
Preoperative lymphocyte-monocyte ratio is not an independent prognostic factor in M0 (stage I–III) esophageal squamous cell carcinomas
Introduction
Esophageal cancer is the fourth most frequently diagnosed cancer in China (1). Esophageal squamous cell carcinoma (ESCC) represents over 90 percent of esophageal carcinoma in high-risk regions (2). Although there has been considerable progress in the development of treatments and therapeutic interventions, including chemotherapy, radiation, and surgery, the prognosis for ESCC patients is still poor due to the high incidence of local and metastatic recurrence (3). ESCC patients generally have a 5-year overall survival (OS) rate of below 28% (4) and the clinical variables commonly used for outcome prediction are not precise. Therefore, the identification of novel prognostic markers may allow for improved risk stratification for patients with ESCC, which might contribute to the development of individualized treatment strategies.
Besides treatment- and patient-related factors, such as chemoradiotherapy and tumor stage, inflammation or innate immunity is causally related to the prognosis of cancers. Cancer-related inflammation plays a notable role in tumor development through inhibition of apoptosis, DNA damage, regulation of cytokines and inflammatory mediators, antitumor immunity and tumor angiogenesis (5). Inflammatory cells consist of a variety of leukocytes, including lymphocytes, neutrophils, macrophages, mast cells and dendritic cells. Recently, evidence has come to light indicating that inflammatory cells correlate with cancer prognosis in many malignancies. The neutrophil-lymphocyte ratio (NLR) in the circulation is an independent predictive factor for patients with renal cell carcinomas, gastric cancer, hepatocellular carcinomas and colorectal carcinomas (6). It has also been suggested that the peripheral PLR may be a prognostic marker in many malignancies (7). Nevertheless, the prognostic significance of the platelet-lymphocyte ratio (PLR) and NLR in ESCCs is contentious. Recently, the lymphocyte-monocyte ratio (LMR) was shown to be an inexpensive and easy prognostic marker to use in gastric cancer, pancreatic adenocarcinoma, colon cancer, soft tissue sarcoma, diffuse large B-cell lymphoma as well as classical Hodgkin’s lymphoma (cHL) (8). To date, only two clinical research studies have suggested that LMR acts as a prognostic marker in ESCCs (9,10).
In this study, a retrospective analysis of 178 patients was performed to determine whether the preoperative LMR, NLR, and PLR could potentially be used as prognostic biomarkers for ESCCs.
Methods
Study patients
We performed this retrospective study on 178 patients who underwent esophagectomy between April 2006 and December 2012 at the Department of Thoracic Surgery, Tongji Hospital of Huazhong University of Science and Technology. Patients who had historically proven ESCC and underwent R0 resection were recruited. The exclusion criteria were as follows: neoadjuvant treatment, distal metastasis, and perioperative death. The medical records of all subjects were retrospectively reviewed and the clinicopathological information was collected. The tumor stage was determined in accordance with the standard in AJCC cancer staging manual (7th edition, 2010). Patients received postoperative follow-up every 4 months for the first 2 years and the follow-up interval was 6 months afterward. The last follow-up was in May 2015. Basic clinical information and the results from the physical examinations were collected at all visits. Computed tomography, barium meal fluoroscopy, and tumor marker assays were used.
LMR, NLR and PLR assessment
A complete peripheral blood cell count was performed on all patients 1 week before surgery. NLR = absolute neutrophil count/absolute lymphocyte count. LMR = absolute lymphocyte count/absolute monocyte count. PLR = total platelet count/total lymphocyte count.
Statistical analysis
The endpoints were the OS and the disease-free survival (DFS), both measured in months. OS is the time from the surgery to the last follow-up visit or the death of the patient (all causes). DFS is the period after the operation that the patient was tumor-free. Spearman’s rank correlation coefficient was adopted in this study to analyze the relationships between NLR, PLR, and LMR. Pearson’s chi-square test was performed here to assess the correlation between LMR/NLR/PLR and clinicopathological parameters, and NLR, PLR, and LMR were categorized into two groups using the median. Kaplan-Meier curves were used to evaluate OS and DFS. For the analysis of the prognostic parameters with known demographic and clinical prognostic factors, Univariate and multivariate analysis by Cox regression models were used. The enter method was used. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. All data analysis was performed by SPSS (Version 16.0, SPSS Inc., Chicago, USA). P<0.05 was assigned to admit statistical significance.
