
Prognostic significance of serum carcinoembryonic antigen and squamous cell carcinoma antigen in patients with esophageal squamous cell carcinoma undergoing radical esophagectomy
Introduction
Esophageal cancer (EC) is the sixth most common cancer and the fourth leading cause of cancer death in China (1). Epidemiological studies show that the incidence rate of adenocarcinoma is on the rise, and has become the most common subtype of EC in western countries, especially in white patients. On the other hand, squamous cell carcinoma (SCC) comprises approximately 90% of the EC cases in China (2). Esophageal carcinoma has been demonstrated to be one of the most aggressive malignancies and often have a poor prognosis. Although great progress in surgical and oncological treatments have been made, the 5-year survival remains low. Therefore, it is essential to identify reliable prognostic indicators to aid in better treatment recommendations and improve prognosis. The prognostic factors of most gastrointestinal tumors include tumor size, lymph node (LN) metastasis and distant metastasis (3). However, conventional examinations such as computed tomography (CT) or endoscopic ultrasonography have limited role in early detection of microscopic lymph node metastases. Therefore, it is essential to investigate other diagnostic and prognostic markers (4).
Serum tumor markers are biological or biochemical substances produced by abnormal tumor cells or stimulated by tumor cells. They are widely used in tumor diagnosis and prognosis prediction. For esophageal squamous cell carcinoma (ESCC) patients, preoperative tumor markers are closely related to tumor invasion depth, lymph node metastasis and prognosis (5,6). Carcinoembryonic antigen (CEA), squamous cell carcinoma antigen (SCC-Ag), cytokeratin 19 fragments (Cyfra21-1), and CA19-9 are commonly measured in EC patients. Serum CEA is one of the most widely expressed tumor markers in cancer cells, which is used for preoperative evaluation of colorectal cancer and gastric cancer patients (7,8). Extraction and purification of SCC-Ag from uterine SCC for the first time (9), and SCC-Ag level can predict SCC of cervix and esophagus (10). Cyfra21-1 is a sensitive biomarker in malignant disease, especially in squamous cell carcinoma (11), and CA19-9 is particularly elevated in gastrointestinal cancer (12).
CEA and SCC-Ag are common indicators for diagnosis and follow-up of EC, but the sensitivity of biochemical indicators is still not ideal (13,14). Bagaria et al. showed that the combined detection of CEA and CA19-9 was more efficient than single tumor marker in the diagnosis of EC and gastric cancer (8). In this study, the serum CEA and SCC-Ag levels of ESCC patients before operation were measured to clarify its clinical significance as a prognostic factor and its role in guiding treatment.
Methods
Study population, data collection and participant follow-up
From February 2009 to October 2012, 1281 patients with EC underwent therapeutic esophagectomy in Sun Yat-sen University Cancer Center. The exclusion criteria were as follows: (I) patients with tumors of histological subtypes of EC other than ESCC; (II) patients who underwent the sweet approach or for whom the number of removed lymph nodes (RLNs) was less than 15; (III) patients with a history of malignant disease or other primary cancer; (IV) patients with missing data or lost follow-up (exclusion criteria arranged by the investigator). The final study population consisted of 348 patients.
The hematological and biochemical profiles of each ESCC patient were evaluated before surgery. Radioimmunoassay (RIA) was used to detect serum CEA and SCC-Ag levels in our clinical laboratory. The normal upper limits of CEA and SCC-Ag were 5.00 and 1.5 ng/mL respectively. The cutoff values of CEA and SCC-Ag were determined by receiver operating characteristic (ROC) curve and Youden index. The cutoff value for CEA was 2.28 ng/mL, while the cutoff value for SCC-Ag was 0.75 ng/mL. According to the TNM classification of the eighth edition of UICC and AJCC, the pathological stage and LN involvement were evaluated (15).
After operation, it is recommended to follow up once every 3 months in the first 2 years, once every 6 months in the next 3 years, and once a year later. We observed patients from diagnosis to death or to November 2018, including reviewing the clinical attendance records or telephone interviews with the patient or their family. The average follow-up time was 43.0 months (range, 1–93 months).
Statistical analysis
We used χ2 test to compare the classified variables. The continuous variables were compared by variance (ANOVA). Kaplan Meier method was used to draw survival curve and log rank test was used to evaluate the difference of survival rate between the two groups. Cox Regression Analysis is used in multivariate analysis to determine important prognostic factors. The endpoint of the study was OS. All calculations were carried out using SPSS 17.0 software (SPSS, Chicago, IL) and R (version 3.3.0; http://www.rproject.org). P value less than 0.05 was considered significant.
