
HAX-1 overexpression in gastric cancer promotes cell proliferation
Introduction
Gastric cancer is a common malignancy of the digestive tract. According to a World Health Organization (WHO) report, the incidence of gastric cancer ranks fifth worldwide, while the mortality rate of gastric cancer ranks second (1). Furthermore, the 2015 China Cancer Data Report estimates 679,000 new cases of gastric cancer and 498,000 gastric cancer deaths in China each year. Indeed, the incidence and mortality rates of gastric cancer in China are both the second highest among all malignant tumors. Additionally, the number of new gastric cancer cases and deaths in China account for 42.6% and 45.0% of the world’s totals (2), respectively. Many patients with gastric cancer are already at an advanced stage at diagnosis, resulting in a low radical resection rate, poor quality of life and a 5-year survival rate of less than 30% (3). Therefore, it is of great significance to further explore the molecular mechanisms underlying the development and progression of gastric cancer and to identify effective diagnostic and therapeutic targets.
Hematopoietic cell-specific protein 1 (HS-1)-associated protein X-1 (HAX-1) was first discovered as an HS-1 interacting protein. The gene encoding the HAX-1 protein is located on chromosome 1q21.3. HAX-1 consists of 279 amino acids, with a molecular weight of 35 kDa, and contains an N-terminal acidic region, two domains (BH1 and BH2) that are similar in structure to Bcl-2 homology domains, a PEST motif, a C-terminal transmembrane region and an integrin β6-binding region. In recent years, many studies have reported that HAX-1 participates in multiple signaling pathways and pathophysiological processes through interactions with various proteins (4-6). Homozygous mutations of the HAX-1 gene are related to acute congenital neutropenia, with an autosomal recessive inheritance pattern. HAX-1 mutations are also associated with delayed puberty and gonadal dysfunction in women (7,8).
HAX-1 regulates apoptosis through multiple pathways. It exerts an anti-apoptotic effect predominantly by directly or indirectly inhibiting caspase 3/9 activities (9,10). It has been demonstrated that HAX-1 interacts with two-pore ion channels in endocytic lysosomes, regulates the quantity of ion channels to maintain the stability of the lysosomal membrane, and reduces lysosomal permeability and enzyme release, thereby exerting an apoptotic effect (11,12). In addition, HAX-1 is involved in regulating cell migration. HAX-1 forms a tetramer with Galpha13 (a protein that promotes cell migration and induces tumor cell metastasis) through glucocorticoid and Rac, which tightly binds to the cytoskeleton. Overexpression of HAX-1 attenuates the function of intracellular actin stress fibers and focal adhesion plaques, facilitating Galpha13-mediated cell movement. In contrast, silencing endogenous HAX-1 with small interfering RNA (siRNA) greatly reduces the cell movement mediated by Galpha13 (13).
The functions of HAX-1 suggest that this protein plays an important role in the processes of tumor development and metastasis. In fact, studies have found that HAX-1 expression is significantly elevated in malignant tumors, such as glioma, hypopharyngeal carcinoma and rectal cancer, in comparison to normal tissues. Overexpression of HAX-1 has also been related to the chemoresistance of breast cancer cells (14).
Nonetheless, HAX-1 expression in gastric cancer and its role in tumor development and progression remain unclear. Thus, the present study explored the expression status of HAX-1 in gastric cancer and its role in its development and progression. The effect of HAX-1 expression on the growth, proliferation and migration of various gastric cancer cell lines was also investigated.
Methods
Patients and tissue specimens
Tissue specimens were collected from 103 patients with gastric cancer who underwent D2 radical resection between 2014 and 2015 at Nantong University Affiliated Hospital. All patients were diagnosed with stage II and III cancer based on the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system for gastric cancer (Eighth edition). The ages of the patients ranged from 30 to 78 years, with a median age of 59 years. The detailed clinicopathological parameters of the patients are shown in Table 1. Ten pairs of fresh gastric cancer and normal paracancerous tissue specimens were stored at −80 °C in liquid nitrogen until used for quantitative polymerase chain reaction (qPCR). The present study was approved by the Ethics Committee of Nantong University Affiliated Hospital.