Results
Patient characteristics
Of the 178 patients, there were 139 (78.1%) men and 39 (21.9%) women with a median age of 56 years (range, 38–76); 111 (62.4%) had smoked tobacco, 102 (57.3%) of patients had consumed alcohol, 34 (19.1%) were diagnosed with stage III disease and 72 (40.4%) were treated with adjuvant therapy.
The overall median of the absolute white blood cell count was 5.68 (2.51–12.84) ×109/L, 3.29 (0.7–7.11) ×109/L for the absolute neutrophil count, 1.73 (0.74–4.21) ×109/L for the absolute lymphocyte count, 0.43 (0.18–1.30) ×109/L for the absolute monocyte count, and 203 (87–434) ×109/L for the absolute platelet count.
LMR was negatively correlated with NLR (ρ=−0.578, P<0.001) and PLR (ρ=−0.513, P<0.001). NLR was positively correlated with PLR (ρ=0.528, P<0.001).
All ESCC patients were grouped by the median values of NLR, LMR, and PLR. Their clinical characteristics were shown (Table 1). We used the median count of NLR (1.89), PLR (118.91) and LMR (3.88) as cut-off values.
Table 1
| Characteristic | Subgroup | Patients, n (%) | NLR, n | PLR, n | LMR, n | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NLR <1.89 (n=87) | NLR ≥1.89 (n=91) | P value* | PLR <118.91 (n=89) | PLR ≥118.91 (n=89) | P value* | LMR <3.88 (n=89) | LMR ≥3.88 (n=89) | P value* | |||||
| Gender | Male | 139 (78.1) | 65 | 74 | 0.287 | 68 | 71 | 0.587 | 78 | 61 | 0.002 | ||
| Female | 39 (21.9) | 22 | 17 | 21 | 18 | 11 | 28 | ||||||
| Age, years | <56 | 81 (45.5) | 44 | 37 | 0.184 | 38 | 43 | 0.452 | 38 | 43 | 0.452 | ||
| ≥56 | 97 (54.5) | 43 | 54 | 51 | 46 | 51 | 46 | ||||||
| Tobacco smoking | Never | 67 (37.6) | 37 | 30 | 0.188 | 33 | 34 | 0.877 | 24 | 43 | 0.003 | ||
| Ever | 111 (62.4) | 50 | 61 | 56 | 55 | 65 | 46 | ||||||
| Alcohol drinking | Never | 76 (42.7) | 42 | 34 | 0.141 | 39 | 37 | 0.762 | 30 | 46 | 0.015 | ||
| Ever | 102 (57.3) | 45 | 57 | 50 | 52 | 59 | 43 | ||||||
| Tumor length, cm | ≤3 | 80 (44.9) | 46 | 34 | 0.038 | 43 | 37 | 0.366 | 40 | 40 | 1.000 | ||
| >3 | 98 (55.1) | 41 | 57 | 46 | 52 | 49 | 49 | ||||||
| Tumor location | Upper | 20 (11.2) | 11 | 9 | 0.704 | 8 | 12 | 0.562 | 10 | 10 | 0.892 | ||
| Middle | 73 (41.0) | 37 | 36 | 39 | 34 | 35 | 38 | ||||||
| Lower | 85 (47.8) | 39 | 46 | 42 | 43 | 44 | 41 | ||||||
| Differential degree | Well | 103 (57.9) | 49 | 54 | 0.919 | 55 | 48 | 0.541 | 51 | 52 | 0.760 | ||
| Middle | 65 (36.5) | 33 | 32 | 29 | 36 | 34 | 31 | ||||||
| Poor | 10 (5.6) | 5 | 5 | 5 | 5 | 4 | 6 | ||||||
| Tumor stage | I | 75 (42.1) | 42 | 33 | 0.198 | 44 | 31 | 0.106 | 36 | 39 | 0.882 | ||
| II | 69 (38.8) | 32 | 37 | 32 | 37 | 36 | 33 | ||||||
| III | 34 (19.1) | 13 | 21 | 13 | 21 | 17 | 17 | ||||||
| Adjuvant therapy | No | 106 (59.6) | 58 | 48 | 0.059 | 58 | 48 | 0.127 | 50 | 56 | 0.360 | ||
| Yes | 72 (40.4) | 29 | 43 | 31 | 41 | 39 | 33 | ||||||
*, difference between groups was tested by Chi-square test. ESCC, esophageal squamous cell carcinoma; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; LMR, lymphocyte-monocyte ratio.
According to the median value of NLR, 91 patients were assigned to the high NLR group (≥1.89). The tumor length between patients with NLR <1.89 and those with NLR ≥1.89 was significantly different (P=0.038). No statistical significance was observed in these two groups regarding sex, age, alcohol consumption, tobacco smoking, tumor location, tumor stage, differential degree, and adjuvant therapy.
