Type 2 diabetes mellitus does not increase the risk of multiple myeloma: a systematic review and meta-analysis
Introduction
Multiple myeloma (MM) is a malignant disease featuring plasma cell proliferation and accounting for about 15% of lymphoid system tumors (1). In recent years, the incidence of MM has increased significantly, making MM the second commonest hematological disease. MM occurs in 1.6% of all cancer cases and about 10% of blood malignancy cases in the United States. In 2015, America estimated 28,850 new cases and more than 11,000 MM-led deaths. Age and race may be risk factors of MM. Over 85% of MM cases are over 65 years, and the incidence in the blacks is twice of that in the whites (2). With new drugs like bortezomib, thalidomide, lenalidomide, as well as autologous stem cell transplantation (ASCT), the median survival period of MM patients has been greatly improved, but there is no doubt that MM relapses in those showing drug resistance (3). Therefore, it is necessary to explore other risk factors that cause the increase in MM incidence.
Recent studies have shown a worldwide increase in the prevalence of diabetes. Of the 400 million people with diabetes worldwide, 85–95% suffer from type 2 diabetes mellitus (T2DM) (4-6). For a long time, there has been speculation about the possible link between T2DM and the risk of cancer, because some cancers are diagnosed more frequently in diabetics than in non-diabetics (7). A number of original studies and meta-analyses have shown that T2DM is associated with an increased risk of cancers in the pancreas, liver, endometrium, breast, colorectum, and bladder, and a decreased risk of prostate cancer (8).
However, the association between T2DM and MM incidence remains controversial (9-12). Therefore, we conducted this meta-analysis to resolve this question.
Methods
Study design and eligibility criteria
Instructed by professional researchers and following the guidelines for the meta-analysis of Observational Studies in Epidemiology (MOOSE), two investigators searched the related database independently (13). In PubMed, Cochrane Library (https://www.cochranelibrary.com), and EMBASE databases, the following keywords were searched: diabetes, type 2 diabetes mellitus, and myeloma (the latest search on February 28, 2019) (See appendix for more details about the search procedures). The reference lists were checked to identify relevant studies. All studies that met the inclusion criteria (provided below) were subjected to our meta-analysis.
The authors (Zhang and Sha) screened publications using the following inclusion criteria: (I) evaluated and clearly defined exposure to T2DM; (II) reported the risk of MM incidence; (III) the type of study was case-control or cohort; (IV) the risk score was reported as hazard ratio (HR), incidence risk ratio, rate ratio (RR), odds ratio (OR), and 95% confidence interval (CI), or the article provided sufficient information to allow the calculation of OR and 95% CI. If multiple publications were generated by the same study, only the most recent was selected. Any disagreement between the two authors was resolved by a third researcher through efficient discussion.
Excluded were studies (I) including patients not diagnosed with T2DM; (II) duplicates within and between the databases; (III) written in reviews, viewpoints, or reports; and (IV) showing insufficient data.
Data extraction
Data were extracted by two investigators (Zhang and Sha), and the differences were reviewed by a third researcher (Liu) and resolved by consensus. An qualified article included the name of the first author, publishing date, country, study period, study type [cohort (CO) or case-control (CC)], sample size (case group and control group), number of cases, OR, 95% CI, adjusted variables, and confounders in the multivariate analysis (see Tables 1,S1,S2). If necessary, letters were given to the first authors to acquire the detailed data.
