The relationship of EZH2 and HOXA5 with non-small cell lung carcinoma patient survival rate
Introduction
Including adenocarcinoma and squamous cell carcinoma, non-small cell lung cancer (NSCLC) is the primary type of lung cancer responsible for most cancer deaths worldwide (1). Despite recent advancements in surgical and experimental oncology, lung cancer prognosis remains poor, with an average 5-year survival rate of about 11 percent (2). Clinically, NSCLC shows a wide range of behavioral habits that vary from slowly advancing to quickly advancing, and may be extremely metastatic or only invasive locally. The molecular origin of these behavioral differences, however, is not well understood. In 194 cases of NSCLC tumors, the sequences of 4 proteins were analyzed immunohistochemically to identify a series of prognostic factors that can be readily tested with high effectiveness in clinical practice, and then compared with clinical outcomes.
EZH2 is a polycomb repressor complex 2 (PRC2), which catalyzes lysine 27 (H3K27me) methylation of histone H3 and mediates target gene silencing via local chromatin reorganization. Numerous pieces of evidence indicate that EZH2 plays a critical role in the development, growth, and metastasis of cancer (3-5). Recent studies have shown that EZH2 leads to the development of NSCLC (6) and accompanies poor survival (7,8). It has also been shown that EZH2 controls the intrinsic expression of multidrug resistance gene 1 (MDR1), indicating its contribution to drug resistance (9,10). The fundamental molecular events associated with increased expression of EZH2 with cancer metastasis and poor prognosis, however, remain unclear.
Homeobox genes constitute a family of regulatory genes comprising a standard sequence of 183 nucleotides (homeobox) and encoding unique nuclear proteins (homeoproteins) acting as transcription factors (11). HOX genes are called the clustered group of homeobox genes in humans. Homeobox A5 (HOXA5) is a master morphogenesis and cell differentiation regulator that is active in breast cancer as a tumor suppressor gene (12). It is shown that the deregulated expression(s) of a specific HOX gene(s) is associated with cancer growth and progression, such as invasion and metastasis (13). Several studies have recently reported that HOXA5 plays an important role in tumorigenesis in NSCLC by directly controlling the expression of p21 (14). Nevertheless, the biological function and therapeutic importance of HOXA5 is still not well known in the development and advancement of NSCLC and should be further studied.
Some studies report that the expression of Gst-π and survivin in NSCLC promotes multidrug-resistance and inhibits apoptosis.
However, little is known about the role of EZH2, HOXA5, GST-π, and survivin expression in NSCLC tissues, especially in the survival of lung cancer patients at present. This study is likely to aid in clarifying the correlations of EZH2, HOXA5, GST-π, and survivin expression, and clinical features with the overall survival (OS) and prognosis in patients with lung cancer.
Methods
Patients and tumor specimens
A total of 194 patients who underwent complete tumor resection from 2010 to 2013 were chosen for this research at the Harbin Medical University, China. Written informed consent was received from each patient and tissue specimens were prepared according to the Harbin Medical University Ethics Committee guidelines. After tumor removal in 2018, the OS rate was calculated. Specimens from these patients were collected from the University of Harbin Medical University’s Department of Pathology and the Department of Thoracic Surgery. The study also included 18 matched normal lung tissues (10 cm from the tumor) from the 194 specimens. As defined in Table 1, the clinical pathological characteristics of patients is based on the requirements of the World Health Organization (WHO) (14).
Table 1
| Gene | Normal tissues | Adenocarcinoma | Squamous carcinoma | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | + | Positive (%) | n | + | Positive (%) | n | + | Positive (%) | |||
| EZH2 | 5 | 0 | 0 | 37 | 22 | 59.5 | 37 | 23 | 62.2 | ||
| HOXA5 | 5 | 4 | 80.0 | 37 | 14 | 37.8 | 42 | 15 | 35.7 | ||
| Survivin | 4 | 0 | 0 | 10 | 3 | 30.0 | 11 | 3 | 27.3 | ||
| GST-π | 4 | 3 | 75.0 | 8 | 5 | 62.5 | 12 | 12 | 100.0 | ||
HOXA5, homeobox A5.
