
Prognosis value of liver stiffness measurements by 2D-SWE in primary HBV-positive hepatocellular carcinoma following radiofrequency ablation
Introduction
Hepatocellular carcinoma (HCC) is claimed to be one kind of the most common malignancy in background with a high morbidity of hepatitis virus infection, which is also the third commonest cause of cancer-associated mortality in China for its aggressive recurrence and metastasis (1-4). Despite advancement of adjunctive treatment, surgery and transplantation have taken benefits for HCC patients over decades, the survival outcome of HCC still be unsatisfactory with main contributors of relapse or metastasis, especially for patients complicated with severe cirrhosis (5-7). Radiofrequency ablation (RFA) has been developed as an effective treatment approach for HCC patients with severe cirrhosis, which also had benefits in terms of complications and hospitalization duration (8-10). HBV infection related liver fibrosis or cirrhosis has been confirmed as a significant factor relating to prognosis of HCC (11-13). Thus, accurately quantitative evaluation of the fibrosis is curial for evaluating prognosis and furtherly guiding satisfaction of HCC patients. Moreover, stratification of patients according to biomarkers is crucial for individualized treatment and prognostic prediction, such as Child–Pugh score and Model for end-stage liver disease (MELD) score (14,15). However, in clinical, liver fibrosis mainly is assessed through liver biopsy or postoperative historical examination, which is characterized by invasive, risk of complications and diagnostic bias (16-18). So non-invasive novel methods of pretreatment fibrosis measurement are required.
Recently, liver stiffness measurement (LSM) via 2D-shear wave elastography (2D-SWE) has been showed as a reproducible, reliable and common method for liver fibrosis evaluation (19-22). LSM is also used to predict the occurrence of HCC in chronic liver disease patients (23,24). Furthermore, LSM can be used to predict postoperative complication and relapse in HCC patients underwent surgical resection (25-27). However, the significance of LSM measured by 2D-SWE in prediction of survival and clinical staging of HBV positive HCC patients underwent RFA has not been fully and clearly elucidated. Hence, we try to evaluate significance of LSM measured by 2D-SWE in prognosis evaluation for patients underwent RFA for HBV positive HCC.
Methods
Patients
Ethics approval of this study was obtained from the Institutional Review Board of the First Affiliated Hospital of Anhui Medical University, which was also conducted by adhering to the declaration of Helsinki and corresponding guidelines, all subjects were required for written informed consent. Two hundred seventy-three patients with primary single HCC undergone RFA at the First Affiliated Hospital of Anhui Medical University between June 1, 2013 and June 1, 2018 were included in this study. All patients were confirmed as single HCC with HBsAg positive prior RFA. Inclusion criteria included the largest diameter of HCC no more than 5cm, no distant metastasis, no portal vein tumor thrombus and micro-vessels invasion, Child-Pugh level A or B, incompliance to surgery. Patients with severe preoperative infection, malignant hematologic disease, metastatic cancer, other malignancies were excluded. Patients with unavailable and unreliable clinicopathologic data and 2D-SWE measurement and received preoperative adjuvant treatments were also excluded. Clinicopathologic information of all patients were collected, including gender, age, tumor size, liver status, Child-Pugh stage, HBsAg, AFP level, bilirubin, creatinine, international normalized ratio (INR), sodium level, tumor size, and laboratory tests. Histopathological staging was assessed by histopathological study. Pre-RFA bilirubin, creatinine, INR, and sodium level were used to calculate MELD scores.
RFA procedure
Fasting for at least 4–6 hours before RFA for all patients. Following conscious sedation and local anesthesia, RFA procedures were conducted percutaneously under ultrasound (US) guidance. Each tumor was ablated by twice to four times overlapping insertions of single electrode with a 3.5-cm exposed tip (ValleyLab, Burlington, MA). Full ablation was defined as the ablation area overlapping over 1.0 cm width margin of the normal liver parenchyma near the HCC. US was applied to evaluate and observe ablation area in real time. When the ablation area overlapped 0.5–1.0 cm width margin beyond entire HCC, the ablation was finished. After the electrode withdrawn, the needle track was routinely cauterized to avoid tumor seeding and bleeding.