Table 1
| Reference | Design | Cases | Controls | Summary of findings |
|---|---|---|---|---|
| Atchison et al. (9) | CO | 594,815 | 3,906,763 | Increased risk of MM in patients with DM |
| Boursi et al. (10) | CC | 138 | 746 | Decreased risk of MM in patients with DM |
| Carstensen et al. (14) | CO | 56,889 | 4,579,016 | Increased risk of MM in patients with DM |
| Dankner et al. (11) | CO | 159,104 | 1,618,849 | Increased risk of MM in patients with DM |
| Fortuny et al. (15) | CC | 84 | 590 | Increased risk of MM in patients with DM |
| Gini et al. (16) | CO | 32,247 | 46,735 | No association between MM and DM |
| Harding et al. (12) | CO | 872,706 | 20,295,817 | Increased risk of MM in patients with DM |
| Khan et al. (17) | CO | 3,307 | 53,574 | No association between MM and DM |
| Khan et al. (18) | CO | 11,139 | 382,338 | No association between MM and DM |
| Liu et al. (19) | CO | 380,196 | 495,625 | Increased risk of MM in patients with DM |
| La Vecchia et al. (20) | CC | 1,067 | 16,758 | No association between MM and DM |
| Vineis et al. (21) | CC | 175 | 4,212 | Increased risk of MM in patients with DM |
| Wotton et al. (22) | CC | 23,629 | 460,687 | No association between MM and DM |
CO, cohort; CC, case-control; MM, multiple myeloma; DM, diabetes mellitus.
Quality assessment
The methodological scientificity of the included studies was assessed independently by two researchers using the nine-item Newcastle-Ottawa scale (NOS) (http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm) (23). The scale was used for non-randomized case-control and cohort studies, with a maximum of 9. The quality of the included literature was determined by assessing three features: group selection (four questions), comparability (one question), exposure, and outcomes for study participants (three questions). All questions scored 1, except for the comparability between the study groups. In terms of study comparability, a study with a rigorous design or a good control of significant confounding factors was scored separately (maximum 2 points). Any conflicting judgement was resolved by consensus. We considered articles scoring ≥7 points as high-quality; the others were regarded as low-quality.
Statistical analysis
We evaluated the data heterogeneity using the Cochrane Q test and I2, and P<0.1 for the Q statistic or I2>75% suggested high heterogeneity (24,25). If significant heterogeneity existed, the random-effects model (DerSimonian-Laird method) was applied; otherwise, a fixed-effects model (Mantel-Haenszel method) was utilized (26). In each round of sensitivity analysis, one study was excluded to test its impact on the stability of the overall results. Publication bias was evaluated by the Begg’s funnel plot and Egger’s linear regression test with a funnel plot. If P<0.05, it indicated the existence of publication bias (27). Data were analyzed using Stata version 12.0 software (StataCorp LP, College Station, TX, USA), and P value <0.05 was regarded as statistically significant.
Results
Characteristics and quality of included studies
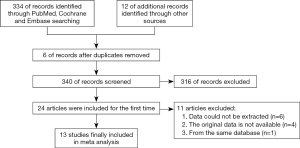
A total of 334 pieces of literature were found after the literature search. The searching processes (including reasons for exclusion) are listed in Figure 1. All included studies were written in English. In the end, 13 studies were deemed suitable and selected for meta-analysis, including 5 case-control studies and 8 cohort studies, with a total of 2,135,496 case groups and 31,861,710 control groups (9-12,14-22). The main characteristics of the included studies are shown in Table 1 (see Tables S1 and S2 for relevant information). Of the 13 studies, seven showed an increased risk of MM in patients with T2DM; one showed the opposite relationship; the remaining five showed no significant association. Three reviewers reached an agreement in scoring study quality, including 6 points for 3 articles, 7 points for 7 articles, and 8 points for 3 articles (see Tables 2 and 3 for details). Hence, 10 studies were of high quality, and 3 were of low quality.
