
| N0 | 1,579 (58.0) | 24 (30.0) | 0 (0) | 2 (33.3) | 6 (22.2) |
| N1 | 756 (27.8) | 37 (46.3) | 0 (0) | 1 (16.7) | 8 (29.6) |
| N2 | 199 (7.3) | 10 (12.5) | 0 (0) | 0 (0) | 6 (22.2) |
| N3 | 105 (3.9) | 8 (10.0) | 0 (0) | 1 (16.7) | 5 (18.5) |
| Unknown | 84 (3.1) | 1 (1.3) | 2 (100.0) | 2 (33.3) | 2 (7.4) |
| ER | 0.001* | <0.001* | 0,032* | <0.001* | |
| Positive | 2,507 (92.1) | 70 (87.5) | 1 (50.0)) | 3 (50.0) | 18 (66.7) |
| Negative | 67 (2.5) | 7 (8.8) | 1 (50.0)) | 1 (16.7) | 4 (14.8) |
| Unknown | 149 (5.5) | 3 (3.8) | 0 (0) | 2 (33.3) | 5 (18.5) |
| PR | 0.015* | 0.041* | 0.035* | 0.002* | |
| Positive | 2,331 (85.6) | 64 (80.0) | 1 (50.0)) | 2 (33.3) | 16 (59.3) |
| Negative | 226 (8.3) | 13 (16.3) | 1 (50.0)) | 2 (33.3) | 7 (25.9) |
| Unknown | 166 (6.1) | 3 (3.8) | 0 (0) | 2 (33.3) | 4 (14.8) |
| HER2 | 0.334 | 0.603 | 0.004* | 0.001* | |
| Positive | 289 (10.6) | 11 (13.8) | 0 (0) | 0 (0) | 4 (14.8) |
| Negative | 2,138 (78.5) | 59 (73.8) | 2 (100.0) | 3 (50.0) | 18 (66.7) |
| Unknown | 296 (10.9) | 10 (12.5) | 0 (0) | 3 (50.0) | 5 (18.5) |
| Subtype | <0.001* | <0.001* | 0.400 | 0.014* | |
| HR−/HER+ | 2,908 (77.0) | 53 (66.3) | 1 (50.0)) | 3 (50.0) | 16 (59.3) |
| HR+/HER+ | 265 (9.7) | 10 (12.5) | 0 (0) | 0 (0) | 3 (11.1) |
| HR+/HER- | 21 (0.8) | 1 (1.3) | 0 (0) | 0 (0) | 1 (3.7) |
| TN | 34 (1.2) | 6 (7.5) | 1 (50.0)) | 0 (0) | 2 (7.4) |
| Unknown | 305 (11.2) | 10 (12.5) | 0 (0) | 3 (50.0) | 5 (18.5) |
| Laterality | 0.978 | 0.216 | 0.032* | 0.054 | |
| Right | 1,253 (46.0) | 38 (47.5) | 0 (0) | 1 (16.7) | 7 (25.9) |
| Left | 1,441 (52.9) | 40 (50.0) | 2 (100.0) | 4 (66.7) | 20 (74.1) |
| Bilateral | 25 (0.9) | 2 (2.5) | 0 (0) | 1 (16.7) | 0 (0) |
| Unknown | 4 (0.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Surgery | <0.001* | 0.016* | <0.001* | <0.001* | |
| No | 185 (6.8) | 41 (51.2) | 1 (50.0) | 5 (83.3) | 15 (55.6) |
| Yes | 2,498 (91.7) | 38 (47.5) | 1 (50.0) | 1 (16.7) | 12 (44.4) |
| Unknown | 40 (1.7) | 1 (1.3) | 0 (0) | 0 (0) | 0 (0) |
| Status | <0.001* | 0.001* | 0.010* | <0.001* | |
| Alive | 2,283 (83.8) | 41 (51.2) | 0 (0) | 1 (16.7) | 12 (44.4) |
| Dead | 440 (16.2) | 39 (48.8) | 2 (100.0) | 5 (83.3) | 15 (55.6) |
*, represent the P value <0.05.