Table 1
| Clinicopathological features | Number of patients | Number of HAX-1 positive patients (%) | χ2 value | P value |
|---|---|---|---|---|
| Age | 0.267 | 0.606 | ||
| <50 years | 39 | 16 (41.0) | ||
| ≥50 years | 64 | 23 (35.9) | ||
| Sex | 0.029 | 0.865 | ||
| Male | 57 | 22 (38.6) | ||
| Female | 46 | 17 (37.0) | ||
| Degree of histological differentiation | 6.083 | 0.014* | ||
| High+ moderate | 53 | 14 (26.4) | ||
| Low | 50 | 25 (50.0) | ||
| Vascular tumor thrombus | 5.431 | 0.020* | ||
| Present | 31 | 17 (54.8) | ||
| Absent | 72 | 22 (30.6) | ||
| TNM stage | 7.481 | 0.006* | ||
| Stage II | 44 | 10 (22.7) | ||
| Stage III | 59 | 29 (49.2) | ||
| Lymph node metastasis | 5.275 | 0.022* | ||
| Present | 80 | 35 (43.8) | ||
| Absent | 23 | 4 (17.4) |
Statistical analyses were performed with Pearson’s χ2 test. *, P<0.05 was considered significant.
Antibodies
The antibodies and reagents used in the present study included mouse anti-HAX-1 polyclonal (BD Biosciences Pharmingen Inc.), mouse anti-Ki-67 monoclonal (Santa Cruz Biotechnology), rabbit anti-human caspase 3 polyclonal (Santa Cruz Biotechnology), mouse anti-human caspase 9 polyclonal (Santa Cruz Biotechnology), and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Sigma-Aldrich) antibodies, and Lipofectamine 2000 (Santa Cruz Biotechnology).
Real-time fluorescence-based qPCR
Total RNA was extracted from homogenized tissues and cells collected by digestion using TRIzol reagent (Invitrogen, USA) in accordance with the manufacturer’s instructions. The RNA concentration was determined using an ultramicro nucleic acid/protein analyzer; RNA integrity was examined by agarose gel electrophoresis. Total RNA was reverse transcribed into complementary DNA (cDNA) using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA), which was then subjected to real-time, fluorescence-based qPCR analysis. The sequences of the specific primers used in the real-time, fluorescence-based qPCR were as follows: HAX-1 forward primer, 5'-ATGAGCCTCTTTGATCTCTTCC-3', HAX-1 reverse primer, 5'-CTACCGGGACCGGAACCAAC-3'; GAPDH forward primer, 5'- CCTGGATACCGCAGCTAGGA-3', GAPDH reverse primer, 5'- GCGGCGCATACGAATGCCCC -3'. Relative quantitative analysis was performed using a 7500 Fast fluorescence quantitative PCR system (ABI, USA).
Immunohistochemical examination
Tissue specimens were fixed with 4% formaldehyde, routinely embedded in paraffin, sectioned, dewaxed and hydrated. After endogenous peroxidases were blocked with 3% hydrogen peroxide, the tissue specimens were blocked for 30 min with a working solution of normal goat serum. The samples were then incubated at 4 °C with primary antibodies (working concentrations of 1:200) overnight followed by corresponding secondary antibodies for 1 h at room temperature (RT). Following chromogenic reaction with diaminobenzidine (DAB) and counterstaining with hematoxylin, the specimens were dehydrated and mounted.
Immunohistochemical evaluation criteria
Positive HAX-1 protein expression was mainly represented by nuclear staining. Specifically, positive HAX-1 protein expression was defined as the appearance of apparent brownish-yellow granules in the nucleus and cytoplasm. Positive expression of caspase-3 and caspase-9 proteins was defined as the appearance of apparent brownish-yellow granules in the cell membrane or cytoplasm. In the negative control, the primary antibody was replaced with a homologous IgG monoclonal antibody. The results were evaluated according to immunoreactivity scores, which were determined based on staining intensity and the percentage of positive cells. The staining intensity of tumor cells was scored using the following criteria: 0 points, no staining; 1 point, light yellow; 2 points, brownish yellow; and 3 points, dark brown. The scoring criteria for the percentage of positive cells were as follows: 0 points, <5%; 1 point, 5–25%; 2 points, 26–50%; 3 points, 51–75%; and 4 points, >75%. The staining intensity score was multiplied by the score for the percentage of positive cells. A product of ≥4 points indicated high expression of HAX-1, caspase-3 and caspase-9 proteins. Ki-67 expression was assessed based on the rate of positivity: a rate of >50% was considered a high level of expression, whereas a rate of ≤50% was considered a low level of expression.