Table 2
| Study (reference) | Exposed populations represent or to some extent represent communities | The non-exposed and exposed groups came from the same population | Ascertainment of exposure(secure record or structured interview) | Demonstration that outcome of interest was not present at start of study | The exposed and non-exposed groups were matched and/or adjusted for factors | Outcome defined with independent validation | Follow-up long enough for outcomes to occur | The loss of follow-up rate is within the range of bias | Overall score |
|---|---|---|---|---|---|---|---|---|---|
| Atchison et al. (9) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Carstensen et al. (14) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Dankner et al. (11) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Gini et al. (16) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Harding et al. (12) | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 6 |
| Khan et al. (18) | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 7 |
| Khan et al. (17) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 6 |
| Liu et al. (19) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
A study could be awarded a maximum of one star for each item except for the item control for important factor or additional factor. The definition/explanation of each column of the Newcastle Ottawa Quality Assessment Scale is available from (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). One means study adequately fulfilled a quality criterion (two for cohort fully matched and adjusted), 0 means it did not. Quality scale does not imply that items are of equal relevant importance
Table 3
| Study (reference) | Case defined with independent validation | Representativeness of the cases | Selection of controls from community | Statement that controls have no history of outcome | Cases and controls matched and/or adjusted by factors | Ascertain exposure by blinded structured interview | Same method of ascertainment for cases and controls | Same response rate for both groups | Overall score |
|---|---|---|---|---|---|---|---|---|---|
| Boursi et al. (10) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Fortuny et al. (15) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| La Vecchia et al. (20) | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 6 |
| Vineis et al. (21) | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 7 |
| Wotton et al. (22) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
A study could be awarded a maximum of one star for each item except for the item control for important factor or additional factor. The definition/explanation of each column of the Newcastle Ottawa Quality Assessment Scale is available from (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). One means study adequately fulfilled a quality criterion (two for cohort fully matched and adjusted), 0 means it did not. Quality scale does not imply that items are of equal relevant importance.
Quality control
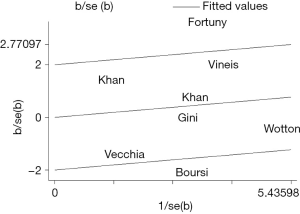
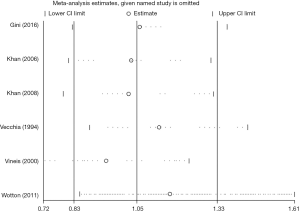
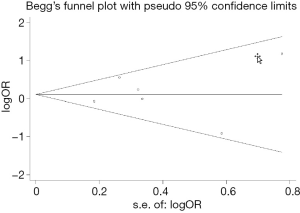
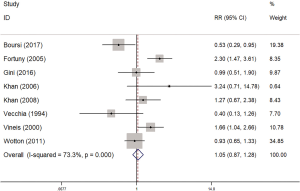
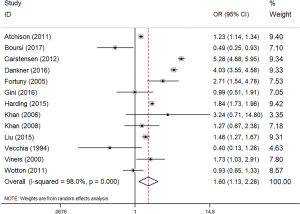
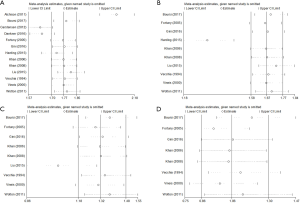
Overall, the risk of myeloma was higher in patients with diabetes (OR =1.60, 95% CI: 1.13–2.26, I2=98%, P=0.000) (Figure 2). As shown in Figure 2, I2 value was 98%, indicating a high heterogeneity in the data of the included studies. So, a random-effects model was adopted for statistical analysis. First, through the first round of sensitivity analysis, we excluded three studies with high heterogeneity (9,11,14). The remaining studies showed a high heterogeneity (I2=81.5%, greater than 75%). Therefore, we conducted a second sensitivity analysis and excluded another study with high heterogeneity (12). I2 dropped to 71.5%, indicating a moderate heterogeneity. After the third round of sensitive analysis, a study with a greater impact on heterogeneity was further eliminated (19). In the end, eight studies were left for meta-analysis (Figure 3), but I2 rose to 73.3% (Figure S1). Next, Galbraith heterogeneity map was used to further detect the possible sources of heterogeneity (Figure 4). Finally, six studies were included in this meta-analysis, because none of them significantly influenced the pooled OR and 95% CIs. Therefore, our results were expected to be comparatively reliable and stable (Figure 5). The six studies were submitted to a fixed-effects model.
Association between diabetes and MM
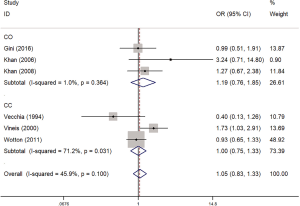
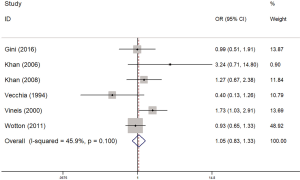
As shown in Figure 6, the incidence of concomitant myeloma in diabetic patients was only 1.05 times higher than that of non-diabetic patients (OR =1.05, 95% CI: 0.83–1.33, I2=45.9%, P=0.100), with no significant difference. A subgroup analysis found that the risk of myeloma detected by case-control studies was higher than that detected by the cohort studies (OR =1.19, 95% CI: 0.76–1.85, I2=1%, P=0.364; OR =1.00, 95% CI: 0.75–1.33, I2=71.2%, P=0.031, respectively) (Figure 7), but this difference was not significant.
