
Bone metabolism in Chinese patients after laparoscopic Roux-en-Y gastric bypass
Introduction
The prevalence of T2D with obesity in China is rising rapidly. Hu et al. reported that China ranks number one with an estimate of 109.6 million adults with diabetes (1). And the prevalence of overweight in Chinese adults were 41.2% in 2014, with an estimated increase of 0.27% per year. In addition to medical and exercise therapy, bariatric surgery is an effective long-term method to achieve weight loss and normal blood glucose levels. Several conditions, namely, essential hypertension, diabetes, asthma, osteoarthritis, and hyperlipidemia, can be improved and even resolved following bariatric surgery (2).
Laparoscopic Roux-en-Y gastric bypass (LRYGB) is a common procedure for achieving weight loss and glycemic control by reducing gastric capacity. LRYGB is a safe and effective procedure to improve glycemic control, obesity, body fat percentage, and blood pressure in patients with type 2 diabetes mellitus (T2DM) and obesity (3). The underlying mechanism for these improvements is not so clear, but hormones such as ghrelin, leptin, GLP-1, GIP, hepatic and peripancreatic fat, inflammatory markers, miRNA, and gut microbiota all play important roles (4-6). However, although gastric volume reduction and bypassing of proximal small bowel can alleviate T2DM, complications can occur. Abnormal bone metabolism is a late complication after LRYGB, and factors such as lower food intake and vitamin D deficiency may lead to abnormal bone mineral density (BMD), which increases the risk of fracture and osteoporosis. Therefore, long-term follow-up and appropriate treatment is necessary after LRYGB.
In this study, we evaluated index changes reflecting bone metabolism after LRYGB, namely blood levels of calcium, vitamin D, and parathyroid hormone (PTH). Evidence indicates that regular supplementation with calcium, vitamin D, and a multivitamin may contribute to maintaining index levels in their normal ranges. Preventing gradual increases in PTH levels (secondary hyperthyroidism) requires further study.
Methods
This was a retrospective study involving data collected between February 2012 and December 2016. The human research review board at Shanghai 6th People’s Hospital approved the study, and all patients provided written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki.
We enrolled 238 patients with T2DM with a body mass index (BMI) >27.5 kg/m2: 111 women and 127 men with a mean age of 46.91±12.03 years and a mean BMI of 31.37 kg/m2. The highest BMI was 44.9 kg/m2 before operation. The inclusion criteria were: (I) T2DM duration ≤15 years with adequate islet function defined as fasting C-peptide by the oral glucose tolerance test >1 ng/mL and a ratio of peak to fasting blood glucose value >2 ng/mL; (II) age 16–65 years; and (III) BMI >27.5 kg/m2. Patients with established diagnoses of type 1 diabetes, latent autoimmune diabetes in adulthood, malignancy, debilitating disease, unresolved psychiatric illness, or substance abuse were excluded from the study.
Weight was determined using an electronic scale; participants were barefoot and wore light clothes. The waist/hip ratio was calculated by waist circumference/hip circumference.
Patients underwent standard LRYGB, which included a 25–30-mL gastric pouch, 100-cm biliopancreatic limb, and 100-cm alimentary limb.
Medical history, current medications, and anthropometric evaluations including BMI, weight, serum calcium, PTH, and 25-hydroxy vitamin D (25OHD) levels were recorded before and after bypass surgery at 3, 6, 12, 24, and 36 months. Postoperatively, we supplemented patients to prevent osteoporosis using 600 mg calcium per day, 0.5 mg vitamin D3 per day, and one multivitamin tablet per day. Among the 238 original patients, 16 patients were selected randomly for analyzed data in 6 months postoperatively regarding the levels of C-terminal crosslinking telopeptide of type I collagen (CTX-1) and bone-specific alkaline phosphatase (BAP) recorded preoperatively and 6, 12, and 24 months postoperatively.
All statistics were calculated using SPSS statistical software (version 20.0; SPSS Inc., Armonk, NY, USA) and GraphPad Prism (version 7.0; GraphPad Software Inc., San Diego, CA, USA). Paired t-tests were used to compare pre- and postoperative values. Pearson’s correlation test was performed to assess the correlation between postoperative BMI, serum calcium, PTH, and 25OHD. Data are presented as mean ± standard deviation. Statistical significance was defined as P<0.05.