Cell culture and transfection
The gastric adenocarcinoma cell lines SGC-7901, MKN-28 and AGS were all purchased from Shanghai Institute of Cell Research, Chinese Academy of Sciences. The cell lines were cultured under standard conditions (37 °C, 5% CO2) in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS).
For HAX-1 expression interference, three small hairpin RNA (shRNA) plasmids were designed were designed and synthesized by Shanghai Genechem Co., Ltd. The sequences of the shRNAs were as follows: HAX-1 shRNA#1, 5'-GATCCCGACTTATTCCTGGGACGTTCTCGAGAACGTCCCAGGAATAAGTCCATTTTTGGAT-3'; HAX-1 shRNA#2, 5'-GATCCCAGCCCAAATCCTATTTCAACTCGAGTTGAAATAGGATTTGGGCTGGTTTTTGGAT-3'; HAX-1 shRNA#3, 5'-GATCCCAGAGGCCATTTCATAGGTTCTCGAGAACCTATGAAATGGCCTCTGG TTTTTGGAT-3'. Plasmid transfection was carried out using LipofectamineTM 2000 in accordance with the manufacturer’s instructions. Cells were cultured for 48 h after transfection. Cellular protein was then extracted, and HAX-1 expression was analyzed.
Western blot analysis
Total protein was extracted, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were washed 3 times with Tris-buffered saline/Tween 20 (TBST) and then blocked with TBST containing 5% nonfat dry milk at RT for 1 h. After sufficient washing with TBST, the membranes were incubated overnight with primary antibodies at 4 °C. The membranes were rinsed 3 times with TBST, incubated with horseradish peroxidase-conjugated secondary antibodies at RT for 1 h, and washed again with TBST. Expression of relevant proteins was examined by electrochemiluminescence assays and imaged. The grayscale values of the protein bands were determined using Kodak Digital Science 1D Image software. GAPDH was used as a reference. HAX-1, Ki-67, caspase 3 and caspase 9 protein expression in SGC-7901 gastric cancer cells that had been transfected for 48 h was assessed using the method described above. The experiment was repeated 3 times.
Cell cycle analysis
Cells were seeded into 6-well plates and cultured until adherence. After treatment with DS2 for 24 h, the cells were trypsinized, centrifuged, collected, suspended in precooled 70% ethanol, and fixed overnight. The cells were centrifuged to remove the fixative, washed twice with phosphate-buffered saline (PBS) and stained at RT in the dark by resuspension in PBS containing 50 mg/L RNase and 100 mg/L propidium iodide (PI). DNA contents were measured by flow cytometry. A total of 20,000 cells were examined per sample, and the cell cycle was analyzed using FlowJo software.
Examination of cell proliferation using the cell counting Kit-8 (CCK-8) assay
Logarithmically growing SGC-7901 gastric cancer cells were seeded into 96-well culture plates at a density of 5×103 cells/well and routinely cultured for 24 h. The experimental group was transfected with HAX-1 shRNA; the negative control group was transfected with a negative shRNA construct. The blank control group consisted of cells that had not been subjected to treatment. The cells were harvested at 0, 6, 12, 24 and 48 h after transfection. Each well of cells was incubated with 10 µL of CCK-8 solution at 37 °C for 2 h. Absorbance (A) at 450 nm was measured using a microplate reader. The experiment was repeated 3 times.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 software. Count data were compared using the χ2 test and the measurement data using the t-test. Survival analysis was performed using the Kaplan-Meier method and the log-rank test. Multivariate analysis was performed using the Cox proportional hazards model. The significance level was set at α=0.05.
Results
HAX-1 expression is upregulated in gastric cancer
We first investigated HAX-1 mRNA expression in 10 matching pairs of gastric cancer and adjacent non-tumor tissues. qPCR analysis showed that HAX-1 mRNA expression was significantly upregulated in gastric cancer tissues compared to adjacent non-tumor tissues (Figure 1A). Moreover, immunohistochemical results revealed that HAX-1 protein was mainly expressed in the cytoplasm, exhibiting brownish-yellow patchy staining. Among the 103 gastric cancer tissue specimens, the rate of high HAX-1 expression was 37.8% (39/103). In paracancerous normal tissues, 25 of 103 cases showed high expression levels of HAX-1 protein, for a rate of 24.3% (25/103) (Figure 1B). HAX-1 protein expression was significantly increased in gastric cancer tissues compared to paracancerous normal tissues (P=0.035).