LSM by 2D-SWE
All patients underwent LSM examination immediately prior to RFA treatment. Aixplorer ultrasound system (Supersonic Imagine, France) with a convex broadband probe (SC6-1, 1–6 MHz) was used to perform LSM measurement by 2D-SWE technology in accordance with the manufacturer’s instructions. All LSM measurements were conducted by one experienced sonographer blind to the patients’ information. Patients were placed in a supine position with the right arm in maximum abduction, and expose right intercostal space for scanning right liver lobe. Valid LSM was defined as 10 effective measurements for each patient. The result of LSM was expressed as the mean (M) of effective measurements in kilopascals (kPa). The Sonographer were blinded to all data of patient.
Outcome evaluation
All patients were followed up by outpatient visiting or telephone visiting in a regular interval frequent. Enhanced imaging evaluations were usually conducted every 12 months. Clinical following-up periods lasted from the day of surgery to either the day of death or May 2019. Overall survival (OS) was evaluated as the primary outcome of this study, which was defined as the period from date of RFA to time of disease-specific death. The secondary outcome was the Recurrent-free survival (RFS). Recurrences were consisted of intrahepatic local recurrence, extrahepatic recurrence and intrahepatic distant recurrence.
Statistical analysis
Statistical analyses were conducted by using the SPSS 20.0 (IBM, USA). P<0.05 (two sided) was confirmed as statistically significant. The optimal cutoff value of the LSM to predict survival was determined by receiver operating characteristic (ROC) curve analysis. The association between qualitative variables and LSM was analyzed by using the χ2 test or Fisher’s exact test, whereas the association between LSM quantitative values was evaluated by independent student’s t-test. The OS and RFS and survival curve were analyzed in the Kaplan-Meier analyses by using the log-rank test. The Cox regression model was applied to evaluate the hazard ratio (HR) and to conduct multivariate analysis.
Results
Baseline clinical and pathological characteristics
Baseline characteristics of 273 primary HCC patients enrolled in this study were shown in Table 1. There were 168 males and 95 females in whole group of HCC patients, with 58.2±2.1-year average age. A total of 185 (67.6%) patients had liver cirrhosis. The most (190, 69.6%) of all patients had a Child–Pugh class A liver function. Among the whole group of HCC patients. Otherwise. Median preoperative LSM measured by 2D-SWE was 13.6 (4.3–46.2) kPa, which was positively significantly related to liver cirrhosis (both P<0.05, by χ2 test).
Table 1
| Characteristics | All patients (n=273) |
|---|---|
| Demographic factors | |
| Age, years | 58.2±2.1 |
| Male | 172 (67.9%) |
| BMI, kg/m2 | 25.5±3.1 |
| Liver cirrhosis | 185 (67.7%) |
| Child–Pugh class, A/B | 190 (69.6%)/82 (30.4%) |
| MELD scores | 17.9±9.4 |
| Tumor factors | |
| Tumor size, cm | 2.9 (1.4–5.0) |
| Alpha-fetoprotein, ng/mL | 22.7 (1.2–3,100.0) |
| Laboratory tests | |
| Alanine aminotransferase, IU/L | 35.4±21.5 |
| Albumin, g/L | 38.3±5.7 |
| Total bilirubin, mg/dL | 0.87±0.43 |
| Platelet count, 109/L | 124±62 |
| Prothrombin time, INR | 1.08±0.11 |
| Noninvasive fibrosis measurement | |
| LSM, kPa | 13.6 (4.3–46.2) |
| Patients with an LSM ≥13.4 kPa | 187 (68.5%) |
HCC, hepatocellular carcinoma; RFA, radiofrequency ablation; BMI, body mass index; LSM, liver stiffness measurement; MELD, model for end-stage liver disease scores.
Prognostic value of LSM for OS of HCC after RFA
Based on the results of ROC curve analysis, An LSM measured by 2D-SWE of 13.4 kPa (AUC, 0.81; 95% CI: 0.70–0.92; P<0.01) was confirmed as the cutoff level predicting survival outcome, with a sensitivity of 91.4% and a specificity of 65.2%, respectively (Figure 1).