Results
The patients’ baseline characteristics are shown in Table 1. Comparing pre- and 3-month postoperative values in all patients, calcium decreased (P<0.05), 25OHD did not change (P>0.05), and PTH increased significantly (P<0.05) in Tables 2, 3 and 4. In the 16 patients in the subgroup, CTX-1 increased in the first year and decreased in the second year, postoperatively (P>0.05); BAP increased gradually 6, 12, and 24 months postoperatively (P>0.05). BMI decreased sharply comparing pre- and 3-month postoperative values (preoperative mean BMI: 31.37±3.52, N=238; postoperative mean BMI: 25.20±2.86, N=218; P<0.05). And a female patient had a low BMI (19.1 kg/m2) 3 months after RYGB. Postoperative BMI decreased and remained stable, indicating that patients maintained a steady weight without weight regain, and indicated a good postoperative result (Table 5). After surgery, BMI was related to 25OHD (P<0.05), and had an inverse relationship with PTH (P<0.05). We saw no relationship between calcium and BMI (Table 6).
Table 1
| Variable | At baseline (obesity and T2D) |
|---|---|
| N | 238 |
| Sex, male/female | 111/127 |
| Age, year | 46.91±12.03 |
| T2D duration, year | <15 |
| WHR | 0.98±0.06 |
| BMI, kg/m2 | 31.37±3.52 |
| 25OHD, μg/mL | 15.03±6.14 |
| Ca2+, mmol/L | 2.31±0.11 |
| PTH, ng/L | 39.74±15.22 |
Table 2
| Time | n | Serum calcium | F | P |
|---|---|---|---|---|
| Pre | 235 | 2.31±0.11 | 12.844 | <0.05 |
| 3 months | 216 | 2.35±0.12 | ||
| 6 months | 209 | 2.31±0.12 | ||
| 12 months | 195 | 2.29±0.11 | ||
| 24 months | 123 | 2.27±0.10 | ||
| 36 months | 68 | 2.25±0.11 |
Table 3
| Time | n | 25OHD | F | P |
|---|---|---|---|---|
| Pre | 234 | 15.03±6.14 | 1.957 | >0.05 |
| 3 months | 216 | 15.87±6.93 | ||
| 6 months | 209 | 16.63±7.15 | ||
| 12 months | 193 | 16.94±8.18 | ||
| 24 months | 125 | 15.77±8.35 | ||
| 36 months | 68 | 16.95±7.42 |
Table 4
| Time | n | PTH | F | P |
|---|---|---|---|---|
| Pre | 221 | 39.74±15.22 | 21.328 | <0.05 |
| 3 months | 216 | 41.79±15.81 | ||
| 6 months | 207 | 43.04±16.35 | ||
| 12 months | 193 | 46.10±14.63 | ||
| 24 months | 125 | 55.59±20.05 | ||
| 36 months | 68 | 54.36±21.49 |
Table 5
| Time | n | BMI | F | P |
|---|---|---|---|---|
| Pre | 221 | 31.37±3.52 | 95.01 | <0.05 |
| 3 months | 216 | 25.60±2.86 | ||
| 6 months | 207 | 24.48±2.90 | ||
| 12 months | 193 | 24.22±2.89 | ||
| 24 months | 125 | 24.77±3.30 | ||
| 36 months | 68 | 24.83±3.15 |
Table 6
| Parameters | r | P |
|---|---|---|
| 25OHD | −0.0179 | <0.05 |
| Ca | 0.053 | >0.05 |
| PTH | −0.009 | <0.05 |
P<0.05 means significant different. 25OHD and PTH has a negative association with BMI. Ca2+ has no relationship with BMI.
Discussion
LRYGB is used to treat obesity and also improves hypertension and T2DM. LRYGB has been proven clinically effective for obesity. A female patient had a low BMI (19.1 kg/m2) 3 months after RYGB. However, postoperative complications stress the importance of follow-up. Low serum 25OHD levels and poor calcium absorption appeared to be related to secondary hyperparathyroidism in women who had undergone RYGB (7). We found consistently decreased calcium levels and increased PTH postoperatively during the 3-year follow-up in our study, no changes in 25OHD, and no relationship between calcium and BMI.