Sensitivity analysis and publication bias
The sensitivity analysis was implemented to evaluate the contribution of every study to the polled estimation. Seven studies were omitted, and the pooled OR was reevaluated based on the remaining studies that showed a low heterogeneity (Figures 2,6). Begg’s and Egger’s tests were utilized to evaluate the publication bias and the funnel plots were displayed in Figure 8. In the pooled analysis of OR, the P values of Begg’s and Egger’s tests were all above 0.05, indicating that no publication bias existed in this study and our findings were statistically robust.
Discussion
Since 1980, the age-standardized prevalence of diabetes has increased by little or remained unchanged among adults in each country. But the number of diabetic adults has grown by four folds as the global population explodes (28). Diabetes frequently arises from immune and hormonal abnormalities (29). Malignant tumors are associated with T2DM-favoring conditions (30). Therefore, more and more researchers are concerned about whether patients with T2DM are confronted with a higher risk of second cancer. Previously, Castillo et al. conducted a meta-analysis in which adjusted OR value was used to evaluate the relationship between T2DM and the risk of non-Hodgkin’s lymphoma, leukemia and myeloma (31). Up to now, no independent study has been conducted to clarify the relationship between T2DM and MM. Based on the data from previous studies, our meta-analysis found that T2DM is not a risk factor of MM. Although only six studies were included in this analysis, their inner consistency made our findings reliable.
Many factors, like congenital genetic defects and controllable environmental assaults, can increase the risk of MM. T2DM is more common in people aged over 40, especially those obese. Obesity is a risk factor for MM. To a certain extent, patients with T2DM should have a higher incidence of MM. But we found that T2DM does not serve as a risk factor for myeloma. We hypothesize that this may be related to the use of hypoglycemic drugs.
As we all know, metformin is the first choice for T2DM. A population-based observational study showed that patients who used single metformin presented a lower incidence of cancer (5.4% vs. 8.1%) than those who used other hypoglycemic drugs (16). Long-term use of metformin also lowers the morbidity and mortality of cancers in patients with diabetes (32). At the same time, metformin can prolong the progress of monoclonal gammopathy of undetermined significance (MGUS) to MM to a certain extent (10). Besides, metformin can curb the transition of MGUS to MM (33). The reason may be that metformin activates the AMPK pathway, inhibits gluconeogenesis, and induces glucose into muscle cells, all helpful to reduce circulating glucose and insulin (34). Cancer cells usually require high levels of glucose uptake, but metformin can reduce circulating glucose (35). At the same time, metformin can lead to weight loss (36). All these mechanisms are beneficial to reduce the risk of MM in patients with T2DM.
However, in addition to metformin, many other hypoglycemic drugs are also commonly used for T2DM. Insulin and insulin-like growth factors promote tumor development by stimulating epithelial cell proliferation (37,38). The use of insulin is more common in patients with poor blood glucose control or compliance. Sulfonylureas increases circulating insulin level, probably resulting in cancer. Hsieh et al. showed that metformin reduced the risk of cancer by 25% compared to sulfonylureas (39). These differences in the relationship between drugs and cancer risk mean that we need to be cautious with our findings.
Studies have shown that the incidence of MM varies with race, with Asians showing a lower incidence than Caucasians (40). Since the beginning of 2007, genome-wide association studies (GWAS) have found more than 70 susceptible loci of T2DM, such as KCNJ11, TCF7L2, IRS1, MTNR1B, PPARG2, IGF2BP2, CDKN2A, HHEX, FTO, etc. (41). A recent study explored the relationship between common T2DM-related variants and the risk of MM, demonstrating that T2DM-related variant genes KCNQ1, CDKM2A-2B, IGF-1, and MADD were linked to the increase in MM incidence, and that KCNJ11, THADA, LTA, and FTO genes were associated with the decrease in MM incidence. These genes may influence the risk of MM through non-insulin-related mechanisms (42). Therefore, for newly diagnosed T2DM, relevant genetic testing may be required.