Results
Patient characteristics and OS
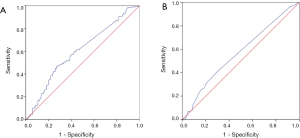
A total of 348 patients were included in the analysis. Preoperative peripheral venous blood of the patients was extracted. CEA and SCC-Ag levels in peripheral venous blood were detected by ELISA. The reagent was purchased from CanAg Company of Sweden. The determination procedure was carried out according to the operation instructions strictly. According to ROC curve and Youden index (Youden index = sensitivity + specificity-1), the cut-off value of CEA was 2.28 ng/mL, and that of SCC-Ag is 0.75 ng/mL (Figure 1). In addition, the area under the curve of CEA is 0.600 (95% CI: 0.541–0.660; P=0.001; Figure 1A), and that of SCC-Ag is 0.567 (95% CI: 0.507–0.628; P=0.030; Figure 1B). According to the critical value, patients were divided into two groups to determine the effect of CEA and SCC-Ag on prognosis.
The relationship between patient demographics and the cutoff value of CEA and SCC-Ag is summarized in Table 1. In general, there were significant differences in TNM stage, gender, surgical complications and prognosis among patients with different CEA levels. Preoperative SCC-Ag level was significantly correlated with T (tumor) stage, preoperative radiotherapy (CRT) and prognosis (P<0.05) (Table 1). However, there was no significant correlation between serum CEA, SCC-Ag and other clinical characteristics (P>0.05) (Table 1). At the time of this analysis, the median OS was 65.0 months (95% CI: 50.4–79.6 months). In addition, the survival time of patients with high and low serum CEA and SCC-Ag was analyzed and compared. For serum levels of CEA ≤2.28 ng/mL, the median OS was 85.0 months, whereas for patients’ serum levels of CEA >2.28 ng/mL, the median OS was 39.0 months. Similarly, the median OS of patients with serum SCC-Ag ≤0.75 ng/mL was 71.0 months, while that of patients with serum SCC-Ag >0.75 ng/mL was 43.0 months.
Table 1
| Demographics | The level of CEA | The level of SCC-Ag | |||||
|---|---|---|---|---|---|---|---|
| ≤2.28 ng/mL | >2.28 ng/mL | P | ≤0.75 ng/mL | >0.75 ng/mL | P | ||
| Number (n) | 219 | 129 | 260 | 88 | |||
| Age (years), n (%) | 0.575 | 0.115 | |||||
| ≤60 | 128 (58.4) | 71 (55.0) | 154 (59.2) | 45 (51.1) | |||
| >60 | 91 (41.6) | 58 (45.0) | 106 (40.8) | 43 (48.9) | |||
| Gender, n (%) | 0.014 | 0.129 | |||||
| Male | 164 (74.9) | 111 (86.0) | 200 (76.9) | 75 (85.2) | |||
| Female | 55 (25.1) | 18 (14.0) | 60 (23.1) | 13 (14.8) | |||
| T stage, n (%) | 0.080 | 0.014 | |||||
| T0 | 18 (8.2) | 2 (1.6) | 18 (6.9) | 2 (2.3) | |||
| T1 | 26 (11.9) | 15 (11.6) | 37 (14.2) | 4 (4.5) | |||
| T2 | 44 (20.1) | 28 (21.7) | 55 (21.2) | 17 (19.3) | |||
| T3 | 131 (59.8) | 84 (65.1) | 150 (57.7) | 65 (73.9) | |||
| N stage, n (%) | 0.025 | 0.118 | |||||
| N0 | 113 (51.6) | 60 (46.5) | 139 (53.5) | 34 (38.6) | |||
| N1 | 65 (29.7) | 28 (21.7) | 65 (25.0) | 28 (31.8) | |||
| N2 | 33 (15.1) | 29 (22.5) | 42 (16.2) | 20 (22.7) | |||
| N3 | 8 (3.7) | 12 (9.3) | 14 (5.4) | 6 (6.8) | |||
| Grade, n (%) | 0.103 | 0.383 | |||||
| Gx | 20 (9.1) | 3 (2.3) | 20 (7.7) | 3 (3.4) | |||
| G1 | 29 (13.2) | 18 (14.0) | 32 (12.3) | 15 (17.0) | |||
| G2 | 104 (47.5) | 68 (52.7) | 130 (50.0) | 42 (47.7) | |||
| G3 | 66 (30.1) | 40 (31.0) | 78 (30.0) | 28 (31.8) | |||
| TNM stage, n (%) | 0.011 | 0.080 | |||||
| 0 | 18 (8.2) | 2 (1.6) | 18 (6.9) | 2 (2.3) | |||
| I | 11 (5.0) | 3 (2.3) | 14 (5.4) | 0 (0) | |||
| II | 67 (30.6) | 36 (27.9) | 73 (28.1) | 30 (34.1) | |||
| III | 115 (52.5) | 76 (58.9) | 141 (54.2) | 50 (56.8) | |||
| IV | 8 (3.7) | 12 (9.3) | 14 (5.4) | 6 (6.8) | |||
| Location, n (%) | 0.810 | 0.545 | |||||