Eighty-nine patients were assigned in the high PLR group (≥118.91). There was no statistical significance in sex, age, tobacco smoking, alcohol consumption, tumor location, length, stage, differential degree and adjuvant therapy between the two groups.
According to the median value of LMR, eighty-nine patients were separated into the high LMR group (≥3.88), while the others were assigned in the low LMR group. Data showed a significant difference regarding sex (P=0.002), tobacco smoking (P=0.003) and alcohol consumption (P=0.015) between the groups. No statistical significance was found in age, tumor location, length, stage, differential degree and adjuvant therapy between the two groups.
Survival and prognostic value of NLR, PLR, and LMR in ESCC patients
The median follow-up was 39 months (range, 3–88 months). During this period, a total of 63 (35.4%) patients died and 75 (42.1%) patients had tumor recurrence. For all patients, the median DFS and OS were 37 and 39 months, respectively. The median DFS and OS of patients with tumor recurrence were 16 and 24 months, respectively.
Kaplan-Meier analysis showed lower but not significant OS (mean 39.1 vs. 43.8 months, P=0.087) and DFS (mean 35.1 vs. 40.4 months, P=0.115) in patients with high NLRs (Figure 1). Marginal reductions in OS (mean 40.6 vs. 42.2 months, P=0.330) and DFS (mean 36.3 vs. 39.1 months, P=0.259) were observed in the high versus low PLR group, but these differences were not significant. A worse but not significant prognosis was shown in patients with low preoperative LMR for OS (mean 40.1 vs. 42.6 months, P=0.187) and DFS (mean 36.8 vs. 38.6 months, P=0.400) compared with those with a high LMR.
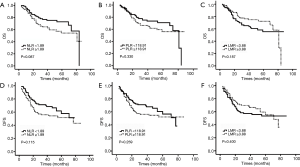
As demonstrated in the univariate analysis, tumor length (P=0.037), tumor stage, (P=0.024; P<0.001) and adjuvant therapy (P<0.001) were significantly associated with the OS of patients (Table 2). By running multivariate analysis, we found that only tumor stage was independently associated with unfavorable OS (HR =1.943, 95% CI: 1.020–3.704, P=0.043; HR =3.374, 95% CI: 1.712–6.650, P<0.001, respectively). Neither NLR (HR =1.097, 95% CI: 0.598–2.010, P=0.765), PLR (HR =0.910, 95% CI: 0.511–1.622, P=0.749) nor LMR (HR =0.733, 95% CI: 0.397–1.353, P=0.321) were related to the OS of ESCCs.
Table 2
| Characteristic | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Gender (female) | 0.613 (0.311–1.208) | 0.158 | – | – | |
| Age (≥56 years) | 0.922 (0.562–1.513) | 0.748 | – | – | |
| Tobacco smoking (ever) | 1.061 (0.633–1.779) | 0.823 | – | – | |
| Alcohol drinking (ever) | 1.135 (0.687–1.875) | 0.622 | – | – | |
| Tumor length (>3 cm) | 1.758 (1.036–2.984) | 0.037 | 1.392 (0.800–2.420) | 0.242 | |
| Tumor location | |||||
| Upper | 1.000 | – | – | – | |
| Middle | 1.925 (0.671–5.521) | 0.223 | – | – | |
| Lower | 2.257 (0.798–6.386) | 0.125 | – | – | |
| Differential degree | |||||
| Well | 1.000 | – | – | – | |
| Middle | 1.154 (0.684–1.947) | 0.590 | – | – | |
| Poor | 0.992 (0.304–3.233) | 0.989 | – | – | |
| Tumor stage | |||||
| I | 1.000 | – | 1.000 | – | |
| II | 2.070 (1.100–3.893) | 0.024 | 1.943 (1.020–3.704) | 0.043 | |
| III | 3.770 (1.977–7.192) | <0.001 | 3.374 (1.712–6.650) | <0.001 | |
| Adjuvant therapy (yes) | 3.425 (2.026–5.790) | <0.001 | – | – | |
| NLR ≥1.89 | 1.549 (0.933–2.572) | 0.091 | 1.097 (0.598–2.010) | 0.765 | |
| PLR ≥118.91 | 1.279 (0.777–2.105) | 0.333 | 0.910 (0.511–1.622) | 0.749 | |
| LMR ≥3.88 | 0.716 (0.434–1.182) | 0.192 | 0.733 (0.397–1.353) | 0.321 | |
Note: adjuvant therapy was not included in the multivariable analysis, because it depends on the tumor stage. NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; LMR, lymphocyte-monocyte ratio; CI, confidence interval.