MM risk in the case-control studies was significantly lower than that in the cohort studies, presumably due to the error caused by the relatively small number of cases and recall bias. After all, some patients have a vague definition of diabetes, and they do not consider that an increase in blood glucose without drug control (which has reached the diagnostic criteria for diabetes and is temporarily regulated by diet and exercise) also supports the diagnosis of diabetes. These biases could increase or decrease the risk of parallel occurrence of diabetes and myeloma.
In this study, some limitations might exist. Firstly, our search was limited to published studies (with unpublished or ongoing studies not included), and the articles published in English. Secondly, the study included people from different regions, the gender distribution was uneven among the study groups, and the observation time for diabetes was not consistent. Thirdly, data were missing during the extraction of MM patients. All these limitations should be overcome in future better-designed studies.
Conclusions
This meta-analysis showed that T2DM is not a risk factor of MM. More large-scale, prospective randomized controlled studies are needed to verify the association between the two.
Table S1
| Author | Year | Country | Study design | Study period | Age (year) | DM assessment | MM assessment | Source of cohort | Total N [N of case] | Adjusted variables | OR | 95% CI | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atchison et al. (9) | 2011 | US | CO | 1969–1996 | 18–100 | Hospital discharge records | hospital records and Social Security Administration mortality files | Veterans Affairs Hospital | 4,501,578 [4,641] | Age, year, race, number of hospital visits | 1.23 | 1.14–1.34 | 8 |
| Carstensen et al. (14) | 2012 | Danish | CO | 1995– 2009 | >30 | The National Diabetes Register | The Danish Cancer Registry | Entire Danish population | 4,635,905 [4,618] | Age, year, sex | 5.28 | 4.68–5.95 | 7 |
| Dankner et al. (11) | 2016 | Israelis | CO | 2002–2012 | 21–89 | Meet the 6 criteria in the paper | The Israel National Cancer Registry | The largest health maintenance organization | 1,777,953 [1,167] | Age, ethnicity, and socioeconomic status | 4.03 | 3.55–4.58 | 7 |
| Gini et al. (16) | 2016 | Northern Italy | CO | 2002–2009 | 40–84 | Meet the 3 criteria in the paper | Cancer registry | Population-based | 32,247 [15] | Age, sex | 0.99 | 0.51–1.91 | 7 |
| Harding et al. (12) | 2015 | Australian | CO | 1997–2008 | >30 | The National Diabetes Services Scheme | Australian Cancer Database | The National Diabetes Services Schem | 872,706 [1,013] | Age, sex, year | 1.84 | 1.73–1.96 | 6 |
| Khan et al. (18) | 2006 | Japan | CO | 1988–1997 | 40–79 | Self-administered questionnaire | Population-based and hospital-based cancer registries | The Japan Collaborative Cohort (JACC) Study | 56,881 [12] | Age, BMI, smoking, drinking | 3.24 | 0.71–14.80 | 7 |
| Khan et al. (17) | 2008 | European | CO | 1992–2000 | >30 | Self-reported | Histologically confirmed | 23 study centers in ten European countries | 393,477 [281] | Age, sex, region | 1.27 | 0.67–2.38 | 6 |
| Liu et al. (19) | 2015 | Sweden | CO | 1964–2010 | >39 | Swedish Hospital Discharge Register, the Outpatient Register and the primary health care in Stockholm and Skåne County | The nationwide Swedish Cancer Register | Several national Swedish registers | 380,196 [432] | Age, sex, year, region, socioeconomic status | 1.46 | 1.27–1.67 | 8 |
Table S2
| Author | Year | Country | Study design | Study period | DM assessment | MM assessment | Source of case, N | Source of control, N | Adjusted variables | OR | 95% CI | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boursi et al. (10) | 2017 | UK | CC | 1995–2013 | General practitioners diagnosed | READ codes | The Health Improvement Network, 138 | The health improvement network, 746 | Age, sex, year | 0.49 | 0.25–0.93 | 7 |