| Upper third | 43 (19.6) | 23 (17.8) | 50 (19.2) | 16 (16.2) | |||
| Middle third | 100 (45.7) | 57 (44.2) | 113 (43.5) | 44 (50.0) | |||
| Lower third | 76 (34.7) | 49 (38.0) | 97 (37.3) | 28 (31.8) | |||
| Surgical approach, n (%) | 0.868 | 0.714 | |||||
| Ivor-Lewis | 27 (12.3) | 17 (13.2) | 32 (12.3) | 12 (13.6) | |||
| 3-incision | 192 (87.7) | 112 (86.8) | 228 (87.7) | 76 (86.4) | |||
| Complications, n (%) | 0.044 | 0.502 | |||||
| No | 169 (77.2) | 86 (66.7) | 190 (73.1) | 65 (73.9) | |||
| Yes | 50 (22.8) | 43 (33.3) | 70 (26.9) | 23 (26.1) | |||
| Neoadjuvant therapy, n (%) | 0.283 | 0.006 | |||||
| No | 182 (83.1) | 113 (87.6) | 213 (81.9) | 82 (93.2) | |||
| Yes | 37 (16.9) | 16 (12.4) | 47 (18.1) | 6 (6.8) | |||
| Postoperative CRT, n (%) | 0.907 | 0.225 | |||||
| No | 142 (64.8) | 85 (65.9) | 173 (66.5) | 54 (61.4) | |||
| Yes | 77 (35.2) | 44 (34.1) | 87 (33.5) | 34 (38.6) | |||
| Prognosis, n (%) | <0.001 | 0.036 | |||||
| Alive | 124 (56.6) | 46 (35.7) | 136 (52.3) | 34 (38.6) | |||
| Death | 95 (43.4) | 83 (64.3) | 124 (47.7) | 54 (61.4) | |||
CEA, carcinoembryonic antigen; SCC-Ag, squamous cell carcinoma antigen; CRT, chemoradiation therapy; Gx, uncertain differentiation; G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated.
Preoperative serum levels of CEA and SCC-Ag and ESCC patient survival
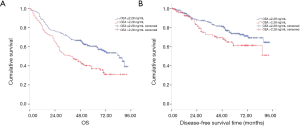
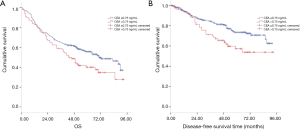
During the follow-up period, 87 cases recurred in 348 cases and 178 died of all causes. According to Kaplan Meier survival curve, patients with CEA ≤2.28 ng/mL had better OS and DFS than those with CEA >2.28 ng/mL (log rank =16.09, P<0.001; log rank =4.11, P=0.043; Figure 2A,B, respectively). Meanwhile, patients with serum SCC-Ag ≤0.75 ng/mL had more favorable OS and DFS than those with SCC-Ag >0.75 ng/mL (log rank =5.23, P=0.022; log rank =6.71, P=0.010; Figure 3A,B).
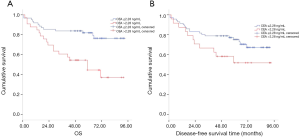
We conducted Cox regression analysis to assess whether serum CEA and SCC-Ag levels were associated with survival and recurrence after adjustment for potential confounders. Single factor analysis showed that serum CEA, SCC-Ag, N-phase and postoperative CRT levels were significantly correlated with OS (all P<0.05) (Table S1). Univariate analysis also showed that gender, the serum level of CEA and SCC-Ag, surgical approaches, N stage, and postoperative CRT were significantly associated with DFS (all P<0.05) (Table S1). After adjusting for the confounding covariates, multivariate Cox regression analysis showed that patients with serum levels of CEA >2.28 ng/mL had higher overall mortalities compared to those of patients with serum levels of CEA ≤2.28 ng/mL (HR 1.76; 95% CI: 1.39–2.39; P<0.001). In addition, higher T stage, N metastasis, and complications were independent factors associated with poor survival (Table 2). Similarly, multivariate analysis showed that SCC-Ag >0.75 ng/mL was an independent prognostic factor (HR 1.86; 95% CI: 1.17–2.96; P=0.009), while postoperative CRT was an independent prognostic factor (HR 0.60; 95% CI: 0.36-0.82; P<0.001) (Table 2). We further investigated the relationship between preoperative serum CEA and SCC-Ag levels and survival in patients with pT3N0M0 ESCC who did not receive adjuvant therapy. Kaplan–Meier analysis demonstrated that pT3N0M0 patients with a serum level of CEA >2.28 ng/mL had a poor outcome compared to that in patients with CEA ≤2.28 ng/mL (log rank =11.56, P=0.001; Figure 4A), while the relationship between pT3N0M0 patient prognosis and the serum level of SCC-Ag was not statistically significant (log rank =2.92, P=0.087; Figure 4B).