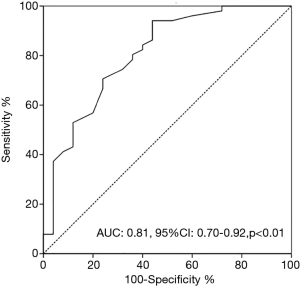
At the endpoint (mean 36.6±12.3 months), 88 (32.2%) out of all 273 patients had died. In 82 patients with an LSM level measured by 2D-SWE less than 13.4 kPa, 19 (23.2%) patients died, with an estimated mean overall survival of 62.6 months. Among 191 patients with an LSM values ≥13.4 kPa, 66 (34.6%) patients died, with an estimated mean overall survival of 48.5 months. The difference of mean OS between groups was statistically significant (hazard ratio, 5.12; 95% CI: 1.38–16.52; P=0.01, Figure 2A, Table 2).
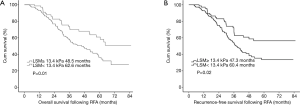
Table 2
| Variables | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Demographic factors | |||||||
| Age ≥55 years | 1.01 | 0.82–1.63 | 0.21 | ||||
| Sex (male) | 0.67 | 0.53–2.86 | 0.42 | ||||
| BMI ≥25 kg/m2 | 1.11 | 0.78–2.62 | 0.17 | ||||
| Liver cirrhosis | 2.53 | 0.96–8.76 | 0.08 | ||||
| Child-Pugh stage | 1.42 | 0.46–4.21 | 0.47 | ||||
| MELD scores | 2.17 | 1.12–4.18 | 0.02 | 1.87 | 1.01–3.56 | <0.01 | |
| Tumor factors | |||||||
| AFP (ng/mL) | 1.01 | 0.98–1.03 | 0.07 | ||||
| Tumor size (cm) | 1.61 | 0.83–2.77 | 0.63 | ||||
| Laboratory tests | |||||||
| ALT | 2.17 | 0.98–9.63 | 0.51 | ||||
| ALB | 0.67 | 0.13–1.94 | 0.32 | ||||
| TB | 3.88 | 1.12–9.37 | 0.03 | 3.16 | 1.25–8.62 | 0.03 | |
| PLT | 1.00 | 0.99–1.01 | 0.06 | ||||
| PT | 1.01 | 0.99–1.03 | 0.21 | ||||
| Fibrosis status | |||||||
| LSM ≥13.4 kPa | 5.12 | 1.38–16.52 | 0.01 | 3.68 | 1.22–9.86 | <0.01 | |
OS, overall survival; HCC, hepatocellular carcinoma; CI, confidence interval; HR, hazard ratio; BMI, body mass index; AFP, alpha-fetoprotein; ALT, alanine aminotransferase; ALB, albumin; TB, total bilirubin; PLT, platelet count; PT, prothrombin time; LSM, liver stiffness measurement; MELD, model for end-stage liver disease scores.
Multivariate analysis enrolled age and sex of patients, BMI, liver cirrhosis, tumor size, MELD scores, laboratory tests and LSM measured by 2D-SWE into the COX regression model, which showed that LSM ≥13.4 kPa (hazard ratio, 3.88; 95% CI: 1.26–9.35; P<0.01) remained the independent risk factor of OS (Table 2).
Prognostic value of LSM for RFS of HCC after RFA
Among 273 HCC patients successfully treated by RFA, recurrent developed in 73 (26.7%) patients. Fifty-six (29.3%) in group of 191 patients with an LSM ≥13.4 kPa had tumor recurrent whereas 13 (15.9%) in 82 patients with an LSM <13.4 kPa had a recurrence. In univariate analysis, patients with a high LSM level had a poorer mean RFS than those with a low LSM (60.4 vs. 47.3 months, P=0.02), and the LSM ≥13.4kPa also was an independent risk factor for RFS of HCC patients in multivariate analysis (HR, 2.87; 95% CI: 1.03–9.15; P<0.01) (Table 3, Figure 2B).