Table S2
| Variables | Control, N=2,723 (%) | Double, N=59 (%) | Three, N=21 (%) | Four, N=3 (%) |
|---|---|---|---|---|
| Survival (months) | 29.94±20.344 | 17.29±17.048 | 12.05±16.209 | 6.33±4.726 |
| Age at diagnosis, y | 0.075 | 0.039* | 0.573 | |
| <40 | 32 (1.2) | 2 (3.4) | 1 (4.8) | 0 (0) |
| 40–59 | 684 (25.1) | 15 (25.5) | 8 (38.1) | 1 (33.3) |
| 60–79 | 1,509 (55.4) | 36 (61.0) | 9 (42.9) | 1 (33.3) |
| ≥80 | 498 (18.3) | 6 (10.2) | 3 (14.3) | 1 (33.3) |
| Race | 0.001* | 0.206 | 0.439 | |
| White | 2,199 (80.8) | 39 (66.1) | 15 (71.4) | 3 (100.0) |
| Black | 373 (13.7) | 13 (22.0) | 4 (19.0) | 0 (0) |
| Other | 131 (4.8) | 7 (11.9) | 2 (9.5)) | 0 (0) |
| Unknown | 20 (0.7) | 0 (0) | 0 (0) | 0 (0) |
| Grade | 0.038* | 0.257 | 0.020* | |
| I | 325 (11.9) | 4 (6.8) | 1 (4.8) | 0 (0) |
| II | 1,345 (49.4) | 14 (23.7) | 6 (28.6) | 1 (33.3) |
| III | 848 (31.1) | 25 (42.4) | 8 (38.1)) | 0 (0) |
| IV | 4 (0.1) | 1 (1.7) | 0 (0) | 0 (0) |
| Unknown | 201 (7.4) | 15 (25.4) | 6 (28.6) | 2 (66.7) |
| T stage | <0.001* | <0.001* | 0.501 | |
| T1 | 1,237 (45.4) | 2 (3.4) | 2 (9.5) | 1 (33.3) |
| T2 | 1,096 (40.2) | 14 (23.7) | 7 (33.3) | 0 (0) |
| T3 | 68 (2.5) | 3 (5.1) | 4 (19.0) | 0 (0) |
| T4 | 173 (6.4) | 25 (42.4) | 5 (23.8) | 1 (33.3) |
| Unknown | 149 (5.5) | 15 (25.4) | 3 (14.3) | 1 (33.3) |
| N stage | <0.001* | 0.242 | 0.811 | |
| N0 | 1,579 (58.0) | 15 (25.4) | 8 (38.1) | 1 (33.3) |
| N1 | 756 (27.8) | 28 (47.5) | 7 (33.3) | 2 (66.7) |
| N2 | 199 (7.3) | 6 (10.2) | 0 (0) | 0 (0) |
| N3 | 105 (3.9) | 6 (10.2) | 3 (14.3) | 0 (0) |
| Unknown | 84 (3.1) | 4 (6.8) | 3 (14.3) | 0 (0) |
| ER | <0.001* | 0.001* | <0.001* | |
| Positive | 2,507 (92.1) | 46 (78.0) | 16 (76.2) | 1 (33.3) |
| Negative | 67 (2.5) | 6 (10.2) | 3 (14.3) | 2 (66.7) |
| Unknown | 149 (5.5) | 7 (11.9) | 2 (9.5) | 0 (0) |
| PR | 0.002* | <0.001* | <0.001* | |
| Positive | 2,331 (85.6) | 40 (67.8) | 12 (57.1) | 0 (0) |
| Negative | 226 (8.3) | 12 (20.3) | 7 (33.3) | 3 (100.0) |
| Unknown | 166 (6.1) | 7 (11.9) | 2 (9.5) | 0 (0) |
| HER2 | <0.001* | <0.001* | 0.003* | |
| Positive | 289 (10.6) | 12 (20.3) | 6 (28.6) | 2 (66.7) |
| Negative | 2,138 (78.5) | 38 (64.4) | 10 (47.6) | 1 (33.3) |
| Unknown | 296 (10.9) | 9 (15.3) | 5 (23.8) | 0 (0) |
| Subtype | 0.001* | <0.001* | <0.001* | |
| HR−/HER+ | 2,908 (77.0) | 35 (59.3) | 7 (33.3) | 0 (0) |
| HR+/HER+ | 265 (9.7) | 10 (16.9) | 6 (28.6) | 1 (33.3) |
| HR+/HER− | 21 (0.8) | 2 (3.4) | 0 (0) | 1 (33.3) |
| TN | 34 (1.2) | 3 (5.1) | 3 (14.3) | 1 (33.3) |
| Unknown | 305 (11.2) | 9 (15.3) | 5 (23.8) | 0 (0) |
| Laterality | 0.736 | 0.296 | 0.130 | |
| Right | 1,253 (46.0) | 31 (52.5) | 9 (42.9) | 1 (33.3) |
| Left | 1,441 (52.9) | 25 (42.4) | 10 (47.6) | 1 (33.3) |
| Bilateral | 25 (0.9) | 3 (5.1) | 2 (9.5) | 1 (33.3) |
| Unknown | 4 (0.1) | 0 (0) | 0 (0) | 0 (0) |