| Fortuny et al. (15) | 2005 | Spain | CC | 1998–2002 | Personal interviews | Histology, immunohistochemistry test and flow cytometry | At four centres in Spain, 84 | At four centres in Spain, 590 | Age, gender, study centre | 2.71 | 1.54–4.78 | 7 |
| La Vecchia et al. (20) | 1994 | Northern Italy | CC | 1983–1992 | Self-administered questionnaire | The National Cancer Institute | Greater Milan area, 1,067 | Greater Milan area, 16,758 | Age, sex, education, smoking, BMI | 0.40 | 0.13–1.26 | 6 |
| Vineis et al. (21) | 2000 | Italy | CC | 1990–1993 | Personal interviews | Cancer registry and hospital records | 11 Italian areas, 175 | 11 Italian areas, 4,212 | Age, sex, centre | 1.73 | 1.03–2.91 | 7 |
| Wotton et al. (22) | 2011 | UK | CC | 1993–2008 | Records from National Health Service hospital and ORLS | Records from National Health Service hospital and ORLS | Oxford Record Linkage Study (ORLS), 23,629 | Oxford Record Linkage Study (ORLS), 460,687 | Age, sex, calendar year of first recorded admission and district of residence | 0.93 | 0.65–1.33 | 8 |
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.36). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thordardottir M, Lindqvist EK, Lund SH, et al. Obesity and risk of monoclonal gammopathy of undetermined significance and progression to multiple myeloma: a population-based study. Blood Adv 2017;1:2186-92. [Crossref] [PubMed]
- Michels TC, Petersen KE. Multiple Myeloma: Diagnosis and Treatment. Am Fam Physician 2017;95:373-83. [PubMed]
- Uckun FM, Qazi S, Demirer T, et al. Contemporary patient-tailored treatment strategies against high risk and relapsed or refractory multiple myeloma. EBioMedicine 2019;39:612-20. [Crossref] [PubMed]
- Selvin E, Parrinello CM, Sacks DB, et al. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med 2014;160:517-25. [Crossref] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-858. [Crossref] [PubMed]
- Bragg F, Holmes MV, Iona A, et al. Association Between Diabetes and Cause-Specific Mortality in Rural and Urban Areas of China. JAMA 2017;317:280-9. [Crossref] [PubMed]
- Chowdhury TA, Jacob P. Challenges in the management of people with diabetes and cancer. Diabet Med 2019;36:795-802. [Crossref] [PubMed]
- Noto H. Unfolding link between diabetes and cancer. J Diabetes Investig 2017; [Epub ahead of print]. [PubMed]
- Atchison EA, Gridley G, Carreon JD, et al. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer 2011;128:635-43. [Crossref] [PubMed]
- Boursi B, Mamtani R, Yang YX, et al. Impact of metformin on the progression of MGUS to multiple myeloma. Leuk Lymphoma 2017;58:1265-7. [Crossref] [PubMed]
- Dankner R, Boffetta P, Balicer RD, et al. Time-Dependent Risk of Cancer After a Diabetes Diagnosis in a Cohort of 2.3 Million Adults. Am J Epidemiol 2016;183:1098-106. [Crossref] [PubMed]
- Harding JL, Shaw JE, Peeters A, et al. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care 2015;38:264-70. [Crossref] [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [Crossref] [PubMed]
- Carstensen B, Witte DR, Friis S. Cancer occurrence in Danish diabetic patients: duration and insulin effects. Diabetologia 2012;55:948-58. [Crossref] [PubMed]
- Fortuny J, Benavente Y, Bosch R, et al. Type 2 diabetes mellitus, its treatment and risk for lymphoma. Eur J Cancer 2005;41:1782-7. [Crossref] [PubMed]
- Gini A, Bidoli E, Zanier L, et al. Cancer among patients with type 2 diabetes mellitus: A population-based cohort study in northeastern Italy. Cancer Epidemiol 2016;41:80-7. [Crossref] [PubMed]