The mean preoperative 25OHD level in our patients was 15.03±6.14 µg/mL. 25OHD levels <20 µg/mL indicate vitamin D deficiency, and 21–29 µg/mL indicates vitamin D insufficiency (8). Based on these values, 16.4% of obese patients are diagnosed with vitamin D insufficiency, and 79.0% with vitamin D deficiency. It shows that 0.5 µg alfacalcidol daily for patients is not enough to avoid vitamin D deficiency. Vitamin D levels are related to skin color, diet, and decreased sun exposure, in China. Low plasma 25OHD is associated with insulin resistance and low-grade inflammation (9). Lower vitamin D levels in obese patients with T2DM are a consistent problem that requires attention. Preoperative mean calcium levels in obese patients are 2.31±0.11 mmol/L and mean PTH is 39.74±15.22 ng/L. Low vitamin D levels can be seen with PTH and calcium levels in the normal range, indicating that vitamin D deficiency is not related to bone metabolism before bariatric surgery. Wortsman et al. found that decreased bioavailability of vitamin D3 from cutaneous and dietary sources could explain obesity-associated vitamin D insufficiency and deficiency (10).
Lower fat intake affects absorption of vitamin D, which is fat-soluble, in the ileum and jejunum, and LRYGB bypasses the duodenum and proximal jejunum, the primary sites of calcium absorption (11). LRYGB dramatically reduces caloric intake with concomitant changes in eating behavior. Patients restart their diet as liquids only, and gradually progress to half-liquid then full diet, which takes at least 3 months. During this period, patient’s fat and vitamin D intake is limited. In patients with obesity with preoperative vitamin D deficiency, limited intake may worsen this situation. Although poor digestion and/or poor absorption may affect the serum levels of 25OHD and calcium, serum 25OHD remained stable in our study because of supplementation. Recent guidelines suggesting 1,200–1,500 mg/d of calcium citrate and 3,000 IU/day of vitamin D (12). As Figure 1 and Table 3 show, the multivitamin and alfacalcidol supplementation in our study prevented declining 25OHD levels; however, patients were still vitamin D deficient. Helping patients maintain sufficient vitamin D levels is important after LRYGB.
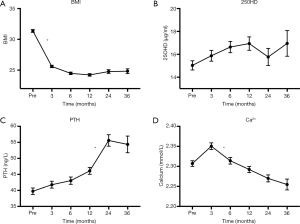
In our study, PTH was negatively correlated with serum 25OHD (P<0.05). Long-term vitamin D deficiency causes secondary hyperparathyroidism, high bone turnover, bone loss, and increases the risk of fractures (13). In our study, serum 25OHD remained low with no further rapid decrease postoperatively. Studies report that secondary hyperparathyroidism develops once 25OHD levels are <30 µg/mL (14). Vitamin D deficiency may not affect bone turnover directly, but it might regulate bone turnover by PTH. A certain degree of increased PTH may compensate for keeping normal bone turnover rate (15). Higher PTH may also increase the risk of fractures. Recommended supplementation of 1,200 mg calcium and 0.5 µg alfacalcidol is not enough for avoid postoperative secondary hyperparathyroidism, though it maintained serum 25OHD levels in our study. However, we saw no indication of increased bone loss among patients developing mild secondary hyperparathyroidism 24 months postoperatively (16). Yu et al. reported that many LRYGB patients require significantly higher doses of vitamin D3 (as much as 50,000 IU/day) to maintain sufficient vitamin D levels and avoid secondary hyperparathyroidism (17).
Calcium levels may decrease following LRYGB because of both vitamin D deficiency and lower calcium absorption post-LRYGB. In addition, fat malabsorption following diet changes impacts serum calcium. Lower fat intake increases free fatty acid levels, which combine with calcium to form insoluble calcium soap, which is excreted, further worsening calcium loss. Additionally, PTH regulates calcium homeostasis. Unfortunately, we saw lower calcium levels in our patients, despite the presence of secondary hyperparathyroidism and calcium supplementation. In our study, serum calcium levels approached the lower limit of the normal range (2.25–2.75 mmol/L) at 6, 12, 24, and 36 months, but did not exceed the normal range. Some researchers consider that preoperative LRYGB calcium absorption efficacy is sufficiently high that postoperative values decline to within the normal range (18). Both pre- and postoperative serum calcium levels were in the normal range (2.25–2.75 mmol/L) with slight fluctuations in the first 3 months after surgery (Figure 1 and Table 2).
Regarding bone loss, some researchers believe that there is an association between drastic weight loss and bone loss (19); however, in our study, we found no relationship between BMI and calcium levels (P>0.05). Weight loss has not been confirmed as the mechanism for bone loss post-LRYGB; however, continuously increasing PTH levels are associated with a high risk of fracture and bone loss, and has a negative association with BMI. When BMI decreases, PTH levels may continue to increase without changes in 25OHD levels. Therefore, decreased BMI may be a factor in bone loss after bariatric surgery.