Table 2
| Variables | OS | DFS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Cutoff of CEA | |||||||
| ≤2.28 ng/mL | 1 | 1 | |||||
| >2.28 ng/mL | 1.76 | 1.30–2.39 | <0.001 | 1.47 | 0.92–2.36 | 0.109 | |
| Cutoff of SCC-Ag | |||||||
| ≤0.75 ng/mL | 1 | 1 | |||||
| >0.75 ng/mL | 1.70 | 0.81–1.61 | 0.444 | 1.86 | 1.17–2.96 | 0.009 | |
| T stage | |||||||
| T0 | 1 | 1 | |||||
| T1 | 0.35 | 0.16–0.81 | 0.013 | 0.08 | 0.02–0.96 | 0.047 | |
| T2 | 0.55 | 0.27–1.13 | 0.103 | 0.23 | 0.02–2.41 | 0.219 | |
| T3 | 0.60 | 0.31–1.67 | 0.131 | 0.22 | 0.02–2.25 | 0.200 | |
| N stage | |||||||
| N0 | 1 | 1 | |||||
| N1 | 2.14 | 1.46–3.14 | <0.001 | 2.07 | 0.18–23.45 | 0.556 | |
| N2 | 3.31 | 2.25–4.89 | <0.001 | 4.72 | 0.45–49.16 | 0.194 | |
| N3 | 5.60 | 3.20–9.79 | <0.001 | 3.37 | 1.97–34.64 | 0.306 | |
| Surgical approach | |||||||
| Ivor-Lewis | 1 | 1 | |||||
| 3-incision | 1.03 | 0.66–1.63 | 0.885 | 0.75 | 0.89–1.75 | 0.075 | |
| Complications | |||||||
| No | 1 | 1 | |||||
| Yes | 1.54 | 1.20–2.16 | 0.013 | 0.73 | 0.40–1.31 | 0.283 | |
| Neoadjuvant therapy | |||||||
| No | 1 | 1 | |||||
| Yes | 0.73 | 0.61–1.58 | 0.460 | 0.68 | 0.48–1.22 | 0.089 | |
| Postoperative CRT | |||||||
| No | 1 | 1 | |||||
| Yes | 0.85 | 0.78–2.04 | 0.350 | 0.98 | 0.56–2.77 | 0.595 | |
HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen; SCC-Ag, squamous cell carcinoma antigen; CRT, chemoradiation therapy.
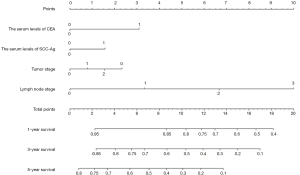
The nomogram constructed for predicting the prognosis is depicted in Figure 5. The model consisted of the serum level of CEA and SCC-Ag, T stage and N stage. The predicted C index of Harrell survival rate was 0.70. The calibration curves showed a good agreement between the probability of the survival rate by a nomogram and the ideal probability (Figure S1). As shown in the model, the survival rate of ESCC patients with high preoperative serum CEA and SCC levels may be relatively low.
Discussion
The aim of this research was to investigate the relationship between CEA, SCC-Ag and the prognosis of ESCC. A ROC curve was applied to verify the cutoff point of CEA and SCC-Ag for OS prediction Our study identified values of 2.28 ng/mL as the optimum cutoff point for CEA while 0.75 ng/mL as the optimum cutoff point for SCC-Ag, in predicting prognosis in patients with ESCC.
CEA is one of the most widely used and easily obtained tumor markers in gastrointestinal cancer, especially colorectal cancer (7,8,16,17). In addition, detecting the level of serum tumor markers is a routine preoperative examination for ESCC patients. Due to the relatively low sensitivity and specificity of CEA and SCC-Ag to ESCC, the specific function of these tumor markers in ESCC patients is not clear clinically. Our research showed that serum CEA >2.28 ng/mL was an independent risk factor for poor OS, and SCC-Ag >0.75 ng/mL was an independent negative prognostic factor for DFS. Lee et al. reported that patients with CEA levels >10 ng/mL had higher rates of lymph node involvement and deeper tumor infiltration than other patients (7). Mihmanli et al. found that the elevation of CEA level was related to the depth of invasion and pathological stage (16). In addition, Munck-Wikland et al. reported that the increase of CEA level is related to the distant metastasis of EC, which may reflect the metastasis potential of EC cells (17). Therefore, an elevated level of preoperative CEA served as a poor prognostic marker for ESCC patients after surgical resection. In addition, it should be noted that regular postoperative monitoring of CEA may help to identify patients with metastatic diseases after surgical treatment. A previous study reported that CEA had a higher positivity rate for ESCC than that of cytokeratin-18 and CA19-9, and the authors believe that CEA level is helpful for the detection of postoperative recurrence of gastric cancer and EC and the evaluation of therapeutic response (18).
