
Establishment of surface marker expression profiles for colorectal cancer stem cells under different conditions
Introduction
Colorectal cancer is ranked as the fourth leading cancer for incidence and the second leading cause for cancer mortality worldwide in both sexes combined in 2018 (1). Generally, 50% of people will be diagnosed with a colorectal adenoma by the age of 50, and 10% of diagnosed patients will progress to malignancy (2). The incidence of colorectal cancer increases with age in both Western and Asian populations (3). While the mortality of colorectal cancer has declined in the past decades, 50% of patients still develop metastases (4). Colorectal cancer is a tissue growth that first begins on the inner linings of the colon or rectum, also known as a polyp (5). Therefore, many non-metastatic colorectal cancers can be surgically removed at this early stage. When a polyp grows abnormally and subsequently becomes cancerous, it can form a tumor on the wall of the colon or rectum, and then grows into lymph vessels to spread into other organs (6). Since colorectal cancer exhibits well-defined early stage characteristics and slow progression to metastasis, eliminating the cancer stem cell population becomes a critical cancer treatment.
Cancer stem cells were initially identified in acute myeloid leukemia, and were characterized as a CD34+CD38− subpopulation (7). Cancer stem cells were later found in other kinds of tumors, such as the CD44+CD24− subpopulation in breast cancer (8). For colorectal cancer stem cells, CD44+, CD133+, and epithelial cell adhesion molecule (EpCAM)high have been identified as reliable markers (9-11). Identifying cancer stem cell markers is not only beneficial for targeted therapy development (12), but also critical for detecting circulating tumor cells (CTCs) (13). The advantage of detecting cancer stem cells in CTCs is that we can trace tumor progression in response to treatment, since CTCs are present in the blood when cancer cells are transitioning from their origin to distant organs (14). It has been demonstrated that CTCs isolated from colorectal cancer patients displayed strong expression of CD133, EpCAM, or CD44 (13,15,16), suggesting that detecting colorectal cancer stem cells in CTCs may help early detection of metastasis and subsequently lower mortality. Identifying CTCs is beneficial for colorectal cancer patients as well, since a simple blood draw is all that is needed to detect CTCs and patients would not need to be subjected to magnetic resonance imaging.
The morphology and immunostaining pattern of CTCs is mainly used for identification, and the heterogeneity of CTCs becomes a major difficulty for CTC detection. In addition, only a small aliquot of blood will be obtained from a patient for CTC detection and may subsequently be cultured to verify. In cell culture, if the initial seeding number is too small, surface marker expression levels can be affected. In this study, we studied how the expression of surface markers of colorectal cancer stem cells changed in different culture conditions. We investigated the expression of CD44 and CD133 of a colorectal cancer stem cell line, Caco-2. Different initial seeding cell concentrations of Caco-2 were cultured, and the cell morphology and the expression of surface markers were observed. Caco-2 was treated with eicosapentaenoic acid (EPA), dimethyl sulfoxide (DMSO), and ethylenediaminetetraacetic acid (EDTA) to mimic conditions after colorectal cancer treatments. By characterizing the changes of surface marker expression of Caco-2 cells in different cell culture conditions, our results might provide useful information for improving the accuracy and sensitivity of CTC detection in the future.
Methods
Cell line and culture
Colorectal cancer cell line, Caco-2, was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Caco-2 cells were cultured in normal medium: DMEM (Dulbecco’s Modified Eagle Medium, GIBCO, Thermo Fisher Scientific Inc., Waltham, MA, USA), high glucose medium, containing 20% FBS (Fetal bovine serum, Thermo Fisher Scientific Inc.), 0.02 mM L-Glutamine (Thermo Fisher Scientific Inc.), 0.1× non-essential amino acid (Thermo Fisher Scientific Inc.), 0.01 mM sodium pyruvate (Hyclone, GE Healthcare, Pittsburge, PA, USA), and 1.5 g/L sodium bicarbonate (Sigma, St. Louis, MO, USA).
Different culture conditions
For different initial seeding concentration experiments, Caco-2 was all cultured in normal medium. The initial seeding concentration is 1×106, 7.5×105, 5×105, and 2.5×105 cells/mL were tested. Cells were cultured for 3 days, and subsequently collected for flow cytometric analysis.
