Primary pleural synovial sarcoma in an adolescent: a case report
Introduction
Synovial sarcoma (SS) is a spindle cell tumour with uncertain histogenesis showing variable mesenchymal and epithelial differentiation, accounting for 2.5–10% of all soft tissue sarcoma (1,2). SS is reported as the most common non-rhabdomyosarcomatous soft tissue sarcoma in children and adolescents with a pathognomonic chromosomal translocation t(x;18)(p11;q11) (1). Although a wide anatomic distribution has been documented, primary SS in the pleural cavity is extremely rare, often involving the underlying parenchyma. Due to its rarity in this location and variable histogenesis, the diagnosis of SS is a clinical challenge. As far as we are aware, 12 cases of primary pleural SSs in children and adolescents under the age of 18 have been previously reported in the English-language literature (Table S1) (3-11), albeit the cases in one series study without individual information was not included (12). We report herein an additional case of primary pleural SS in a 14-year-old boy and review the literature on the subject. And the following case was presented in accordance with the CARE Guideline (13).
Case presentation
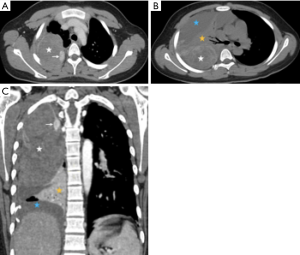
A 14-year-old Asian-Chinese boy initially presented another facility with spontaneous right-sided chest pain and dyspnea on July 1, 2019. There was no history of exposure to asbestos or underlying disease. A total of 700 mL bloodstained fluid was aspirated after a massive effusion in the right chest cavity revealed on the images of computed tomography (CT). The patient was then transferred to the emergency department of our hospital on July 6, 2019, and the bloodstained exudate was 1,000 mL on that day. The items in routine haematological investigations were within normal limits, except for the lower level of eosinophil. Blood and sputum cultures revealed no bacterial growth. Contrast-enhanced CT (Figure 1) showed a heterogeneously enhancing solid mass occupied the upper two-thirds of the right hemithorax, compressing the right lung tissue. The wall of the mass was enhanced, especially at the mural nodule and thickened areas. In addition, there was massive pleural effusion surrounding the pulmonary atelectasis in the lower half of the hemithorax. There is no evidence of hilar or mediastinal lymphadenopathy. Although negative results of preoperative serum tumour markers [human chorionic gonadotropin (HCG), carcinoembryonic antigen (CEA), alpha fetal protein (AFP), carbohydrate antigen (CA) 72-4, CA125, CA199, CA153] were observed, the embryonal tumour was suspected. Subsequently, open thoracotomy was performed on July 10, 2019. The mass was mainly located in the dorsal part of the hemithorax with direct infiltration of the lower lobe. The mass was easily peeled off the chest wall and dissected from the upper and middle lobe, which were re-expanded completely following removal of the mass and the lower lobe. The lymph nodes in paratracheal (upper and lower) and subcarinal regions were also dissected.
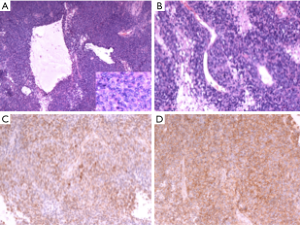
Grossly, the tumour was partially encapsulated and received in two fragments, the larger one is 9 cm × 6 cm × 4 cm, and the smaller one is 5.5 cm × 4.5 cm × 3 cm. Cut sections mainly showed a soft, grey red and solid appearance. Focally greyish yellow and white area measuring 3 cm × 2.5 cm × 2 cm was under the margin of the larger fragment. Histologic examination showed that tumour had hemorrhagic suffusion and necrosis without invading lymph nodes, and was classified as FNCLCC 3. Microscopic examination [hematoxylin-eosin (H&E) staining) of the tumor demonstrates a high degree of cellularity and mitotic figures. Glandular structures and a hemangiopericytoma-like vascular pattern are present (Figure 2A,B). Immunohistochemical analysis revealed positive reaction for B-cell lymphoma 2 (Bcl-2) (Figure 2C), CD99 (Figure 2D), cytokeratin (CK) and vimentin, and focally positive reaction for epithelial membrane antigen (EMA), transducing-like enhancer of split 1 (TLE1). There was evidence of a high proliferation index [Ki-67(MIB-1)] in around 40% of cells. No immunoreactivity with CD34, S100 protein, desmin, a-SMA, chromogranin A, ALK, and WT-1 was observed. The tumour was negative for t(X;18) translocation using Fluorescence in situ hybridization (FISH) analysis.