CEA is an acidic glycoprotein found in thin films of colon and rectal cancer and in embryonic mucosa cells. It can be widely found in the digestive system cancer of endodermal origin, in the digestive tract tissue of normal embryo, and in the serum of normal human. SCC-Ag is an important tumor marker of squamous cell carcinoma. It is separated from cervical squamous cell carcinoma and exists in the cytoplasm of squamous cell carcinoma. When the cells undergo malignant transformation and tumor growth, the synthesis or expression of these antigens is significantly increased due to the de inhibition of the corresponding coding genes. Secondly, genes that were not expressed were reactivated during tumorigenesis. Thirdly, abnormal glycosylation results in special degradation products of protein. What’s more, some aspects of antigen synthesis are abnormal, such as labnormal ectopic expression of embryo antigen or differentiation antigen. In addition, overexpression of gene products, especially signaling molecules leads to tumor antigen elevation (19,20). Based on these factors, serum CEA and SCC-Ag were increased in ESCC patients. Because CEA and SCC-Ag are considered to reflect systemic inflammatory response (SIR) on the basis of hypercytokinemia caused by tumor host interaction, the prognosis of CEA and SCC-Ag may be significantly different (21).
Previous studies have reported that elevated CEA and SCC-Ag levels are independent predictors of poor OS in EC patients, and the best cutoff time is uncertain (6,22,23). Ma et al. found that serum SCC-Ag, CEA, CA19-9 were significantly correlated with lymph node metastasis. According to the normal upper limit at their institute, they stated that the cutoff for CEA was 5 µg/L while the cutoff for SCC-Ag was 1.5 µg/L (24). Similarly, Oki et al. defined the cut-off value of CEA as 5 ng/mL because it is the upper limit of normal level (22). Cao et al. suggested that high preoperative levels of CYFRA21-1 and SCC-Ag have a negative impact on the survival of stage II ESCC patients, and those authors also defined the cut off as the normal upper limit of 1.5 µg/L for SCC-Ag (6). Zhang et al. reported that CEA level is an independent predictor of the prognosis of ESCC patients treated with CRT at the same time. According to 95% CIs of cancer-free patients in China, the critical value of CEA is 3.3 ng/mL (23). Huang et al. constructed a prognostic model that combined C-reactive protein and CEA, which proved to be a more precise prognostic factor in ESCC patients. These authors concluded that the optimum cutoff point for CEA was 4.2 ng/mL for predicting cancer- specific survival (CSS) as verified by ROC curves, and the AUC was 0.618 for CEA (25). In clinical practice, we found that ESCC patients with early and medial stages of the disease usually present with serum levels of CEA and SCC-Ag in the normal range. Therefore, according to ROC analysis, the critical values of CEA and SCC-Ag are 2.28 and 0.75 ng/mL, respectively, which are lower than previous studies. We reasoned that we did not defined the cutoff as the normal upper limit, as most of the patients included in our study were in the early and medial stages of the disease and were primarily eligible for surgical treatment, which justifies the lower cutoff values.
The relationship between serum SCC-Ag concentration and patient survival was also discussed earlier (5,26,27). Mroczko et al. found that SCC-Ag and CEA had no significant effect on the prognosis of ESCC (27). However, Kosugi et al. found that a high level of serum SCC-Ag before esophagectomy predicted a negative prognosis in patients after esophagectomy. They found a significant correlation between SCC-Ag and tumor invasion depth, lymph node status, TNM stage and vascular invasion (5). Shimada et al. obtained similar results, in which high preoperative SCC-Ag concentration is an important prognostic factor (28). SCC-Ag production has been proven to be enhanced by epidermal growth factor in cervical cancer cells, suggesting that high SCC levels may be involved in elevated tumor proliferative ability (29). Additionally, as an inhibitory serpin, SCC-Ag may function as a regulator of the differentiation of normal squamous epithelium or as an inhibitor of apoptosis in cancer tissue (30).
Considering that there are controversies about whether pT3N0M0 ESCC patients should undergo adjuvant treatment after surgery (31), we further analyzed the association between the serum levels of CEA and prognosis for pT3N0M0 ESCC patients who did not receive adjuvant therapy. We found that pT3N0M0 patients with preoperative serum level of CEA >2.28 ng/mL had a poor prognosis compared to that patients with lower levels of CEA. Therefore, we recommended that pT3N0M0 patients with CEA >2.28 ng/mL preoperatively should receive adjuvant treatment even after radical esophagectomy. We further constructed a nomogram using clinical parameters of T stage, N stage and preoperative serum CEA and SCC-Ag levels. The values “0” and “1” represent values below and above the cutoff, respectively. We evaluate an acceptable calibration curve by looking at the nomograph prediction probability versus actual probability (Figure S1), and a concordance index (c-index) of 0.70 was obtained. These results suggested that our nomogram has an acceptable predictive accuracy (31). This nomogram may contribute to clinical decision-making in terms of postoperative treatment. According to the nomogram, higher serum levels of CEA have a more prominent effect on patient outcome than SCC-Ag, and ESCC patients with high serum levels of both CEA and SCC may suffer a relatively low survival rate. For instance, a patient with pT3N0M0 staging and levels of both CEA and SCC-Ag that are higher than the cutoff (value =1) would score 6.8 total points and have a 5-year survival rate of only approximately 50% (Figure 5). Adjuvant treatment would be recommended for patients with this profile to improve survival even if lymph node involvement was not detected.