Tumor length (P=0.046), tumor stage (P=0.031; P<0.001) and adjuvant therapy (P<0.001) were found to be significantly associated with DFS (Table 3). In multivariate analysis, only tumor stage was independently associated with unfavorable DFS (HR =1.734, 95% CI: 0.972–3.904, P=0.062; HR =3.467, 95% CI: 1.876–6.438, P<0.001, respectively). Similar analyses of NLR (HR =1.065, 95% CI: 0.620–1.828, P=0.820), PLR (HR =1.023, 95% CI: 0.607–1.726, P=0.932) and LMR (HR =0.850, 95% CI: 0.491–1.473, P=0.562) showed no associations with DFS.
Table 3
| Characteristic | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Gender (female) | 0.718 (0.401–1.284) | 0.264 | – | – | |
| Age (≥56 years) | 0.805 (0.511–1.267) | 0.348 | – | – | |
| Tobacco smoking (ever) | 1.142 (0.712–1.831) | 0.583 | – | – | |
| Alcohol drinking (ever) | 0.966 (0.612–1.524) | 0.881 | – | – | |
| Tumor length (>3 cm) | 1.618 (1.009–2.594) | 0.046 | 1.284 (0.785–2.099) | 0.320 | |
| Tumor location | |||||
| Upper | 1.000 | – | – | – | |
| Middle | 1.663 (0.696–3.977) | 0.253 | – | – | |
| Lower | 1.533 (0.645–3.641) | 0.333 | – | – | |
| Differential degree | |||||
| Well | 1.000 | – | – | – | |
| Middle | 1.048 (0.646–1.700) | 0.850 | – | – | |
| Poor | 1.935 (0.821–4.563) | 0.131 | – | – | |
| Tumor stage | |||||
| I | 1.000 | – | 1.000 | – | |
| II | 1.861 (1.058–3.276) | 0.031 | 1.734 (0.972–3.094) | 0.062 | |
| III | 3.824 (2.132–6.859) | <0.001 | 3.467 (1.867–6.438) | <0.001 | |
| Adjuvant therapy (yes) | 3.506 (2.183–5.632) | <0.001 | – | – | |
| NLR ≥1.89 | 1.438 (0.909–2.275) | 0.120 | 1.065 (0.620–1.828) | 0.820 | |
| PLR ≥118.91 | 1.296 (0.822–2.042) | 0.264 | 1.023 (0.607–1.726) | 0.932 | |
| LMR ≥3.88 | 0.824 (0.524–1.298) | 0.405 | 0.850 (0.491–1.473) | 0.562 | |
Note: adjuvant therapy was not included in the multivariable analysis, because it depends on the tumor stage. NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; LMR, lymphocyte-monocyte ratio; CI, confidence interval.
Discussion
Since the first indication that chronic inflammation may cause many tumors about 150 years ago, it is widely recognized that inflammation and innate immunity are strongly associated with tumor development (11). In the current investigation, a retrospective study was performed on 178 ESCC patients who received a radical resection and the association between NLR/PLR/LMR with clinical outcome was assessed. This study is the first to show that LMR was not independently associated with DFS or OS in ESCC patients who had a radical resection. Furthermore, our data indicate that NLR and PLR are also not independently related to OS or DFS in ESCCs.
Contrary to prior research, we demonstrated that an elevated LMR was not significantly related to the increase in OS and DFS in operable ESCC patients. Huang et al. studied 348 patients who had undergone esophagectomy for ESCCs and showed that patients with LMR ≤2.93 had a significantly poorer 5-year cancer-specific survival (CSS) than those with LMR >2.93 (21.2% vs. 59.3%, P<0.001) (9). Han et al. analyzed 218 patients with ESCC who received radical surgery and showed that, for both OS and DFS, preoperative LMR was an independent prognostic factor (10).
We assessed the potential prognostic significance of PLR and NLR. Despite an inverse association between NLR/PLR and prognosis in various tumors, their roles in esophageal cancer are confusing. Feng et al. (12) showed that PLR and NLR acted as markers of OS in ESCCs, and Yoo et al. (13) and Sharaiha et al. (14) proposed that an increasing NLR was related to poor OS and DFS in esophageal cancer. Conversely, other studies found that the predictive value of preoperative NLR and/or PLR for CSS was low in esophageal cancer patients (15,16). In the current study, increased NLR or PLR did not prove to be an independent prognostic factor.