According to the morphological and immunohistochemical analysis, the tumour was diagnosed as biphasic SS. The patient was discharged 1 week after surgical resection without complications, and adjuvant chemotherapy has been arranged in another tumor hospital. Currently, the patient is clinically well 6 months after surgery, with no evidence of recurrent disease.
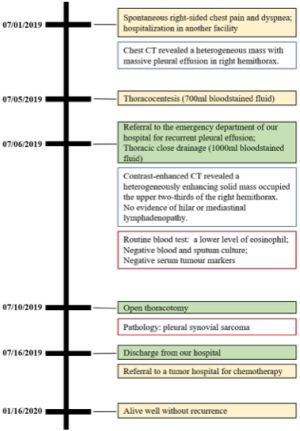
The whole course of interventions and follow-up has been drawn into a timeline figure, and the interested reader can find it in Figure S1.
Discussion
Primary pleural SS is a quite rare anatomical subset of SS, which is believed to arise from pluripotential mesenchymal tissue rather than synovial cell because they occur in places such as the pleura where synovial structures do not exist and can show epithelial differentiation (2,3), therefore the terminology of SS is actually a bit of a misnomer. In addition, an absence of extrathoracic tumor should be confirmed prior to the diagnosis of primary pleural SS, since SS tends to intrathoracic metastasis, especially to the lung (3,6,8,9).
Due to the lack of detailed individual information, the cases in Baheti et al.’s study were not included in the current analysis (12). Thus, primary pleural SSs in children and adolescents under the age of 18 documented in the English literature were 12 cases with no sexual bias (3-11). Typical symptoms include a short term of chest pain and/or dyspnea. Ipsilateral pleural effusion, especially bloodstained exude, is also well observed. It was the most commonly presented as a well-defined mass with patchy low density and heterogeneous enhancement without regional lymphadenopathy or calcification (3,4,6-9,11) on CT images. Only two cases presented as a multiloculated cyst (5,10), which have been mistaken as hydatid cyst before the operation. The radiologic manifestations of pleural SS overlapped with many other neoplasms in the lung and pleura (9), including primary and metastatic lung cancer, malignant mesothelioma, and other rare parenchymal sarcomas (e.g., leiomyosarcoma, sarcomatoid carcinoma, and malignant fibrous histiocytoma). The absence of regional lymphadenopathy tends to against a diagnosis of lung cancer. A history of asbestos exposure or the presence of contralateral pleural plaques favors that of mesothelioma (9). However, no reliable radiologic or clinical features can help to differentiate pleural SS from the other rare parenchymal sarcomas. In the present case, the initial symptoms were as typical as the description in previous reports, including chest pain, dyspnea and hemothorax. On CT images, majority of the tumor appeared to be pleural-based and extended into the right hemithorax without infiltration of the chest wall or cortical bone destruction. The absence of extrathoracic tumor and mediastinal lymphadenopathy only lead to the diagnosis of regional primary parenchymal neoplasm without definite localization. Interestingly, the term intrathoracic or pleuropulmonary SS was frequently used to describe the lesions occurring either pleura or pulmonary for avoiding the dilemma of precise localization in previous studies (8,9,12,14).