Currently in China, there is no consensus regarding who should receive supplementation or the quantities, but supplementation is generally recommended in patients with a high risk of fracture. The complication of gradually increasing PTH and secondary hyperthyroidism remains a concern. Although postoperative multivitamin supplementation helped to some degree, the ideal dose of vitamin D is undetermined. A study from China showed that 1,600 IU/d effectively improved bone loss, and that a low dose of vitamin D supplementation (400 IU/d) showed no obvious improvement in BMD (20). The 2013 guidelines stated that oral doses of 3,000 IU/d vitamin D should be provided post-LRYGB with elemental calcium supplementation as high as 1,200–1,500 mg/d to prevent or minimize secondary hyperthyroidism (21). Another study showed that 1,600 IU/d is a safe dose for Chinese patients; however, vitamin D allergy cannot be ignored (19). Overall, studies stress the importance of regular monitoring of calcium, 25OHD, and PTH, and determining the ideal doses of vitamin D3 and calcium supplementation post-LRYGB.
BAP, which is a tetrameric membrane glycoprotein derived from osteoblasts, is a biomarker of bone formation and bone turnover, and levels are increased in vitamin D deficiency (22,23). Bruno et al. showed that increased BAP 6 and 18 months after RYGB was correlated with decreased leptin levels (24). High BAP levels indicate bone loss, and low calcium levels in bone activates osteoblasts, which causes an imbalance between osteoblasts and osteoclasts and results in osteoporosis and a high risk of fracture. Although BAP was in the normal range in our patients, with no significant increase, we saw a gradual increase when comparing pre- and postoperative levels. Because we screened only 16 patients at 6 months post-LRYGB, BAP change was not statistically significant (P>0.05) (Figure 2). Increasing PTH and BAP indicate that patients may have vitamin D deficiency, which emphasizes the importance of full supplementation post-LRYGB.
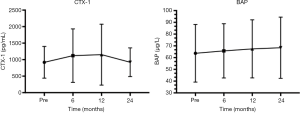
CTX-1 is a bone resorption marker and a degradation product of type I collagen. CTX-1 levels increase rapidly in women with osteoporosis, indicating that osteoclast activity is significantly enhanced (25). CTX-1 in our 16 patients increased rapidly in the first 6 months after surgery, and this finding is associated with a greater risk of fracture. However, in the second-year post-LRYGB, CTX-1 decreased, although the change was not significant (P>0.05). We believe this nonsignificant change resulted from the supplementation regime we used in this study; however, the sample size was small, which may have affected the result.
Unfortunately, BMD and other related bone turnover markers are not measured routinely and remain to be added to regular sequential examinations in our surgical center. Decreased BMD and increased bone turnover markers reflect bone loss (4); however, the relationship between indices remains undefined.
There are several limitations in our study. One of the limitations is the retrospective design, which can introduce selection bias and involve incomplete data collection. Bias can be decreased with a secondary propensity-matched analysis, but uncaptured or unknown factors may affect the outcomes. We also did not compare different levels of supplementation. Second, our sample size of 16 patients was too small to confirm our BAP and CTX-1 results, and BMD and other bone turnover markers are not measured routinely. Finally, our follow-up duration was short. Despite these limitations, our results emphasize the importance of investigating bone metabolism in Chinese obese patients after LRYGB.
Conclusions
LRYGB benefits patients with obesity and type 2 diabetes mellitus. Achieving and maintaining weight loss is an important benefit of bariatric surgery. However, postoperative stable and insufficient 25OHD levels, decreased serum calcium levels, and increasing PTH levels necessitate supplementation to prevent bone loss. Long-term-follow-up and vitamin D and calcium supplementation is important and beneficial, nutritionally. Future directions for research include determining ideal vitamin D and calcium supplementation doses, and adding bone index evaluations as part of routine examinations may help clarify why LRYGB can lead to bone loss, and whether other bariatric methods share the same mechanism. This information may provide a better understanding of the relationship between bone metabolism and bariatric surgery.