Patients aged 28–82, with a median age of 58 years, and complete clinical pathological data with follow-up to December 2, 2013, were included.
Immunohistochemical analysis
Paraffin-embedded, formalin-fixed tissues were immunostained for EZH2, HOXA5, survivin, and GST-π to determine the expression levels of EZH2, HOXA5, survivin and GST-π in NSCLC tissues. Immunohistochemical staining was conducted overnight at 4 °C with a rabbit anti-HOXA5 antibody (1:100, Bioss, Beijing) on an automated staining system (TechMate 500, DakoCytomation). For immunohistochemical measurement of EZH2, HOXA5, survivin, and GST-π expression, the signal was amplified and visualized with diaminobenzidine chromogen, followed by counterstaining with hematoxylin. For EZH2, HOXA5, survivin and GST-π, an immunohistochemistry (IHC) score of 2+ or more was defined as positive, and IHC scores of 0 and 1+ were defined as negative.
Statistical processing SPSS 19.0 statistical software for data processing was used. The same index expression in different organizations and relationships with different clinical pathological characteristics were analyzed using a chi-square test. Using EZH2, HOXA5 multivariable logistic regression analysis, the influence factors of survivin and GST-PI-positive expression and the correlation of protein expression was calculated using the Spearman correlation analysis. Inspection level of alpha =0.05.
Results
EZH2, HOXA5, and survivin expression in normal lung tissues and NSCLC tissues
EZH2, HOXA5, survivin, and GST-π were expressed in NSCLC tissues and normal lung tissue, as shown in Table 1. Using χ2 test two comparison, EZH2 and HOXA5 showed significant difference (P<0.05), but there were obvious differences between lung adenocarcinoma and squamous carcinomas (P>0.05); survivin showed significant difference in normal lung tissue and NSCLC tissues (P>0.05), while GST-π showed no obvious difference between normal lung and NSCLC tissues (P>0.05), but there was a significant difference between lung adenocarcinoma and squamous carcinoma (P<0.05).
The relationship of EZH2, HOXA5, GST-π, and survivin expression with patient survival
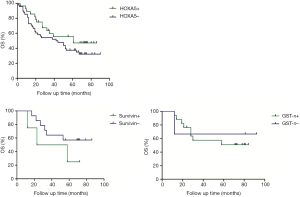
The association between EZH2, HOXA5, GST-π, and survivin expression with patient survival was assessed. Kaplan-Meier survival analysis was conducted to investigate the correlation between EZH2, HOXA5, GST-π, and survivin expression and NSCLC patient prognosis. The EZH2 positive expression of lung cancer patients survival time was 59.0 months on average, and the negative expression in lung cancer patients with an average survival time was 66.5 months; HOXA5 positive expression of lung cancer patients survival time was 61.0 months on average, and negative expression in lung cancer patients with an average survival time was 43.0 months; survivin positive expression of lung cancer patients survival time was 40.5 months on average, and the negative expression in lung cancer patients with an average survival time was 21.3 months; GST-PI-positive expression in lung cancer patients with an average survival time was 69.0 months, and the average negative expression in lung cancer patients survival time was 66.5 months. HOXA5 and survivin expression was associated with the disease-free survival (DFS) and the OS of lung cancer patients (P<0.05, Figure 1).
EZH2, HOXA5, survivin, and GST-PI relationship expression and clinicopathological characteristics of lung cancer
EZH2 expression and patients’ gender, age, smoking history, gross tumor type, and differentiation were not significantly different (P>0.05), and were associated with lymph node metastasis and TNM stages (P<0.01); HOXA5, GST-PI expression and patients’ gender, age, smoking history, gross tumor type, degree of differentiation, lymph node metastasis and TNM stages were not significantly different (P>0.05); survivin expression was related to tumor TNM stage (P<0.05), while patients’ gender, age, smoking history, gross tumor type, degree of differentiation, lymph node metastasis, and other factors were not significantly different (P>0.05, Table 2).