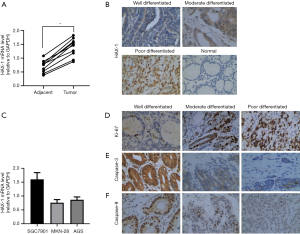
In addition, we examined HAX-1 mRNA expression in 3 human gastric cancer cell lines, SGC-7901, MKN-28 and AGS cells, using qPCR. As HAX-1 expression was significantly higher in SGC-7901 cells, this gastric cancer cell line was selected for subsequent experiments (Figure 1C).
HAX-1 expression correlates with the clinicopathological features of gastric cancer
We analyzed relationships between HAX-1 protein expression and clinicopathological parameters. According to the results, HAX-1 protein expression was associated with the degree of tumor differentiation (P=0.014), vascular tumor thrombus (P=0.020), TNM stage (P=0.006) and lymph node metastatic status (P=0.022). In contrast, HAX-1 protein expression was not related to the age or sex of patients (P>0.05) (Table 1).
HAX-1 expression correlates with Ki-67
We also examined Ki-67, caspase-3 and caspase-9 protein expression in gastric cancer tissues by immunohistochemistry (Figure 1D,E,F). Spearman rank correlation showed that HAX-1 protein expression correlated positively with expression of the proliferation-related protein Ki-67 (r=0.951, P<0.01) and negatively with expression of the apoptosis-related proteins caspase-3 (r=−0.983, P<0.01) and caspase-9 (r=−0.950, P<0.01). These results indicate that HAX-1 may promote the proliferation of tumor cells by inhibiting apoptosis. Taken together, upregulated expression of HAX-1 may be a strong determinant of a poor prognosis in gastric cancer.
HAX-1 expression correlates with poor prognosis in patients with gastric cancer
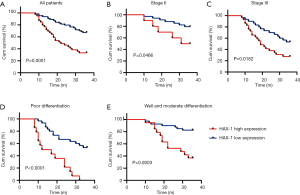
Based on Kaplan-Meier survival analysis, gastric cancer patients with high HAX-1 expression had significantly shortened survival times compared to patients with low HAX-1 expression. Among the 103 patients with gastric cancer analyzed, those with high HAX-1 expression had a postoperative 3-year overall survival (OS) rate of 41.5%, whereas those with low HAX-1 expression had a 3-year OS of 64.6% (P=0.0001) (Figure 2A). In patients with stage II and III poorly differentiated and moderately well differentiated gastric cancer, those with high HAX-1 expression had a shorter 3-year OS than those with low HAX-1 expression (P<0.05) (Figure 2B,C,D,E). Moreover, multivariate analysis using the Cox proportional hazards model revealed that HAX-1 protein expression (P=0.037) and TNM stage (P=0.021) were independent factors influencing the prognosis of gastric cancer patients.
HAX-1 promotes the proliferation of gastric cancer cells
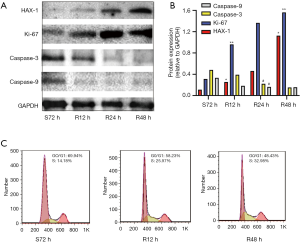
We next performed a serum starvation-release experiment in which SGC-7901 gastric cancer cells were subjected to serum starvation for 72 h after which the serum supply was restored and changes in cell cycle distribution were analyzed by flow cytometry. After 72 h of serum starvation, SGC-7901 cells were arrested at the G1 phase. Once serum was added, the cells were released from G0/G1 phase arrest and gradually entered S and G2. We further examined HAX-1, Ki-67, caspase-3 and caspase-9 protein expression using western blot analysis. After serum stimulation, expression of HAX-1 and Ki-67 was gradually elevated as cell proliferation increased, but caspase-3 and caspase-9 protein expression declined gradually (Figure 3A,B,C). These results suggest that HAX-1 may act as a positive regulator of the cell cycle, exerting proliferation-promoting and anti-apoptotic effects by inhibiting caspase-3 and caspase-9 protein expression.
Knockdown of HAX-1 inhibits the proliferation of gastric cancer cells
To inhibit HAX-1 expression, SGC-7901 gastric cancer cells were transfected with plasmids, and qPCR was performed to select the HAX-1 shRNA with the highest interference efficiency. HAX-1 mRNA expression was lowest, and the interference efficiency was thus the highest, in the HAX-1 shRNA#2-transfected group (Figure 4A,B). Therefore, HAX-1 shRNA#2 was used in subsequent experiments. According to Western blot results, HAX-1 protein expression was decreased but caspase-3 and caspase-9 protein expression increased with the inhibition of cell proliferation (Figure 4C,D). In addition, flow cytometry revealed that knockdown of HAX-1 expression increased the percentage of cells in G0/G1 phase (from 67.34% to 70.27%) and reduced the percentage of cells in S phase (from 14.75% to 13.66%) (Figure 4E).