In addition, although the utility of fluorodeoxyglucose positron emission tomography-CT (FDG PET-CT) in SS has been emphasized in previous studies (11,12,14,15), limited patients received this examination with a variable degree of FDG uptake, and only one adolescent received FDG PET-CT with a high maximum standardized uptake up to 31.7 (11). Currently, it is hard to get a conclusive result, and this examination was also not performed in our case
Pleural SSs share the same histologic, histochemistry, immunohistochemistry with SSs in other locations (1,8), appearing as monophasic, biphasic and poorly differentiated tumors (3-7,10,11). The chromosomal translocation t(x;18) (p11: q11) that has been detected in more than 90% of SS, currently represents the most specific clue for definitive diagnosis (16). However, in the present case, the convincing gene rearrangement was failed to be detected by FISH analysis. The final diagnosis was made by morphological and immunohistochemical results. Initially, the characteristic glandular structure and a hemangiopericytoma-like appearance depicted on hematoxylin-eosin staining section favoured the diagnosis of SS, Immunohistochemical findings including a positive reaction for Bcl-2, CD99, CK and vimentin, and a focally positive reaction for EMA and TLE1 (2,17), also confirmed the diagnosis.
On the other side, primary pleural SS appears to be more aggressive than its soft tissue counterpart with most patients dying within 1–3 years (3,12,17), albeit one patient was alive 8 years after the initial diagnosis with regional and metastatic disease (3). The treatment for primary pleural SS remains unclear. Whenever feasible, radical resection is still the preferred choice followed by chemotherapy and/or radiotherapy. Sometimes, repeat resections were performed for recurrence or the case that were difficult to completely resect at once (18). In addition, radiofrequency thermal ablation (19), phototherapy and hyperthermia therapy as alternatives may also be considered (3,20). Currently, this 14-year boy is alive without recurrence for the initial six months with multimodality therapy (radical resection and postoperative chemotherapy), however, the long-term outcome is still uncertain and longer follow-up is essential.
Conclusions
Primary pleural SS in adolescents is a very rare, aggressive neoplasm that is difficult to confirm based on imaging, but it should be considered in the differential diagnosis of intrathoracic mass without regional lymphadenopathy in adolescents, especially the cases with recurrent hemorrhagic pleural effusion. Even though it was failed to detect convincing gene rearrangement, characteristic morphological structures and immunohistochemical analysis were aided to make the final diagnosis in the current case. However, six-month follow-up is not enough and the long-term outcome remains to be seen, since a worse prognosis of SS in pleura than that in other locations (1,3,12,17).
Table S1
| No. | Year | Author | Case | Symptoms | Radiologic findings | Gross findings | Microscopy subtype | Chromosome | Therapy | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1996 | Gaertner E, et al. (3). | 17F | Dysphagia, chest pain | XR: left pleural effusion, consolidation, mediastinal shift; CT: mass with focal contrast enhancement occupied lower 2/3 s of hemithorax | 21 cm, pseudocapsule, necrosis, haemorrhage, pleural thickening | Biphasic pattern | NA | Surgery | Rapid recurrence, and DOD within 12 months |
| 2 | 17F | Chest pain | XR: left pleural thickening with a central mass | 9 cm, necrosis, haemorrhage, pleural thickening, focal calcification, pleural thickening | Biphasic pattern | NA | Surgery, chemotherapy, radiation | Local recurrence, DOD at 18 months | ||