Table 2
| Parameter | EZH2 | HOXA5 | Survivin | GSTP1 | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | + | − | χ2 | P | n | + | − | χ2 | P | n | + | − | χ2 | P | n | + | − | χ2 | P | ||||
| Gender | |||||||||||||||||||||||
| Male | 58 | 34 | 24 | 0.575 | 0.448 | 55 | 18 | 37 | 1.235 | 0.266 | 14 | 3 | 11 | 0.263 | 0.608 | 16 | 15 | 1 | 1.985 | 0.159 | |||
| Female | 16 | 13 | 3 | 24 | 11 | 13 | 7 | 3 | 4 | 4 | 2 | 2 | |||||||||||
| Age | |||||||||||||||||||||||
| ≤50 | 17 | 12 | 5 | 0.885 | 0.347 | 18 | 6 | 12 | 0.114 | 0.735 | 3 | 0 | 3 | 0.243 | 0.622 | 3 | 2 | 1 | 0.008 | 0.930 | |||
| >50 | 57 | 33 | 24 | 61 | 23 | 38 | 18 | 6 | 12 | 17 | 15 | 2 | |||||||||||
| Smoking use | |||||||||||||||||||||||
| Yes | 50 | 31 | 19 | 0.002 | 0.967 | 46 | 13 | 33 | 3.383 | 0.066 | 13 | 3 | 10 | 0.045 | 0.831 | 13 | 12 | 1 | 0.349 | 0.555 | |||
| No | 24 | 15 | 9 | 33 | 16 | 17 | 8 | 3 | 5 | 7 | 5 | 2 | |||||||||||
| Tumor differentiation | |||||||||||||||||||||||
| Well | 11 | 9 | 2 | 2.122 | 0.145 | 11 | 4 | 7 | 0.001 | 0.980 | 1 | 0 | 1 | 0.420 | 0.517 | 8 | 8 | 0 | 0.801 | 0.371 | |||
| Moderate poor | 63 | 37 | 26 | 68 | 25 | 43 | 20 | 6 | 14 | 12 | 9 | 3 | |||||||||||
| Lymph node metastasis | |||||||||||||||||||||||
| Yes | 31 | 27 | 4 | 12.804 | 0.000** | 33 | 10 | 23 | 1.001 | 0.317 | 11 | 5 | 6 | 3.226 | 0.072 | 8 | 7 | 1 | 0.067 | 0.796 | |||
| No | 43 | 20 | 23 | 46 | 19 | 27 | 10 | 1 | 9 | 12 | 10 | 2 | |||||||||||
| TNM stage | |||||||||||||||||||||||
| I–II | 51 | 25 | 26 | 12.050 | 0.001** | 58 | 22 | 36 | 0.140 | 0.708 | 18 | 3 | 15 | 5.143 | 0.023* | 17 | 14 | 3 | 1.064 | 0.302 | |||
| III–IV | 23 | 21 | 21 | 7 | 14 | 3 | 3 | 0 | 3 | 3 | 0 | ||||||||||||
*, P<0.05; **, P<0.01.
The results of cox model analysis
Cox model single-factor and multiple-factor regression analyses of gender, age, smoking history gross tumor type, degree of tumor differentiation, lymph node metastasis, and TNM stage of patients with lung cancer were performed. The results show that gross tumor type and gender were independent factors for survival in patients with lung cancer (Table 3).