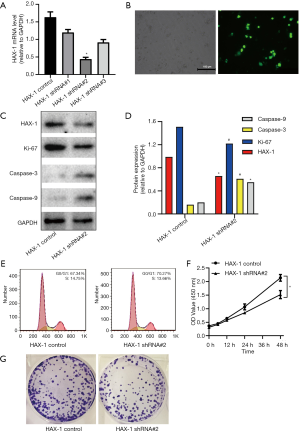
Furthermore, CCK-8 cell proliferation analysis showed decreased absorbance in the HAX-1 shRNA#2 group compared with that in the control group at 6, 12, 24, 48 and 72 h. These results indicate that knockdown of HAX-1 expression inhibited the proliferation of SGC-7901 cells (Figure 4F). By colony formation assay, we found that the rate of colony formation was significantly attenuated in knockdown of HAX-1(Figure 4G).
Discussion
Gastric cancer has been studied in depth. However, the efficacy of gastric cancer treatments is far from ideal. Therefore, the identification of new diagnostic markers and therapeutic targets is of great significance for improving the clinical efficacy of treatment for gastric cancer. HAX-1 is a complex multifunctional protein. It participates in the regulation of cellular processes such as apoptosis, proliferation and migration and is closely related to tumorigenesis and metastasis (15). Studies have found that HAX-1 protein expression is upregulated in various tumor tissues. For example, elevated HAX-1 expression is closely related to a more invasive clinical phenotype and poor prognosis in colorectal cancer and esophageal squamous cell carcinoma (16-18).
In the present study, an immunohistochemical approach was applied to examine expression of HAX-1 in gastric cancer tissues. The results showed that HAX-1 expression was significantly elevated in tumor tissues compared with adjacent noncancerous tissues. Expression of HAX-1 was also found to be closely related to the degree of histological differentiation, vascular tumor thrombus, TNM stage and lymph node metastasis in gastric cancer. In addition, HAX-1 correlated with a poor prognosis, and multivariate analysis demonstrated that HAX-1 might be an independent prognostic factor affecting the survival of patients with gastric cancer. The results of immunohistochemical analysis also indicated that expression of HAX-1 correlated with expression of Ki-67, caspase-3 and caspase-9. These results indicate that HAX-1 may play an important role in the progression and metastasis of gastric cancer and is related to the proliferation and apoptosis of gastric cancer cells.
Previous studies have shown that HAX-1 regulates the proliferation and apoptosis of tumor cells through a variety of mechanisms. It was found that the high expression of HAX-1 promoted the growth, migration and invasion of liver cancer cells, while silence of HAX-1 produced the opposite results. Silencing PIK3CA enhanced the inhibitory effects of HAX-1 silencing on the viability, migration, and invasion of liver cancer cells, and HAX-1 could affect liver cancer cells metastasis and angiogenesis by affecting Akt phosphorylation and FOXO3A expression (19). In the triple-negative breast cancer (TNBC) a result suggested that miR-223 increased the sensitivity of TNBC stem cells to TRAIL-induced apoptosis by targeting HAX-1 (20). A study on prostate cancer showed that HAX-1 knockout in the prostate cancer cell line DU145 promoted tumor cell apoptosis and caspase-9 activation; thus, HAX-1 inhibits tumor cell apoptosis through caspase-9 inactivation (9). We further investigated the effects of HAX-1 on cell proliferation and apoptosis in the gastric cancer cell line SGC-7901, revealing that as cell proliferation increased, HAX-1 expression increased accordingly but that caspase-3 and caspase-9 expression decreased. After knocking down expression of HAX-1, gastric cancer cell proliferation was inhibited, gastric cancer cell apoptosis was increased, and caspase-3 and caspase-9 expression was elevated. The above findings demonstrate that HAX-1 is related to the proliferation and apoptosis of gastric cancer cells. HAX-1 may play a regulatory role through the caspase family.