| 3 | 9M | Dyspnea, chest pain, fever | XR: right pleural-based mass | 5 cm, pseudocapsule, necrosis, focal calcification, | Biphasic pattern | NA | Surgery, chemotherapy, radiation | AWD at 8 years with regional and metastatic disease | ||
| 4 | 1997 | Jawahar DA, et al. (4). | 18F | Dyspnea, dry cough, chest pain | XR: a homogenous density in lower two-thirds of the right hemithorax; CT: heterogeneous mass in right inferior hemithorax | 17 cm, partial pseudocapsule | Biphasic pattern | Translocation (X; 18) | Surgery, radiation | Local recurrence at 5 months; AWD 16 months |
| 5 | 2003 | Yildirim E, et al. (5). | 9M | NA | CT: septated effusion with solid components occupied 3/4 of the left hemithorax | Cystic mass | NA | NA | Surgery, chemotherapy (ifosfamide, etoposide) | Alive without disease at 30 months |
| 6 | 2003 | Ng SB, et al. (6). | 15M | Chest pain, cough, fever | XR: a right-sided hydropneumothorax and an area of ill-defined soft tissue density in the lower half of the right lung. CT: mass in the right posterior mediastinum | 20 cm, solid and cystic areas, haemorrhage, myxoid change | Monophasic pattern | Translocation t(X; 18) (p11.2; q11.2) | Biopsy, surgery, chemotherapy, radiation; resection | Recurrence at 20 months; AWD 21 months |
| 7 | 2005 | Nishio J, et al. (7). | 18M | Chest pain | XR/CT: right pleural effusion, 8cm diaphragmatic pleural-based mass | NA | Monophasic | Translocation t(X; 18) (p11.2; q11.2); SYT-SSX1 fusion; a ring chromosome | Surgery, radiation; resection | Recurrence at 11 months; AWD 2 years |
| 8 | 2005 | Bégueret H, et al. (8). | 16M | NA | NA | NA | Surgery, radiation; resection | Recurrence at 32 months; AWD 36 months | ||
| 9 | 2006 | Frazier AA, et al. (9). | 17F | Chest pain | XR: Left-sided pleural-based mass, multilobular and sharply marginated | Mottled red soft-tissue mass, partly friable, with cystic and hemorrhagic zones; no capsule | NA | NA | Pleural decortication | NA |
| 10 | 17F | Dyspnea, back pain | XR: Mass fills 75% of the lower hemithorax; CT: located in left pleura, 15 cm × 20 cm, heterogeneous enhancement, nodularity mixed with areas of low attenuation, no record of lung nodules, pleural effusion present | Gray soft-tissue mass, zones of haemorrhage and necrosis; capsule present | NA | NA | Surgery | NA | ||
| 11 | 2008 | Tailor J, et al. (10). | 16M | Shortness of breath, chest pain, dry cough, haemoptysis | XR: left pleural effusion (lower 2/3 of the hemithorax), mediastinal shift; CT: multiloculated pleural cyst with thickened enhancing septae within it. Visceral and parietal pleural thickened and enhanced without no identifiable mass lesion | The pleural cavity filled with bloodstained fluid containing thick septae | Monophasic pattern | Translocation (X; 18) (SYT-SSX) | Surgery | AWD 6 months |
| 12 | 2016 | Won JH, et al. (11). | 17F | Chest pain | XR: a round mass in the left upper hemithorax; CT: a well-defined heterogeneous enhancing mass abutting the pleura, no calcification, hemothorax; PET/CT: SUVmax =31.7 | 8.0 cm × 6.5 cm × 5.5 cm well-circumscribed but unencapsulated tumor. Whiting-yellow, soft, and fleshy with cystic degenerative changes and hemorrhage, no calcification | Monophasic pattern | NA | Biopsy Surgery, radiation, chemotherapy; resection | Recurrence at 28 months; died from sepsis at 37 months |
| 13 | 2019 | Our case | 14M | Chest pain; dyspnea | CT: a heterogeneously enhancing solid mass occupied the upper two-thirds of the right hemithorax; massive pleural effusion | The tumour was partially encapsulated and received in two fragments, the larger one is 9 cm × 6 cm × 4 cm, and the smaller one is 5.5 cm × 4.5 cm × 3 cm. Cut sections mainly showed a soft, grey red and solid appearance | Biphasic pattern | (-) | Surgery, chemotherapy | Alive without disease 6 months |