There were 53 patients who received neoadjuvant CRT in the cohort. Antitumor therapy may have an effect on the concentration of related tumor markers, only the serum CEA and SCC-Ag levels after treatment are included in the analysis. We found that neoadjuvant CRT could lower the serum levels of tumor markers to some extent, especially for CEA. However, the results were not presented in detail because of the limited size. We reasoned that neoadjuvant therapy plus surgery could serve as a protective factor for OS in EC patients (32), which may be related to the reduced tumor markers.
Inaccurate LN dissection and pathological evaluation may result in inappropriate pathologic nodal staging and treatment, a phenomenon called stage migration (33). In order to reduce the possibility of non-standard lymphadenectomy leading to incorrect pathological staging, our study only included patients who received the Ivor Lewis approach (44 cases) and the three-incision approach (304 cases). According to NCCN guidelines for the treatment of EC (in 2016), at least 15 lymph nodes should be removed during radical resection of EC (34). In the current study, patients with RLNs <15 were excluded to ensure accurate pN staging and radical resection. This is the first study to adopt these inclusion criteria and discuss the relationship between tumor marker level and prognosis.
The strength of the current study is that we studied ESCC patients from a single institution using relatively standard surgical techniques to ensure accurate pathological staging and the radical resection. The representative sample and the considerably high follow-up rate guarantee the reliability of our results. Nevertheless, there are several limitations that should be considered. First, this is a single center retrospective analysis, which requires a larger cohort of prospective studies to confirm our results. Second, we excluded patients of other histological types. Therefore, caution is needed in extrapolating our findings to these patients. In addition, we included patients who received primary resection and preoperative CRT. However, there were few subjects in the preoperative CRT group. A subgroup analysis was not performed.
Conclusions
In conclusion, we demonstrate that high levels of preoperative serum CEA and SCC-Ag are independent and significant predictors of postoperative ESCC. Higher serum levels of CEA had a more prominent effect on patient outcome than SCC-Ag, and ESCC patients with high serum levels of both CEA and SCC may suffer a relatively low survival rate, which may help clinicians to decide on the adjuvant treatment after surgery. Future prospective studies with larger populations are needed to validate our findings and to explore the potential mechanisms underlying the prognostic value of tumor markers.
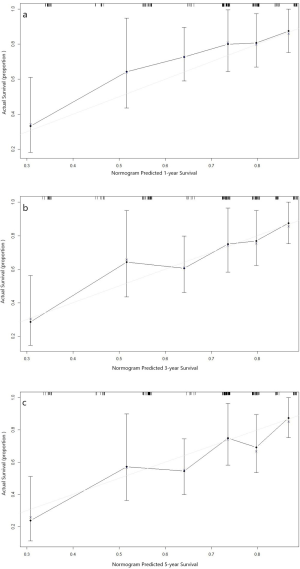
Table S1
| Variables | OS | DFS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Cutoff of CEA | |||||||
| ≤2.28 ng/mL | 1 | 1 | |||||
| >2.28 ng/mL | 1.81 | 1.35–2.43 | <0.001 | 1.55 | 1.01-2.37 | 0.045 | |
| Cutoff of SCC-Ag | |||||||
| ≤0.75 ng/mL | 1 | 1 | |||||
| >0.75 ng/mL | 1.45 | 1.05–1.99 | 0.024 | 1.78 | 1.14-2.77 | 0.011 | |
| Age (years) | |||||||
| ≤60 | 1 | 1 | |||||
| >60 | 1.27 | 0.94–1.70 | 0.115 | 0.66 | 0.42-1.03 | 0.069 | |
| Gender | |||||||
| Female | 1 | 1 | |||||
| Male | 1.40 | 0.94–2.07 | 0.094 | 1.71 | 0.98-3.33 | 0.057 | |
| T stage | |||||||
| T0 | 1 | 1 | |||||
| T1 | 0.60 | 0.27–1.40 | 0.213 | 0.35 | 0.10-1.22 | 0.100 | |
| T2 | 1.04 | 0.52–2.07 | 0.923 | 0.83 | 0.31-2.25 | 0.711 | |
| T3 | 1.07 | 0.56–2.04 | 0.841 | 1.05 | 0.42-2.62 | 0.916 | |
| N stage | |||||||
| N0 | 1 | 1 | |||||
| N1 | 1.90 | 1.30–2.76 | 0.001 | 2.16 | 1.29-3.63 | 0.004 | |
| N2 | 3.36 | 2.29–4.93 | <0.001 | 3.41 | 1.96-5.95 | <0.001 | |
| N3 | 5.19 | 3.05–8.83 | <0.001 | 4.52 | 1.97-10.36 | <0.001 | |
| Grade | |||||||
| G0 | 1 | 1 | |||||
| G1 | 0.76 | 0.36–1.59 | 0.463 | 0.64 | 0.21-1.95 | 0.428 | |
| G2 | 1.17 | 0.62–2.18 | 0.633 | 1.23 | 0.49-3.10 | 0.659 | |
| G3 | 1.07 | 0.56–2.04 | 0.845 | 1.15 | 0.44-2.98 | 0.777 | |
| Location | |||||||
| Upper third | 1 | 1 | |||||
| Middle third | 1.07 | 0.72–1.59 | 0.740 | 1.01 | 0.58-1.77 | 0.965 | |
| Lower third | 0.97 | 0.64–1.46 | 0.866 | 0.87 | 0.48-1.57 | 0.647 | |