Table 3
| Factors | Categories | Multivariate | |
|---|---|---|---|
| OR (95% CI) | P | ||
| Gender | Male/female | 1.094 (0.513–2.331) | 0.816 |
| Age | ≤50/>50 | 0.611 (0.324–1.151) | 0.127 |
| Smoking use | Yes/no | 0.785 (0.393–1.568) | 0.492 |
| Tumor differentiation | Well/moderate-poor | 0.847 (0.344–2.087) | 0.718 |
| Lymph node metastasis | No/yes | 0.410 (0.187–0.902) | 0.027* |
| TNM stage | I–II/III–IV | 2.665 (0.854–5.681) | 0.197 |
| EZH2 expression | Negative/positive | 0.248 (0.066–0.935) | 0.040* |
| HOXA5 expression | Negative/positive | 0.274 (0.086–0.870) | 0.028* |
| Survivin expression | Negative/positive | 0.460 (0.153–1.379) | 0.166 |
*, P<0.05.
Discussion
We detected the expression of EZH2, HOXA5, and SURVIVIN in NSCLC by IHC, and we found that there were significant differences in the expression of EZH2 and HOXA5 in normal lung tissue, adenocarcinoma, and squamous cell carcinoma (P<0.05). However, there was no significant difference in the expression between adenocarcinoma and squamous cell carcinoma (P>0.05). There were significant differences in expression of survivin in normal lung tissue, adenocarcinoma, and squamous cell carcinoma (P<0.05); however, the expression of adenocarcinoma and squamous cell carcinoma (P>0.05) was not significantly different. Studies have shown that abnormal expression of HOXA5 plays an important role in the development of NSCLC, and is differently expressed in different tissues. The expression of HOXA5 is down-regulated by DNA methylation in cancer, which is related to tumor stage, tumor size, and lymph node metastasis (8). EZH2 is carcinogenic, even though it is overexpressed in many human cancers (9), the regulation of expression and the role in NSCLC is still unclear. Our experiments further identified these results and found that the expression of HOXA5 in normal tissues was significantly higher than that in lung cancer; conversely, the expression of EZH2 in lung cancer was significantly higher than that in normal lung tissues.
We analyzed the relationship between the expression of EZH2, HOXA5, and survivin, with the clinicopathological characteristics of NSCLC. The expression of EZH2 was related to lymph node metastasis and TNM stage (P<0.01); the expression of HOXA5 was not related to gender, age, smoking history, general classification, degree of differentiation, lymph node metastasis, or TNM stage (P>0.05); the expression of survivin was related to lymph node metastasis, TNM stage (P<0.05), but not to gender, age, smoking history, general classification, or degree of differentiation (P>0.05, Table 2). Similarly, it is (9) found that EZH2 was related to tumor stage and grade, tumor size, and lymph node metastasis in renal clear cell carcinoma. EZH2 can thus be used as a clinical factor to predict the prognosis and progress of renal clear cell carcinoma. This result was consistent with our own.
Using the Kaplan-Meier method, the survival of patients with EZH2 expression was shown to be significantly shorter than that of patients without EZH2 expression, whereas the survival of patients with HOXA5 expression was significantly longer than that of patients without HOXA5 expression. The survival of patients with a high expression of survivin was significantly shortened. Tang et al. (10) found that HOXA5 gene methylation indicated the shortened survival of patients and a reduced survival rate, but it was not as a single risk factor of prognosis. Geng et al. (6) found that high expression of EZH2 suggested poor prognosis and low survival of patients with lung cancer and was closely related to tumor size, high expression of VEGF-A, and activation of AKE pathway.
Using the Cox regression model, we analyzed patients’ gender, age, smoking status, tumor differentiation, lymph node metastasis, and TNM stage.