| Surgery | <0.001* | <0.001* | <0.001* | |
| No | 185 (6.8) | 43 (72.9) | 16 (76.2) | 3 (100.0) |
| Yes | 2,498 (91.7) | 15 (25.4) | 3 (14.3) | 0 (0) |
| Unknown | 40 (1.7) | 1 (1.7) | 2 (9.5) | 0 (0) |
| Status | <0.001* | <0.001* | 0.018* | |
| Alive | 2,283 (83.8) | 31 (52.5) | 9 (42.9) | 1 (33.3) |
| Dead | 440 (16.2) | 28 (47.5) | 12 (57.1) | 2 (66.7) |
*, represent the P value <0.05.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.43). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Since SEER is a publicly available database with anonymized data, no ethical review was required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Staruch RM, Rouhani MJ, Ellabban M. The surgical management of male breast cancer: Time for an easy access national reporting database? Ann Med Surg (Lond) 2016;9:41-9. [Crossref] [PubMed]
- Gaitanidis A, Alevizakos M, Tsalikidis C, et al. Refusal of Cancer-Directed Surgery by Breast Cancer Patients: Risk Factors and Survival Outcomes. Clin Breast Cancer 2018;18:e469-76. [Crossref] [PubMed]
- Callari M, Cappelletti V, De Cecco L, et al. Gene expression analysis reveals a different transcriptomic landscape in female and male breast cancer. Breast Cancer Res Treat 2011;127:601-10. [Crossref] [PubMed]
- Chen S, Liu Y, Yang J, et al. Development and Validation of a Nomogram for Predicting Survival in Male Patients With Breast Cancer. Front Oncol 2019;9:361. [Crossref] [PubMed]
- Lautrup MD, Thorup SS, Jensen V, et al. Male breast cancer: a nation-wide population-based comparison with female breast cancer. Acta Oncol 2018;57:613-21. [Crossref] [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [Crossref] [PubMed]
- Ess SM, Herrmann C, Bouchardy C, et al. Impact of subtypes and comorbidities on breast cancer relapse and survival in population-based studies. Breast 2018;41:151-8. [Crossref] [PubMed]
- Wu Q, Li J, Zhu S, et al. Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget 2017;8:27990-6. [PubMed]
- Li Y, Su P, Wang Y, et al. Impact of histotypes on preferential organ-specific metastasis in triple-negative breast cancer. Cancer Med 2020;9:872-81. [Crossref] [PubMed]
- Gerratana L, Fanotto V, Bonotto M, et al. Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metastasis 2015;32:125-33. [Crossref] [PubMed]
- Soni A, Ren Z, Hameed O, et al. Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol 2015;143:471-8. [Crossref] [PubMed]
- Wu SG, Sun JY, Yang LC, et al. Patterns of distant metastasis in Chinese women according to breast cancer subtypes. Oncotarget 2016;7:47975-84. [PubMed]
- Fang J, Xu Q. Differences of osteoblastic bone metastases and osteolytic bone metastases in clinical features and molecular characteristics. Clin Transl Oncol 2015;17:173-9. [Crossref] [PubMed]