| Surgical approach | |||||||
| Ivor-Lewis | 1 | 1 | |||||
| 3-incision | 1.09 | 0.70–1.71 | 0.669 | 1.32 | 0.81-2.87 | 0.233 | |
| Complications | |||||||
| No | 1 | 1 | |||||
| Yes | 1.26 | 0.91–1.74 | 0.161 | 0.79 | 0.47-1.32 | 0.360 | |
| Neoadjuvant therapy | |||||||
| No | 1 | 1 | |||||
| Yes | 1.34 | 0.90–1.99 | 0.144 | 1.22 | 0.68-2.21 | 0.503 | |
| Postoperative CRT | |||||||
| No | 1 | 1 | |||||
| Yes | 0.74 | 0.91–2.34 | 0.129 | 0.96 | 0.42-2.86 | 0.669 | |
HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen; SCC-Ag, squamous cell carcinoma antigen; CRT, chemoradiation therapy; Gx, uncertain differentiation; G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated.
Acknowledgments
We would also like to thank all investigators helped in data collection and analysis. We are also grateful to all who reviewed and commented on an early draft of the paper.
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.20). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from all patients. This study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center (approval number: GZR 2018-120). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China, 2013. Cancer Lett 2017;401:63-71. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Sperduto PW, Fang P, Li J, et al. Survival and prognostic factors in patients with gastrointestinal cancers and brain metastases: have we made progress? Transl Res 2019;208:63-72. [Crossref] [PubMed]
- Lindenmann J, Fink-Neuboeck N, Koesslbacher M, et al. The influence of elevated levels of C-reactive protein and hypoalbuminemia on survival in patients with advanced inoperable esophageal cancer undergoing palliative treatment. J Surg Oncol 2014;110:645-50. [Crossref] [PubMed]
- Kosugi S, Nishimaki T, Kanda T, et al. Clinical significance of serum carcinoembryonic antigen, carbohydrate antigen 19-9, and squamous cell carcinoma antigen levels in esophageal cancer patients. World J Surg 2004;28:680-5. [Crossref] [PubMed]
- Cao X, Zhang L, Feng GR, et al. Preoperative Cyfra21-1 and SCC-Ag serum titers predict survival in patients with stage II esophageal squamous cell carcinoma. J Transl Med 2012;10:197. [Crossref] [PubMed]
- Lee JC, Lee SY, Kim CY, et al. Clinical utility of tumor marker cutoff ratio and a combination scoring system of preoperative carcinoembryonic antigen, carbohydrate antigen 19-9, carbohydrate antigen 72-4 levels in gastric cancer. J Korean Surg Soc 2013;85:283-9. [Crossref] [PubMed]
- Bagaria B, Sood S, Sharma R, et al. Comparative study of CEA and CA19-9 in esophageal, gastric and colon cancers individually and in combination (ROC curve analysis). Cancer Biol Med 2013;10:148-57. [PubMed]
- Huang EY, Huang YJ, Chanchien CC, et al. Pretreatment carcinoembryonic antigen level is a risk factor for para-aortic lymph node recurrence in addition to squamous cell carcinoma antigen following definitive concurrent chemoradiotherapy for squamous cell carcinoma of the uterine cervix. Radiat Oncol 2012;7:13. [Crossref] [PubMed]
- Kubik S, Moszynska-Zielinska M, Fijuth J, et al. Assessment of the relationship between serum squamous cell carcinoma antigen (SCC-Ag) concentration in patients with locally advanced squamous cell carcinoma of the uterine cervix and the risk of relapse. Prz Menopauzalny 2019;18:23-6. [Crossref] [PubMed]
- Kanaji N, Kadota K, Tadokoro A, et al. Serum CYFRA 21-1 but not Vimentin is Associated with Poor Prognosis in Advanced Lung Cancer Patients. Open Respir Med J 2019;13:31-7. [Crossref] [PubMed]
- Polat E, Duman U, Duman M, et al. Diagnostic value of preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 in colorectal cancer. Curr Oncol 2014;21:e1-7. [Crossref] [PubMed]
- Guillem P, Triboulet JP. Elevated serum levels of C-reactive protein are indicative of a poor prognosis in patients with esophageal cancer. Dis Esophagus 2005;18:146-50. [Crossref] [PubMed]
- Lukaszewicz-Zajac M, Mroczko B, Kozlowski M, et al. Comparative evaluation of serum C-reactive protein (CRP) levels in the different histological subtypes of esophageal cancer (squamous cell carcinoma and adenocarcinoma of esophagus). J Clin Lab Anal 2012;26:73-81. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Hofstetter WL, et al. Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus 2016;29:897-905.