For different treatment experiments, Caco-2 was treated with EPA, DMSO, and EDTA. For EPA treatment, Caco-2 was cultured in DMEM, high glucose medium containing 10% FBS, 1% penicillin-streptomycin (Thermo Fisher Scientific Inc.), 0.01 mM sodium pyruvate, and 2% EPA (Sigma). For DMSO and EDTA, Caco-2 cells were cultured in normal medium supplementing 2% DMSO (Sigma) or 2% EDTA (Hyclone). Caco-2 cells were cultured in different treatments for 3 days, and then collected for flow cytometric analysis.
Flow cytometric analysis
CD44 (BD Pharmingen, San Diego, CA, USA) and CD133 (BioLegend, San Diego, CA, USA) were tested in this study. Caco-2 cells were washed with phosphate buffered saline (PBS, UniRegion Bio-Tech, Taiwan), and stained with markers for 30 minutes on ice at dark. The samples were then washed with PBS for 3 times, and analyzed by FACScalibur flow cytometer (Becton Dickinson).
Statistical analysis
Flow cytometry analyses were evaluated using CellQuest Pro (BD Biosciences). The expression patterns in the dot plots of Figures 1,2 were from one representative flow cytometry experiment.
Results
Initial seeding concentration corresponds to the co-expression of CD44 and CD133
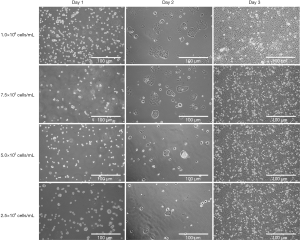
In order to investigate the relationship between the initial seeding concentration of Caco-2 and the expression of CD44 and CD133, four different seeding concentrations were tested. The cell morphology was not affected by initial seeding concentrations (Figure 3). At day 1, cells were round and evenly distributed on the plate. Cells were attached to the bottom of the plate, and morphology became flatter at day 2. Cell clusters were more obvious when the initial seeding concentration were higher (1×106 and 7.5×105 cells/mL), but the clusters could also be observed in the lowest seeding concentration group. At day 3, all the groups reached confluence regardless of the initial seeding concentrations.
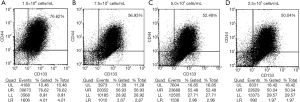
We then analyzed the expression of CD44 and CD133, and the results are shown in Table 1 and Figure 1. When the initial seeding concentration was 1×106 cells/mL, the single and combined expression of CD44 and CD133 were the highest (Figure 1A). When the initial seeding concentration was 7.5×105 cells/mL, the expression of both CD44 and CD133 were reduced dramatically (Figure 1B). The expression of both markers was decreased at the initial seeding concentration of 5.0×105 and 2.5×105 cells/mL, and it was seeding number dependent (Figure 1C,D). The results showed that even when the initial seeding concentration was as low as 2.5×105 cells/mL, the expression of surface markers on Caco-2 cells was still detectable.
Table 1
| Marker | Initial seeding concentration (cell/mL) | |||
|---|---|---|---|---|
| 1.0×106 | 7.5×105 | 5.0×105 | 2.5×105 | |
| CD44+ (%) | 85.28 | 39.38 | 37.21 | 37.91 |
| CD133+ (%) | 77.14 | 42.00 | 40.84 | 41.73 |
| CD44+CD133+ (%) | 76.62 | 56.93 | 52.48 | 50.04 |
Different treatments affect the single and combined expression of markers
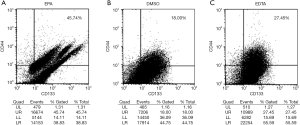
We next investigated the expression of CD44 and CD133 when Caco-2 was treated with different chemicals to mimic the potential clinical situations. When Caco-2 was treated with EPA, the expression of CD44 or CD133 was 0 (Table 2) although the percentage of double positive cells was 45.74% (Figure 2A). When Caco-2 was treated with DMSO, the common diluent for drugs, the percentage of cells expressing only CD44 or CD133 was over 50%, but the proportion of cells expressing both markers dropped to 18% (Figure 2B). When cells were treated with EDTA, the levels of single expression of either marker were nearly as high as those when cells were grown in normal medium. However, the percentage of cells expressing both markers was reduced to 27.45% in the EDTA treated group (Figure 2C).