In summary, the data obtained in the present study demonstrate that HAX-1 expression is elevated in gastric cancer tissues. The expression level of HAX-1 may be related to the clinical progression and poor prognosis of gastric cancer, and HAX-1 is an independent factor affecting the prognosis of patients with gastric cancer. Moreover, HAX-1 may serve as a new prognostic factor and a potential therapeutic target for gastric cancer.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.02.69). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was approved by the Ethics Committee of Nantong University Affiliated Hospital. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015 CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Katai H, Ishikawa T, Akazawa K, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer 2018;21:144-54. [Crossref] [PubMed]
- Yap SV, Koontz JM, Kontrogianni-Konstantopoulos A. HAX-1: a family of apoptotic regulators in health and disease. J Cell Physiol 2011;226:2752-61. [Crossref] [PubMed]
- Chao JR, Parganas E, Boyd K, et al. Hax1-mediated processing of HtrA2 by Parl allows survival of lymphocytes and neurons. Nature 2008;452:98-102. [Crossref] [PubMed]
- Ramsay AG, Keppler MD, Jazayeri M, et al. HS1-associated protein X-1 regulates carcinoma cell migration and invasion via clathrin-mediated endocytosis of integrin alphavbeta6. Cancer Res 2007;67:5275-84. [Crossref] [PubMed]
- Karapınar TH, Yılmaz KD, Oymak Y, et al. HAX1 mutation positive children presenting with haemophagocytic lymphohistiocytosis. Br J Haematol 2017;177:597-600. [Crossref] [PubMed]
- Cekic S, Saglam H, Gorukmez O, et al. Delayed Puberty and Gonadal Failure in Patients with HAX1 Mutation. J Clin Immunol 2017;37:524-8. [Crossref] [PubMed]
- Yan J, Ma C, Cheng J, et al. HAX-1 inhibits apoptosis in prostate cancer through the suppression of caspase-9 activation. Oncol Rep 2015;34:2776-81. [Crossref] [PubMed]
- Lee AY, Lee Y, Park YK, et al. HS 1-associated protein X-1 is cleaved by caspase-3 during apoptosis. Mol Cells 2008;25:86-90. [PubMed]
- Lam AK, Galione A, Lai FA, et al. Hax-1 identified as a two-pore channel (TPC)-binding protein. FEBS Lett 2013;587:3782-6. [Crossref] [PubMed]
- Baumann U, Fernández-Sáiz V, Rudelius M, et al. Disruption of the PRKCD-FBXO25-HAX-1 axis attenuates the apoptotic response and drives lymphomagenesis. Nat Med 2014;20:1401-9. [Crossref] [PubMed]
- Radhika V, Onesime D, Ha JH, et al. Galpha13 stimulates cell migration through cortactin-interacting protein Hax-1. J Biol Chem 2004;279:49406-13. [Crossref] [PubMed]
- Wu G, Zhou W, Pan X, et al. miR-100 Reverses Cisplatin Resistance in Breast Cancer by Suppressing HAX-1. Cell Physiol Biochem 2018;47:2077-87. [Crossref] [PubMed]
- Wu H, Chen J, Wang Q, et al. Abnormal expression of HAX-1 is associated with cellular proliferation and migration in human hypopharyngeal squamous cell carcinoma. Mol Med Rep 2017;16:4664-70. [Crossref] [PubMed]
- Li M, Tang Y, Zang W, et al. Analysis of HAX-1 gene expression in esophageal squamous cell carcinoma. Diagn Pathol 2013;8:47. [PubMed]
- Trebinska A, Rembiszewska A, Ciosek K, et al. HAX-1 overexpression, splicing and cellular localization in tumors. BMC Cancer 2010;10:76. [Crossref] [PubMed]
- Li X, Jiang J, Yang R, et al. Expression of HAX-1 in colorectal cancer and its role in cancer cell growth. Mol Med Rep 2015;12:4071-8. [Crossref] [PubMed]
- Wu Z, Ai X, Hu H, et al. Hematopoietic-substrate-1 associated protein X-1 (HAX-1) regulates liver cancer cells growth, metastasis, and angiogenesis through Akt. Cancer Biol Ther 2019;20:1223-33. [Crossref] [PubMed]
- Sun X, Li Y, Zheng M, et al. MicroRNA-223 Increases the Sensitivity of Triple-Negative Breast Cancer Stem Cells to TRAIL-Induced Apoptosis by Targeting HAX-1. PLoS One 2016;11:e0162754. [Crossref] [PubMed]