- Mihmanli M, Dilege E, Demir U, et al. The use of tumor markers as predictors of prognosis in gastric cancer. Hepatogastroenterology 2004;51:1544-7. [PubMed]
- Munck-Wikland E, Kuylenstierna R, Lindholm J, et al. Carcinoembryonic antigen, CA 19-9 and CA 50 in monitoring human squamous cell carcinoma of the esophagus. Anticancer Res 1990;10:703-8. [PubMed]
- Shu J, Li CG, Liu YC, et al. Comparison of serum tumor associated material (TAM) with conventional biomarkers in cancer patients. Asian Pac J Cancer Prev 2012;13:2399-403. [Crossref] [PubMed]
- Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature 2001;411:380-4. [Crossref] [PubMed]
- Vence L, Palucka AK, Fay JW, et al. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc Natl Acad Sci U S A 2007;104:20884-9. [Crossref] [PubMed]
- Ma Q, Liu W, Jia R, et al. Inflammation-based prognostic system predicts postoperative survival of esophageal carcinoma patients with normal preoperative serum carcinoembryonic antigen and squamous cell carcinoma antigen levels. World J Surg Oncol 2016;14:141. [Crossref] [PubMed]
- Oki S, Toiyama Y, Okugawa Y, et al. Clinical burden of preoperative albumin-globulin ratio in esophageal cancer patients. Am J Surg 2017;214:891-8. [Crossref] [PubMed]
- Zhang HQ, Wang RB, Yan HJ, et al. Prognostic significance of CYFRA21-1, CEA and hemoglobin in patients with esophageal squamous cancer undergoing concurrent chemoradiotherapy. Asian Pac J Cancer Prev 2012;13:199-203. [Crossref] [PubMed]
- Ma Z, Wu X, Xu B, et al. Development of a novel biomarker model for predicting preoperative lymph node metastatic extent in esophageal squamous cell carcinoma. Oncotarget 2017;8:105790-9. [Crossref] [PubMed]
- Huang Y, Liu JS, Feng JF. The combination of preoperative serum C-reactive protein and carcinoembryonic antigen is a useful prognostic factor in patients with esophageal squamous cell carcinoma: a combined ROC analysis. Onco Targets Ther 2015;8:795-803. [Crossref] [PubMed]
- Yoon SM, Shin KH, Kim JY, et al. Use of serum squamous cell carcinoma antigen for follow-up monitoring of cervical cancer patients who were treated by concurrent chemoradiotherapy. Radiat Oncol 2010;5:78. [Crossref] [PubMed]
- Mroczko B, Kozlowski M, Groblewska M, et al. The diagnostic value of the measurement of matrix metalloproteinase 9 (MMP-9), squamous cell cancer antigen (SCC) and carcinoembryonic antigen (CEA) in the sera of esophageal cancer patients. Clin Chim Acta 2008;389:61-6. [Crossref] [PubMed]
- Shimada H, Nabeya Y, Okazumi S, et al. Prediction of survival with squamous cell carcinoma antigen in patients with resectable esophageal squamous cell carcinoma. Surgery 2003;133:486-94. [Crossref] [PubMed]
- Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest 2005;23:338-51. [Crossref] [PubMed]
- Fu J, Wang W, Wang Y, et al. The role of squamous cell carcinoma antigen (SCC Ag) in outcome prediction after concurrent chemoradiotherapy and treatment decisions for patients with cervical cancer. Radiat Oncol 2019;14:146. [Crossref] [PubMed]
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364-70. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. [Crossref] [PubMed]
- Twine CP, Lewis WG, Morgan MA, et al. The assessment of prognosis of surgically resected oesophageal cancer is dependent on the number of lymph nodes examined pathologically. Histopathology 2009;55:46-52. [Crossref] [PubMed]
- Li H, Fang W, Yu Z, et al. Chinese expert consensus on mediastinal lymph node dissection in esophagectomy for esophageal cancer (2017 edition). J Thorac Dis 2018;10:2481-9. [Crossref] [PubMed]