- Khan AE, Gallo V, Linseisen J, et al. Diabetes and the risk of non-Hodgkin's lymphoma and multiple myeloma in the European Prospective Investigation into Cancer and Nutrition. Haematologica 2008;93:842-50. [Crossref] [PubMed]
- Khan M, Mori M, Fujino Y, et al. Site-specific cancer risk due to diabetes mellitus history: evidence from the Japan Collaborative Cohort (JACC) Study. Asian Pac J Cancer Prev 2006;7:253-9. [PubMed]
- Liu X, Hemminki K, Försti A, et al. Cancer risk in patients with type 2 diabetes mellitus and their relatives. Int J Cancer 2015;137:903-10. [Crossref] [PubMed]
- La Vecchia C, Negri E, Franceschi S, et al. A case-control study of diabetes mellitus and cancer risk. Br J Cancer 1994;70:950-3. [Crossref] [PubMed]
- Vineis P, Crosignani P, Sacerdote C, et al. Haematopoietic cancer and medical history: a multicentre case control study. J Epidemiol Community Health 2000;54:431-6. [Crossref] [PubMed]
- Wotton CJ, Yeates DG, Goldacre MJ. Cancer in patients admitted to hospital with diabetes mellitus aged 30 years and over: record linkage studies. Diabetologia 2011;54:527-34. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820-6. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45:139-45. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513-30. [Crossref] [PubMed]
- Vaiserman A, Lushchak O. Developmental origins of type 2 diabetes: Focus on epigenetics. Ageing Res Rev 2019;55:100957. [Crossref] [PubMed]
- Wojciechowska J, Krajewski W, Bolanowski M, et al. Diabetes and Cancer: a Review of Current Knowledge. Exp Clin Endocrinol Diabetes 2016;124:263-75. [Crossref] [PubMed]
- Castillo JJ, Mull N, Reagan JL, et al. Increased incidence of non-Hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: a meta-analysis of observational studies. Blood 2012;119:4845-50. [Crossref] [PubMed]
- Campbell JM, Bellman SM, Stephenson MD, et al. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res Rev 2017;40:31-44. [Crossref] [PubMed]
- Willrich MA, Katzmann JA. Laboratory testing requirements for diagnosis and follow-up of multiple myeloma and related plasma cell dyscrasias. Clin Chem Lab Med 2016;54:907-19. [Crossref] [PubMed]
- Duca FA, Côté CD, Rasmussen BA, et al. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med 2015;21:506-11. [Crossref] [PubMed]
- Pernicova I, Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol 2014;10:143-56. [Crossref] [PubMed]
- Ejtahed HS, Tito RY, Siadat SD, et al. Metformin induces weight loss associated with gut microbiota alteration in non-diabetic obese women: a randomized double-blind clinical trial. Eur J Endocrinol 2018; [Epub ahead of print]. [PubMed]
- Pryor R, Cabreiro F. Repurposing metformin: an old drug with new tricks in its binding pockets. Biochem J 2015;471:307-22. [Crossref] [PubMed]
- Baxter RC. IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer 2014;14:329-41. [Crossref] [PubMed]
- Hsieh MC, Lee TC, Cheng SM, et al. The influence of type 2 diabetes and glucose-lowering therapies on cancer risk in the Taiwanese. Exp Diabetes Res 2012;2012:413782. [Crossref] [PubMed]
- Lu J, Lee JH, Huang SY, et al. Continuous treatment with lenalidomide and low-dose dexamethasone in transplant-ineligible patients with newly diagnosed multiple myeloma in Asia: subanalysis of the FIRST trial. Br J Haematol 2017;176:743-9. [Crossref] [PubMed]
- Ng MC, Shriner D, Chen BH, et al. Meta-analysis of genome-wide association studies in African Americans provides insights into the genetic architecture of type 2 diabetes. PLoS Genet 2014;10:e1004517. [Crossref] [PubMed]
- Ríos R, Lupiañez CB, Campa D, et al. Type 2 diabetes-related variants influence the risk of developing multiple myeloma: results from the IMMEnSE consortium. Endocr Relat Cancer 2015;22:545-59. [Crossref] [PubMed]