Table 3
| Variables | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Demographic factors | |||||||
| Age ≥55 years | 1.02 | 0.85–1.26 | 0.12 | ||||
| Sex (male) | 0.66 | 0.31–1.67 | 0.31 | ||||
| BMI ≥25 kg/m2 | 1.03 | 0.99–1.12 | 0.24 | ||||
| Liver cirrhosis | 2.23 | 1.06–7.56 | 0.04 | 1.88 | 0.85–6.53 | 0.33 | |
| Child-Pugh stage | 1.12 | 0.53–3.51 | 0.27 | ||||
| MELD scores | 1.56 | 0.88–4.66 | 0.06 | ||||
| Tumor factors | |||||||
| AFP (ng/mL) | 1.00 | 0.98–1.01 | 0.21 | ||||
| Tumor size (cm) | 1.31 | 0.93–2.57 | 0.08 | ||||
| Laboratory tests | |||||||
| ALT | 2.07 | 0.88–9.53 | 0.41 | ||||
| ALB | 0.57 | 0.12–2.14 | 0.26 | ||||
| TB | 2.89 | 1.05–8.57 | 0.03 | 2.61 | 1.22–8.31 | 0.06 | |
| PLT | 1.00 | 0.98–1.02 | 0.37 | ||||
| PT | 1.01 | 0.99–1.01 | 0.51 | ||||
| Fibrosis status | |||||||
| LSM ≥13.4 kPa | 3.12 | 1.08–10.25 | 0.02 | 2.87 | 1.03–9.15 | <0.01 | |
RFS, recurrence-free survival; HCC, hepatocellular carcinoma; CI, confidence interval; HR, hazard ratio; BMI, body mass index; AFP, alpha-fetoprotein; ALT, alanine aminotransferase; ALB, albumin; TB, total bilirubin; PLT, platelet count; PT, prothrombin time; LSM, liver stiffness measurement; MELD, model for end-stage liver disease scores.
Discussion
Liver fibrosis has been considered as a significant contributor to progression and metastasis of HCC, especially for patients complicated HBV infection (28-30). In present study, we showed that the LSM value measured by 2D-SWE was an independent prognostic indictor for HBV positive HCC patients underwent RFA. Moreover, we also found that LSM level was significantly associated with liver cirrhosis, thereby concluded that advanced tumor tends to have a high liver stiffness. Furthermore, patients with a high LSM had significant poorer RFS than those with a low LSM, indicating that LSM can not only be applied to evaluate survival, but also the risk of recurrent.
Liver fibrosis acts as a healing response in setting of chronic liver injury, which may chronically lead to liver cirrhosis, thereby severing as a contributor to the development of HCC (31,32). Previously, the extent and degree of liver fibrosis has usually been assessed by tissue pathological exanimation (33,34). However, there are several limitations in effective of pathological evaluation of liver fibrosis, such as unsatisfied accuracy due to both sampling variability and inter-observer variability depend on pathologists, invasive procedure potentially causing risk of complications (35,36). Therefore, several effective noninvasive methods for liver fibrosis evaluation have been published. Until now, LSM, also referred to transient elastography, has been considered as the most widely used measurement to assess liver fibrosis (37).
The diagnostic and prognostic values of LSM by 2D-SWE for liver fibrosis of patients with chronic HBV infection have been extensively evaluated and validated (38-40), For patients with HBV positive HCCs, the presence of liver fibrosis is a well-known risk factor for both the development of post-treatment liver failure and prognosis (15,41). Therefore, considering the results of previous studies and current study, it is possible that the poorer survival of HCCs following RFA in patients with a higher LSM value could be partly due to the presence of liver fibrosis. Therefore, LSM might sever as a valuable noninvasive predictor for prognosis of liver cancer. In previous studies, the most optimal cut-off level of LSM for liver cirrhosis diagnosis was 11.8–15.9 kPa in the HBV and HCV cases (42-44). However, the optimal cut-off level for liver stiffness associated with HCC remains undefined. Previous study showed that chronic hepatitis B patients with a LS value >12 kPa had an observable higher risk of HCC development (24). In addition, HCC patients with a pretreatment LSM value >13 kPa or 13.4 kPa suffered from a higher rate of recurrent (26,45). Optimal cut-off levels were often calculated by using a common analysis of ROC curve. In our study, the optimal cut-off level of LSM predicting survival of HCC following RFA was 13.4 kPa, which was consisted with previous studies. We furtherly confirmed the valid of this cut-off level in recurrent of HCC after RFA.