Table 2
| Marker | Culture condition | ||
|---|---|---|---|
| EPA | DMSO | EDTA | |
| CD44+ (%) | 0 | 52.64 | 77.14 |
| CD133+ (%) | 0 | 70.97 | 85.99 |
| CD44+CD133+ (%) | 45.74 | 18.00 | 27.45 |
EPA, eicosapentaenoic acid; DMSO, dimethyl sulfoxide; EDTA, ethylenediaminetetraacetic acid.
Discussion
In this study, different initial seeding concentrations of Caco-2 and different chemical treatments were tested to investigate the expression of CD44 and CD133 in colorectal cancer cells. The result of different initial seeding concentrations showed that the co-expression of CD44 and CD133 decreased when the initial seeding concentration was reduced in a seeding concentration dependent manner. The result suggested that if the initial seeding concentration was as low as 2.5×105 cell/mL, the percentage of cells that are not expressing both CD44 and CD133 could be close to 50%. When Caco-2 cells were treated with different chemicals, the results demonstrated that EPA but not DMSO or EDTA could effectively inhibit the expression of CD44 or CD133.
It is beneficial for colorectal cancer patients to undergo CTC detection, in order to monitor cancer progression or treatment response. However, heterogeneity and scarcity of the cells are two major obstacles for CTC detection and verification. To overcome the issue of scarcity of CTCs, some researchers have suggested that culturing and characterizing human CTCs can provide better understandings of how treatments can be improved (15,17,18). Because only a small amount of blood is drawn from a patient, researchers have to promote the survival of self-renewing cells in order to characterize CTCs through cancer stem cells. In addition, studies have shown that the volume of blood sample was a limiting factor for flow cytometry-based CTC assays, thus culturing CTCs may increase the accuracy of detection. In order to mimic the potential few colorectal cancer stem cells that might be retrieved from CTC samples, we investigated four different initial seeding concentrations of Caco-2 cells to investigate the sensitivity of flow cytometry. Our results demonstrated that when fewer cancer stem cells were seeded the expression of surface markers was reduced. The flow cytometry results indicated that still ~50% of the cells co-expressed CD44 and CD133, and these cells could be detected when the initial seeding concentration was 2.5×105 cells/mL. Therefore, we provided useful information that cancer stem cells in CTCs could still be detected at the lowest initial concentration we tested. Whether such cancer stem cell concentrations in CTCs are clinically applicable will require further investigation.
Many researchers have demonstrated the effects of EPA and another omega-3 polyunsaturated fatty acid, docosahexaenoic acid (DHA), for their anti-colorectal cancer cell activity in vitro (19). The anti-colorectal cancer cell activity of EPA and DHA has been shown to be accomplished through the promotion of apoptosis and inhibition of colorectal cancer cell proliferation, especially when combined with other chemicals (20). When we treated Caco-2 cells with EPA, we observed poor cell growth. When the surface markers of EPA-treated cells were evaluated, single expression of CD44 or CD133 was 0% but co-expression was 45.74%. The result suggested that the activity of cancer stem cells was not completely inhibited by EPA in this treatment. It was not surprising, since it has been shown that single expression of CD44 or CD133 could be used for the detection of colorectal tumor cells but not cancer stem cells (21). This information could be useful in the future for evaluating colorectal cancer patients who were treated with EPA; colorectal cancer stem cells may still be present to induce recurrence even though tumor cells have been inhibited.
In contrast, expression of either CD44 or CD133 alone was high but co-expression of both markers was low in DMSO and EDTA-treated cells. DMSO is often used as a dissolvent or a control when testing the effects of drugs on colorectal cancer cells (21). It has been shown that, when the platinum drugs are dissolved in DMSO, DMSO reacts with the drugs, which inhibits the drug from promoting colorectal cancer cell death (22). However, it has not been shown that DMSO affects the surface marker expression of colorectal cancer cells. Under the concentration we evaluated in this study, DMSO caused lower co-expression of CD44 and CD133, suggesting the population of colorectal cancer stem cells was reduced but not colorectal tumor cells. More investigation will be required in order to confirm whether DMSO-treated cells lost the self-renewing ability and underwent differentiation. EDTA is a commonly used anticoagulant for blood sample transportation and storage. However, it has been shown that EDTA could reduce EpCAM expression, resulting in degradation of breast cancer cells in CTCs (23). Our results also suggested that EDTA would decrease the co-expression of CD44 and CD133 in Caco-2 cells. Our data provides evidence that another anticoagulant for blood samples should be considered to replace EDTA, in order to reduce the risk of damaging CTCs.