DOD, death of disease; NA, not available; ADW, alive with disease.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.56). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the parents of the patient for publication of this manuscript and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Okcu MF, Munsell M, Treuner J, et al. Synovial sarcoma of childhood and adolescence: a multicenter, multivariate analysis of outcome. J Clin Oncol 2003;21:1602-11. [Crossref] [PubMed]
- Attanoos RL, Pugh MR. The Diagnosis of Pleural tumors other than mesothelioma. Arch Pathol Lab Med 2018;142:902-13. [Crossref] [PubMed]
- Gaertner E, Zeren EH, Fleming MV, et al. Biphasic Synovial Sarcomas Arising in the Pleural Cavity: A Clinicopathologic Study of Five Cases. Am J Surg Pathol 1996;20:36-45. [Crossref] [PubMed]
- Jawahar DA, Vuletin JC, Gorecki P, et al. Primary biphasic synovial sarcoma of the pleura. Respir Med 1997;91:568-70. [Crossref] [PubMed]
- Yildirim E, Dural K, Han S, et al. A case report of a pleural synovial sarcoma misdiagnosed as cyst hydatidosis. J Cardiovasc Surg (Torino) 2003;44:291-2. [PubMed]
- Ng SB, Ahmed Q, Tien SL, et al. Primary Pleural Synovial Sarcoma A Case Report and Review of the Literature. Arch Pathol Lab Med 2003;127:85-90. [PubMed]
- Nishio J, Iwasaki H, Althof PA, et al. Identification of a ring chromosome with spectral karyotyping in a pleural synovial sarcoma. Cancer Genet Cytogenet 2005;160:174-8. [Crossref] [PubMed]
- Bégueret H, Galateau-Salle F, Guillou L, et al. Primary intrathoracic synovial sarcoma: a clinicopathologic study of 40 t(X;18)-positive cases from the French Sarcoma Group and the Mesopath Group. Am J Surg Pathol 2005;29:339-46. [Crossref] [PubMed]
- Frazier AA, Franks TJ, Pugatch RD, et al. From the archives of the AFIP: Pleuropulmonary synovial sarcoma. Radiographics 2006;26:923-40. [Crossref] [PubMed]
- Tailor J, Roy PG, Bowker C, et al. Primary pleural synovial sarcoma presenting as a multiloculated cyst in an adolescent. Pediatr Surg Int 2008;24:597-9. [Crossref] [PubMed]
- Won JH, Chin S, Park JS, et al. Primary Pleural Synovial Sarcoma with Metastatic Cardiac Involvement: A Case Report. Iran J Radiol 2016;13:e41066. [Crossref] [PubMed]
- Baheti AD, Sewatkar R, Hornick JL, et al. Imaging features of primary and recurrent intrathoracic synovial sarcoma: a single-institute experience. Clin Imaging 2015;39:803-8. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Shah D, Odedra P. Primary Pleuropulmonary Synovial Sarcoma on Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography Scan. Indian J Nucl Med 2017;32:340-2. [Crossref] [PubMed]
- Kim GH, Kim MY, Koo HJ, et al. Primary pulmonary synovial sarcoma in a tertiary referral center: Clinical characteristics, CT, and 18F-FDG PET findings, with pathologic correlations. Medicine (Baltimore) 2015;94:e1392. [Crossref] [PubMed]
- Weinbreck N, Vignaud JM, Begueret H, et al. SYT-SSX fusion is absent in sarcomatoid mesothelioma allowing its distinction from synovial sarcoma of the pleura. Mod Pathol 2007;20:617-21. [Crossref] [PubMed]
- Klebe S, Prabhakaran S, Hocking A, et al. Pleural malignant mesothelioma versus pleuropulmonary synovial sarcoma: a clinicopathological study of 22 cases with molecular analysis and survival data. Pathology 2018;50:629-34. [Crossref] [PubMed]
- Katsurada N, Ohnishi H, Ikeda M, et al. Primary pleural synovial sarcoma with repeated resection leading to long-term survival. Respirol Case Rep 2019;7:e00480. [Crossref] [PubMed]
- Lee HK, Kwon HJ, Lee HB, et al. Radiofrequency thermal ablation of primary pleural synovial sarcoma. Respiration 2006;73:250-2. [Crossref] [PubMed]
- Abe K, Maebayashi T, Shizukuishi T, et al. Radiological assessment following thermoradiation therapy for primary pleural synovial sarcoma: case report. Med Oncol 2010;27:1027-30. [Crossref] [PubMed]