In this study, LSM value significantly related to liver cirrhosis. However, the LSM level was significantly correlated to prognosis of HCC after RFA whereas presence of liver cirrhosis was not an independent factor for OS and RFS. In clinical practice, liver cirrhosis was usually evaluated by ultrasonography, histological evaluation and imaging, but its severity cannot be quantitatively evaluated (46). LSM value can be used to detect compensated liver cirrhosis in an accurate style and expressed as continuous quantitative values, which also can overcome ultrasound doctor dependently limitation (47). Furthermore, it has been showed that hepatitis flares and hypertransaminasemia can result in high LSM level in background of low fibrosis. Therefore, the LSM level also can be used to reflect the live function (48,49).
There were several limitations in our study. The single-center, retrospective design might influence the generalization of results, thus contributing to differences between our findings and previous study. A large multi-center prospective study would be performed to confirm the value of LSM. Moreover, in this study, we only enrolled patients with primary single HCC, not multiple or recurrent tumor, so the generalization of results was limited. Furthermore, the optimal cut-off level of LSM measurement was obtained from a patients group in a single center, which also diminished the generalizability of results. Furtherly, studies with a high evidence level may be needed to confirm the significance of LSM in prognostic evaluation of HCC.
Conclusions
In conclusion, a low LSM measured by 2D-SWE predicted a better survival in patients underwent RFA for HCC, which also related to liver cirrhosis. Furtherly, prospective, randomized and control studies with a large scale are required to confirm the significance of LSM in HCC. The LSM by 2D-SWE, as a quantitative assessing tool for liver fibrosis, could sever as an independent prognostic predictor for HBV related HCC following RFA.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure from (available at http://dx.doi.org/10.21037/tcr.2020.03.04). Both authors have no conflicts of interests to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of the First Affiliated Hospital of Anhui Medical University (No. C201908021). Informed consent was obtained. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Feng RM, Zong YN, Cao SM, et al. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond) 2019;39:22. [Crossref] [PubMed]
- Parikh ND, Fu S, Rao H, et al. Risk Assessment of Hepatocellular Carcinoma in Patients with Hepatitis C in China and the USA. Dig Dis Sci 2017;62:3243-53. [Crossref] [PubMed]
- Niu J, Lin Y, Guo Z, et al. The Epidemiological Investigation on the Risk Factors of Hepatocellular Carcinoma: A Case-Control Study in Southeast China. Medicine (Baltimore) 2016;95:e2758. [Crossref] [PubMed]
- Du XF, Zhang LL, Zhang DZ, et al. Clinical significance of serum total oxidant/antioxidant status in patients with operable and advanced gastric cancer. Onco Targets Ther 2018;11:6767-75. [Crossref] [PubMed]
- Poon RT. Prevention of recurrence after resection of hepatocellular carcinoma: a daunting challenge. Hepatology 2011;54:757-9. [Crossref] [PubMed]
- Yoo J, Hann HW, Coben R, et al. Update Treatment for HBV Infection and Persistent Risk for Hepatocellular Carcinoma: Prospect for an HBV Cure. Diseases 2018;6: [Crossref] [PubMed]
- Wang W, Yang C, Zhu K, et al. Recurrence after Curative Resection of HBV-related Hepatocellular Carcinoma: Diagnostic Algorithms on Gadoxetic Acid-enhanced MRI. Liver Transpl 2020; [Epub ahead of print]. [Crossref]
- Crocetti L. Radiofrequency Ablation versus Resection for Small Hepatocellular Carcinoma: Are Randomized Controlled Trials Still Needed? Radiology 2018;287:473-5. [Crossref] [PubMed]