The study has limitations, since only the expression of CD44 and CD133 were investigated when Caco-2 cells were cultured in different conditions; EpCAM expression should be investigated in future studies. More colorectal cancer cell lines should be investigated to understand whether the results we observed were Caco-2-specific. In addition, CTC lines from colorectal cancer patients after different treatments should be evaluated in order to obtain a better understanding of the surface marker profiles.
Conclusions
We showed that the co-expression of CD44 and CD133 was initial seeding concentration dependent, and the co-expression was still above 50% at the lowest seeding concentration we investigated. Our results demonstrated both single and double expression of CD44 and CD133 should be considered when treating colorectal cancer stem cells in different conditions, in order to better understand cancer stem cell properties.
Acknowledgments
Funding: This work was supported financially by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.18). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol 2019;16:713-32. [Crossref] [PubMed]
- Chung RY, Tsoi KKF, Kyaw MH, et al. A population-based age-period-cohort study of colorectal cancer incidence comparing Asia against the West. Cancer Epidemiol 2019;59:29-36. [Crossref] [PubMed]
- Welch HG, Robertson DJ. Colorectal Cancer on the Decline. N Engl J Med 2016;375:804. [PubMed]
- Marley AR, Nan H. Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet 2016;7:105-14. [PubMed]
- Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging 2016;11:967-76. [Crossref] [PubMed]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730-7. [Crossref] [PubMed]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100:3983-8. [Crossref] [PubMed]
- Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A 2007;104:10158-63. [Crossref] [PubMed]
- O'Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007;445:106-10. [Crossref] [PubMed]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2007;445:111-5. [Crossref] [PubMed]
- Desai A, Yan Y, Gerson SL. Concise Reviews: Cancer Stem Cell Targeted Therapies: Toward Clinical Success. Stem Cells Transl Med 2019;8:75-81. [Crossref] [PubMed]
- Agnoletto C, Corra F, Minotti L, et al. Heterogeneity in Circulating Tumor Cells: The Relevance of the Stem-Cell Subset. Cancers (Basel) 2019; [Crossref] [PubMed]
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011;331:1559-64. [Crossref] [PubMed]
- Grillet F, Bayet E, Villeronce O, et al. Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture. Gut 2017;66:1802-10. [Crossref] [PubMed]
- Katoh S, Goi T, Naruse T, et al. Cancer stem cell marker in circulating tumor cells: expression of CD44 variant exon 9 is strongly correlated to treatment refractoriness, recurrence and prognosis of human colorectal cancer. Anticancer Res 2015;35:239-44. [PubMed]
- Gao D, Vela I, Sboner A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014;159:176-87. [Crossref] [PubMed]
- Yu M, Bardia A, Aceto N, et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 2014;345:216-20. [Crossref] [PubMed]
- Volpato M, Hull MA. Omega-3 polyunsaturated fatty acids as adjuvant therapy of colorectal cancer. Cancer Metastasis Rev 2018;37:545-55. [Crossref] [PubMed]
- Lee JY, Sim TB, Lee JE, et al. Chemopreventive and Chemotherapeutic Effects of Fish Oil derived Omega-3 Polyunsaturated Fatty Acids on Colon Carcinogenesis. Clin Nutr Res 2017;6:147-60. [Crossref] [PubMed]
- Tsunekuni K, Konno M, Haraguchi N, et al. CD44/CD133-Positive Colorectal Cancer Stem Cells are Sensitive to Trifluridine Exposure. Sci Rep 2019;9:14861. [Crossref] [PubMed]
- Hall MD, Telma KA, Chang KE, et al. Say no to DMSO: dimethylsulfoxide inactivates cisplatin, carboplatin, and other platinum complexes. Cancer Res 2014;74:3913-22. [Crossref] [PubMed]
- Qin J, Alt JR, Hunsley BA, et al. Stabilization of circulating tumor cells in blood using a collection device with a preservative reagent. Cancer Cell Int 2014;14:23. [Crossref] [PubMed]