- Neuhaus V, Abdullayev N, Hellmich M, et al. Association of Quality and Quantity of Bone Metastases and Computed Tomography Volumetric Bone Mineral Density With Prevalence of Vertebral Fractures in Breast Cancer Patients. Clin Breast Cancer 2016;16:402-9. [Crossref] [PubMed]
- Cosphiadi I, Atmakusumah TD, Siregar NC, et al. Bone Metastasis in Advanced Breast Cancer: Analysis of Gene Expression Microarray. Clin Breast Cancer 2018;18:e1117-22. [Crossref] [PubMed]
- Arciero CA, Guo Y, Jiang R, et al. ER(+)/HER2(+) Breast Cancer Has Different Metastatic Patterns and Better Survival Than ER-/HER2(+) Breast Cancer. Clin Breast Cancer 2019;19:236-45. [Crossref] [PubMed]
- Lee Y, Kang E, Lee AS, et al. Outcomes and recurrence patterns according to breast cancer subtypes in Korean women. Breast Cancer Res Treat 2015;151:183-90. [Crossref] [PubMed]
- Piggott RP, Waters PS, Kerin MJ. The influence of breast cancer subtype on bone metastases development and survival in women with metastatic breast cancer. Ir J Med Sci 2017;186:97-102. [Crossref] [PubMed]
- Kono M, Fujii T, Matsuda N, et al. Somatic mutations, clinicopathologic characteristics, and survival in patients with untreated breast cancer with bone-only and non-bone sites of first metastasis. J Cancer 2018;9:3640-6. [Crossref] [PubMed]
- Kast K, Link T, Friedrich K, et al. Impact of breast cancer subtypes and patterns of metastasis on outcome. Breast Cancer Res Treat 2015;150:621-9. [Crossref] [PubMed]
- Jin L, Han B, Siegel E, et al. Breast cancer lung metastasis: Molecular biology and therapeutic implications. Cancer Biol Ther 2018;19:858-68. [Crossref] [PubMed]
- Qiu J, Xue X, Hu C, et al. Comparison of Clinicopathological Features and Prognosis in Triple-Negative and Non-Triple Negative Breast Cancer. J Cancer 2016;7:167-73. [Crossref] [PubMed]
- Xiao W, Zheng S, Yang A, et al. Breast cancer subtypes and the risk of distant metastasis at initial diagnosis: a population-based study. Cancer Manag Res 2018;10:5329-38. [Crossref] [PubMed]
- Wu SG, Li H, Tang LY, et al. The effect of distant metastases sites on survival in de novo stage-IV breast cancer: A SEER database analysis. Tumour Biol 2017;39:1010428317705082. [Crossref] [PubMed]
- Bacalbasa N, Balescu I, Ilie V, et al. The Impact on the Long-term Outcomes of Hormonal Status After Hepatic Resection for Breast Cancer Liver Metastases. In Vivo 2018;32:1247-53. [Crossref] [PubMed]
- Onal C, Guler OC, Yildirim BA. Treatment outcomes of breast cancer liver metastasis treated with stereotactic body radiotherapy. Breast 2018;42:150-6. [Crossref] [PubMed]
- Li R, Zhang K, Siegal GP, et al. Clinicopathological factors associated with survival in patients with breast cancer brain metastasis. Hum Pathol 2017;64:53-60. [Crossref] [PubMed]
- Lu X, Gao C, Liu C, et al. Identification of the key pathways and genes involved in HER2-positive breast cancer with brain metastasis. Pathol Res Pract 2019;152475. [Crossref] [PubMed]