- Mohkam K, Dumont PN, Manichon AF, et al. No-touch multibipolar radiofrequency ablation vs. surgical resection for solitary hepatocellular carcinoma ranging from 2 to 5cm. J Hepatol 2018;68:1172-80. [Crossref] [PubMed]
- Qi X, Tang Y, An D, et al. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: a meta-analysis of randomized controlled trials. J Clin Gastroenterol 2014;48:450-7. [Crossref] [PubMed]
- Della Corte C, Aghemo A, Colombo M. Individualized hepatocellular carcinoma risk: the challenges for designing successful chemoprevention strategies. World J Gastroenterol 2013;19:1359-71. [Crossref] [PubMed]
- Zampino R, Pisaturo MA, Cirillo G, et al. Hepatocellular carcinoma in chronic HBV-HCV co-infection is correlated to fibrosis and disease duration. Ann Hepatol 2015;14:75-82. [Crossref] [PubMed]
- Li Z, Zhao X, Jiang P, et al. HBV is a risk factor for poor patient prognosis after curative resection of hepatocellular carcinoma: A retrospective case-control study. Medicine (Baltimore) 2016;95:e4224. [Crossref] [PubMed]
- Li GJ, Xu HW, Ji JJ, et al. Prognostic value of preoperative lymphocyte-to-monocyte ratio in pancreatic adenocarcinoma. Onco Targets Ther 2016;9:1085-92. [PubMed]
- Qi M, Chen Y, Zhang GQ, et al. Clinical significance of preoperative liver stiffness measurements in primary HBV-positive hepatocellular carcinoma. Future Oncol 2017;13:2799-810. [Crossref] [PubMed]
- Chin JL, Pavlides M, Moolla A, et al. Non-invasive Markers of Liver Fibrosis: Adjuncts or Alternatives to Liver Biopsy? Front Pharmacol 2016;7:159. [Crossref] [PubMed]
- Amarapurkar D. Role of liver biopsy in management of chronic hepatitis B and chronic hepatitis C. Trop Gastroenterol 2007;28:67-8. [PubMed]
- Wang L, Wang B, You H, et al. Platelets' increase is associated with improvement of liver fibrosis in entecavir-treated chronic hepatitis B patients with significant liver fibrosis. Hepatol Int 2018;12:237-43. [Crossref] [PubMed]
- Bartres C, Lens S. Rev Esp Enferm Dig 2013;105:235. [Elastography of the liver (Fibroscan(R)) in hepatology]. [Crossref] [PubMed]
- Fernandez M, Trepo E, Degre D, et al. Transient elastography using Fibroscan is the most reliable noninvasive method for the diagnosis of advanced fibrosis and cirrhosis in alcoholic liver disease. Eur J Gastroenterol Hepatol 2015;27:1074-9. [Crossref] [PubMed]
- Foucher J, Chanteloup E, Vergniol J, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006;55:403-8. [Crossref] [PubMed]
- Jamialahmadi T, Nematy M, Jangjoo A, et al. Measurement of Liver Stiffness with 2D-Shear Wave Elastography (2D-SWE) in Bariatric Surgery Candidates Reveals Acceptable Diagnostic Yield Compared to Liver Biopsy. Obes Surg 2019;29:2585-92. [Crossref] [PubMed]
- Feier D, Lupsor Platon M, Stefanescu H, et al. Transient elastography for the detection of hepatocellular carcinoma in viral C liver cirrhosis. Is there something else than increased liver stiffness? J Gastrointestin Liver Dis 2013;22:283-9. [PubMed]
- Tatsumi A, Maekawa S, Sato M, et al. Liver stiffness measurement for risk assessment of hepatocellular carcinoma. Hepatol Res 2015;45:523-32. [Crossref] [PubMed]
- Berzigotti A, Reig M, Abraldes JG, et al. Value of transient elastography measured with fibroscan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg 2015;261:e105. [Crossref] [PubMed]
- Jung KS, Kim SU, Choi GH, et al. Prediction of recurrence after curative resection of hepatocellular carcinoma using liver stiffness measurement (FibroScan(R)). Ann Surg Oncol 2012;19:4278-86. [Crossref] [PubMed]
- Cucchetti A, Cescon M, Colecchia A, et al. Adding Liver Stiffness Measurement to the Routine Evaluation of Hepatocellular Carcinoma Resectability Can Optimize Clinical Outcome. Ultraschall Med 2017;38:515-22. [Crossref] [PubMed]
- Xiao G, Zhu F, Wang M, et al. Diagnostic accuracy of APRI and FIB-4 for predicting hepatitis B virus-related liver fibrosis accompanied with hepatocellular carcinoma. Dig Liver Dis 2016;48:1220-6. [Crossref] [PubMed]