Some studies found that HOXA5 was a tumor suppressor gene, and that the abnormal expression of HOXA5 gene inhibited cell invasion, cell metastasis, and pseudopodia formation in highly invasive cancer. HOXA5 impacts metastasis in a variety of cancer cells. For instance, there is an HOXA5 binding site in the P53 promoter region, while transient transfection of HOX/HOXA5 was found to activate the P53 promoter; therefore, the expression of HOXA5 in epithelial cell carcinoma promotes the expression of wild-type P53 (11). Evidence shows that the absence of P53 in breast cancer might be due to the loss of expression HOXA5. The expression of HOXA5 can also be used as a marker for acute myeloid leukemia (AML), and it may have some effects on the prognosis of AML patients. HOXA5 also had an impact on multi-drug resistance in small cell lung cancer and may be a therapeutic target for SCLC (12). While there is a low expression of HOXA5 in stationary endothelial cells, it is highly expressed in an active reproductive vascular endothelial cell. In one study, sustaining the expression of HOXA5 regulated the expression of effector molecules of neovascularization, significantly increased the expression of TSP-2, reduced the expression of VEGF, and inhibited tumor neovascularization. Active HOXA5 in endothelial cells decreased the incidence of tumor inflammation, reduced neovascularization, and inhibited tumor growth (13).
In summary, in the study of NSCLC, the expression of EZH2, HOXA5, and survivin may be useful for predicting the prognosis and progression of NSCLC, and may form a theoretical basis for the further study of anti-NSCLC targeted therapy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.37). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was received from each patient and tissue specimens were prepared according to the Harbin Medical University Ethics Committee guidelines. The institutional ethical approval was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [Crossref] [PubMed]
- Verdecchia A, Francisci S, Brenner H, et al. Recent cancer survival in Europe: a 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol 2007;8:784-96. [Crossref] [PubMed]
- Tao R, Chen Z, Wu P, et al. The possible role of EZH2 and DNMT1 polymorphisms in sporadic triple-negative breast carcinoma in southern Chinese females. Tumour Biol 2015;36:9849-55. [Crossref] [PubMed]
- Reijm EA, Timmermans AM, Look MP, et al. High protein expression of EZH2 is related to unfavorable outcome to tamoxifen in metastatic breast cancer. Ann Oncol 2014;25:2185-90. [Crossref] [PubMed]
- Wan L, Li X, Shen H, et al. Quantitative analysis of EZH2 expression and its correlations with lung cancer patients' clinical pathological characteristics. Clin Transl Oncol 2013;15:132-8. [Crossref] [PubMed]
- Geng J, Li X, Zhou Z, et al. EZH2 promotes tumor progression via regulating VEGF-A/AKT signaling in non-small cell lung cancer. Cancer Lett 2015;359:275-87. [Crossref] [PubMed]
- Xu C, Hao K, Hu H, et al. Expression of the enhancer of zeste homolog 2 in biopsy specimen predicts chemoresistance and survival in advanced non-small cell lung cancer receiving first-line platinum-based chemotherapy. Lung Cancer 2014;86:268-73. [Crossref] [PubMed]
- Behrens C, Solis LM, Lin H, et al. EZH2 protein expression associates with the early pathogenesis, tumor progression, and prognosis of non-small cell lung carcinoma. Clin Cancer Res 2013;19:6556-65. [Crossref] [PubMed]
- Zhou W, Wang J, Man WY, et al. siRNA silencing EZH2 reverses cisplatin-resistance of human non-small cell lung and gastric cancer cells. Asian Pac J Cancer Prev 2015;16:2425-30. [Crossref] [PubMed]
- Tang B, Zhang Y, Liang R, et al. RNAi-mediated EZH2 depletion decreases MDR1 expression and sensitizes multidrug-resistant hepatocellular carcinoma cells to chemotherapy. Oncol Rep 2013;29:1037-42. [Crossref] [PubMed]
- Gehring WJ, Hiromi Y. Homeotic genes and the homeobox. Annu Rev Genet 1986;20:147-73. [Crossref] [PubMed]
- Duriseti S, Winnard PT Jr, Mironchik Y, et al. HOXA5 regulates hMLH1 expression in breast cancer cells. Neoplasia 2006;8:250-8. [Crossref] [PubMed]
- Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer 2002;2:777-85. [Crossref] [PubMed]
- Zhang ML, Nie FQ, Sun M, et al. HOXA5 indicates poor prognosis and suppresses cell proliferation by regulating p21 expression in non small cell lung cancer. Tumour Biol 2015;36:3521-31. [Crossref] [PubMed]