Acknowledgments
Funding: The current study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The human research review board at Shanghai 6th People’s Hospital approved the study, and all patients provided written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hu C, Jia W. Diabetes in China: Epidemiology and Genetic Risk Factors and Their Clinical Utility in Personalized Medication. Diabetes 2018;67:3-11. [Crossref] [PubMed]
- Kleinman NL, Melkonian A, Borden St, et al. The impact of morbid obesity and bariatric surgery on comorbid conditions: a comprehensive examination of comorbidities in an employed population. J Occup Environ Med 2009;51:170-9. [Crossref] [PubMed]
- Zhang P, Zhang H, Han X, et al. Effectiveness and safety of laparoscopic Roux-en-Y gastric bypass for the treatment of type 2 diabetes mellitus. Experimental and therapeutic medicine 2016;11:827-31. [Crossref] [PubMed]
- Hage MP, El-Hajj Fuleihan G. Bone and mineral metabolism in patients undergoing Roux-en-Y gastric bypass. Osteoporos Int 2014;25:423-39. [Crossref] [PubMed]
- Russel SM, Valle V, Spagni G, et al. Physiologic Mechanisms of Type II Diabetes Mellitus Remission Following Bariatric Surgery: a Meta-analysis and Clinical Implications. J Gastrointest Surg 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Sánchez-Alcoholado L, Gutiérrez-Repiso C, Gómez-Pérez AM, et al. Gut microbiota adaptation after weight loss by Roux-en-Y gastric bypass or sleeve gastrectomy bariatric surgeries. Surg Obes Relat Dis 2019;15:1888-95. [Crossref] [PubMed]
- de Vasconcelos RS, Viegas M, Marques TF, et al. Factors associated with secondary hyperparathyroidism in premenopausal women undergoing roux-en-Y gastric bypass for the treatment of obesity. Arq Bras Endocrinol Metabol Brazil 54:233-8.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Endocrine S: Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911-30. [Crossref] [PubMed]
- Wamberg L, Pedersen SB, Rejnmark L, et al. Causes of Vitamin D Deficiency and Effect of Vitamin D Supplementation on Metabolic Complications in Obesity: a Review. Curr Obes Rep 2015;4:429-40. [Crossref] [PubMed]
- Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72:690-3. [Crossref] [PubMed]
- Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg 2004;8:48-55; discussion 54-45.
- Mechanick JI, Youdim A, Jones DB, et al. Clinical Practice Guidelines for the Perioperative Nutritional, Metabolic, and Nonsurgical Support of the Bariatric Surgery Patient - 2013 Update: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Endocr Pract 2013;19:337-72. [Crossref] [PubMed]
- Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 2001;22:477-501. [Crossref] [PubMed]
- Sinha N, Shieh A, Stein EM, et al. Increased PTH and 1.25(OH)2D Levels Associated With Increased Markers of Bone Turnover Following Bariatric Surgery. Obesity (Silver Spring) 2011;19:2388-93. [Crossref] [PubMed]
- Hong W, Zhu HM, Cheng Q, et al. Relationship between serum vitamin D level and bone metabolism with 1389 cases analysis. Chinese Journal of Osteoporosis and Bone Mineral Research 2011;4:224-31.
- Yu EW, Bouxsein ML, Putman MS, et al. Two-Year Changes in Bone Density After Roux-en-Y Gastric Bypass Surgery. J Clin Endocrinol Metab 2015;100:1452-9. [Crossref] [PubMed]
- Yu EW. Bone metabolism after bariatric surgery. J Bone Miner Res 2014;29:1507-18. [Crossref] [PubMed]
- Riedt CS, Brolin RE, Sherrell RM, et al. True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 2006;14:1940-8. [Crossref] [PubMed]
- Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab 2008;93:3735-40. [Crossref] [PubMed]
- Qi QJ, Cai P, Yan QK, et al. Different doses vitamin D to bone metabolism in obese persons after Roux-en-Y gastric bypass. J Reg Anat and Ope Surg 2016;25:116-9.
- Mechanick JI, Youdim A, Jones DB, et al. Clinical Practice Guidelines for the Perioperative Nutritional, Metabolic, and Nonsurgical Support of the Bariatric Surgery Patient—2013 Update: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis 2013;9:159-91. [Crossref] [PubMed]
- Trull, AK, Demers, LM, Holt, DW, et al. Biomarkers of disease: an evidence-based approach. Cambridge, UK: Cambridge University Press; 2002:115-21.
- Lim SM, Kim YN, Park KH, et al. Bone alkaline phosphatase as a surrogate marker of bone metastasis in gastric cancer patients. BMC Cancer 2016;16:385. [Crossref] [PubMed]
- Bruno C, Fulford AD, Potts JR, et al. Serum Markers of Bone Turnover Are Increased at Six and 18 Months after Roux-En-Y Bariatric Surgery: Correlation with the Reduction in Leptin. J Clin Endocrinol Metab 2010;95:159-66. [Crossref] [PubMed]
- Pi YZ, Wu XP, Liu SP, et al. Age-related changes in bone biochemical markers and their relationship with bone mineral density in normal Chinese women. J Bone Miner Metab 2006;24:380-5. [Crossref] [PubMed]