- Wang Q, Blank S, Fiel MI, et al. The Severity of Liver Fibrosis Influences the Prognostic Value of Inflammation-Based Scores in Hepatitis B-Associated Hepatocellular Carcinoma. Ann Surg Oncol 2015;22:S1125-32. [Crossref] [PubMed]
- Suh B, Park S, Shin DW, et al. High liver fibrosis index FIB-4 is highly predictive of hepatocellular carcinoma in chronic hepatitis B carriers. Hepatology 2015;61:1261-8. [Crossref] [PubMed]
- Wang Q, Fiel MI, Blank S, et al. Impact of liver fibrosis on prognosis following liver resection for hepatitis B-associated hepatocellular carcinoma. Br J Cancer 2013;109:573-81. [Crossref] [PubMed]
- Fuchs BC, Hoshida Y, Fujii T, et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology 2014;59:1577-90. [Crossref] [PubMed]
- Petrescu IO, Biciusca V, Taisescu CI, et al. Histological factors that predict the liver fibrosis in patients with chronic hepatitis C. Rom J Morphol Embryol 2016;57:759-65. [PubMed]
- Hasegawa T, Kimura T, Ihara Y, et al. Histological classification of liver fibrosis and its impact on the postoperative clinical course of patients with congenital dilatation of the bile duct. Surg Today 2006;36:151-4. [Crossref] [PubMed]
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002;97:2614-8. [Crossref] [PubMed]
- Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003;38:1449-57. [Crossref] [PubMed]
- Xie QX, Xu N, Jiang XP, et al. Role of FibroScan in liver fibrosis evaluation in patients with chronic hepatitis B virus infection and related influencing factors. Zhonghua Gan Zang Bing Za Zhi 2016;24:659-64. [PubMed]
- Wu S, Kong Y, Piao H, et al. On-treatment changes of liver stiffness at week 26 could predict 2-year clinical outcomes in HBV-related compensated cirrhosis. Liver Int 2018;38:1045-54. [Crossref] [PubMed]
- Kettaneh A, Marcellin P, Douvin C, et al. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol 2007;46:628-34. [Crossref] [PubMed]
- Sporea I, Sirli R, Deleanu A, et al. Liver stiffness measurements in patients with HBV vs HCV chronic hepatitis: a comparative study. World J Gastroenterol 2010;16:4832-7. [Crossref] [PubMed]
- Jeon MY, Lee HW, Kim SU, et al. Subcirrhotic liver stiffness by FibroScan correlates with lower risk of hepatocellular carcinoma in patients with HBV-related cirrhosis. Hepatol Int 2017;11:268-76. [Crossref] [PubMed]
- Akima T, Tamano M, Hiraishi H. Liver stiffness measured by transient elastography is a predictor of hepatocellular carcinoma development in viral hepatitis. Hepatol Res 2011;41:965-70. [Crossref] [PubMed]
- Sultanik P, Sogni P, Meritet JF, et al. Patients with chronic hepatitis B should be screened for hepatocellular carcinoma regardless of liver stiffness measurement. Hepatology 2016;63:672. [Crossref] [PubMed]
- Liu XY, Ma LN, Yan TT, et al. Combined detection of liver stiffness and C-reactive protein in patients with hepatitis B virus-related liver cirrhosis, with and without hepatocellular carcinoma. Mol Clin Oncol 2016;4:587-90. [Crossref] [PubMed]
- Lee SH, Kim SU, Jang JW, et al. Use of transient elastography to predict de novo recurrence after radiofrequency ablation for hepatocellular carcinoma. Onco Targets Ther 2015;8:347-56. [Crossref] [PubMed]
- Ge L, Shi B, Song YE, et al. Clinical value of real-time elastography quantitative parameters in evaluating the stage of liver fibrosis and cirrhosis. Exp Ther Med 2015;10:983-90. [Crossref] [PubMed]
- Wang JH, Chuah SK, Lu SN, et al. Baseline and serial liver stiffness measurement in prediction of portal hypertension progression for patients with compensated cirrhosis. Liver Int 2014;34:1340-8. [Crossref] [PubMed]
- Coco B, Oliveri F, Maina AM, et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat 2007;14:360-9. [Crossref] [PubMed]
- Sagir A, Erhardt A, Schmitt M, et al. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2008;47:592-5. [Crossref] [PubMed]