Our results differ from other studies with reasons likely to include the cut-off values of these ratios, different pathological types, treatment modality and population. No available cut-off value was found in our population by receiver operating characteristic (ROC) curves; thus, we used the median count of NLR, PLR, and LMR as cut-off values. Furthermore, we analyzed the prognostic role of NLR, PLR, and LMR in ESCC patients without neoadjuvant therapy as radiation and/or chemotherapy could have exerted important impacts on systemic inflammation. Therefore, only 19.1% of patients were diagnosed with stage III disease and 42.1% of patients developed tumor recurrence in the current study. Also, we excluded adjuvant therapy from the multivariant analysis as it was dependent on the tumor stage.
Despite the unclear mechanism, many studies have shown that inflammatory and immune cells are related to malignancy. However, it is a complicated process that takes place in the tumor microenvironment (17-19). For example, myeloid growth factors (a production of paraneoplastic syndrome), granulocyte colony stimulating factor derived from cancer cells, and the release of interleukin-6 and tumor necrosis factor-α have also been attributed to neutrophilia in the malignant process. Moreover, unlike colorectal cancer, which is related to ulcerative colitis and esophageal adenocarcinomas that are associated with reflux esophagitis, ESCCs do not arise from chronic or acute inflammation. All of the aforementioned factors may explain the difference in results relating to the different cancers between our study and others.
In our study, the limitations include the single-center design, a relatively small sample size, and retrospective analysis. Also, our subjects were recruited from a single ethnic group (Han Chinese). All of these factors might have caused selection bias and limit sample representativeness. Our findings need to be confirmed in a large-scale multicenter study. Furthermore, median values of NLR, PLR, and LMR were used as cut-off levels, and variant cut-off values served for prognostic markers of ESCC were needed to conduct further analysis. Additional mechanistic studies will also need to be performed.
In conclusion, we demonstrate that LMR could not serve as a prognostic biomarker for patients with operable ESCCs. Moreover, our findings may support further studies to investigate the roles of inflammatory cells in the ESCC microenvironment.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.75). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study had been approved by the Ethics Committee of Tongji Hospital of Huazhong University of Science and Technology (No. TJ-C20061325).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Jomrich G, Paireder M, Gleiss A, Kristo I, Harpain L, Schoppmann SF. Comparison of Inflammation-Based Prognostic Scores in a Cohort of Patients with Resectable Esophageal Cancer. Gastroenterol Res Pract 2017;2017:1678584.
- Hsieh CC, Hsu HS, Li AF, et al. Clinical relevance of PD-L1 and PD-L2 overexpression in patients with esophageal squamous cell carcinoma. J Thorac Dis 2018;10:4433-44. [Crossref] [PubMed]
- Kawamoto T, Nihei K, Sasai K, et al. Clinical outcomes and prognostic factors of chemoradiotherapy for postoperative lymph node recurrence of esophageal cancer. Jpn J Clin Oncol 2018;48:259-64. [Crossref] [PubMed]
- Maddaly R, Subramaniyan A, Balasubramanian H. Cancer Cytokines and the Relevance of 3D Cultures for Studying Those Implicated in Human Cancers. J Cell Biochem 2017;118:2544-58. [Crossref] [PubMed]
- Kong W, Xu H, Cheng J, et al. The Prognostic Role of a Combined Fibrinogen and Neutrophil-to-Lymphocyte Ratio Score in Patients with Resectable Hepatocellular Carcinoma: A Retrospective Study. Med Sci Monit 2020;26:e918824. [Crossref] [PubMed]
- Mo CJ, Hu ZJ, Qin SZ, et al. Diagnostic value of platelet-lymphocyte ratio and hemoglobin-platelet ratio in patients with rectal cancer. J Clin Lab Anal 2020;34:e23153. [Crossref] [PubMed]
- Ichikawa N, Homma S, Yoshida T, et al. An increase in the peripheral lymphocyte-to-monocyte ratio after primary site resection is associated with a prolonged survival in unresectable colorectal carcinoma. Surg Today 2020;50:604-14. [Crossref] [PubMed]
- Huang Y, Feng JF. Low preoperative lymphocyte to monocyte ratio predicts poor cancer-specific survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther 2015;8:137-45. [PubMed]
- Han LH, Jia YB, Song QX, et al. Prognostic significance of preoperative lymphocyte-monocyte ratio in patients with resectable esophageal squamous cell carcinoma. Asian Pac J Cancer Prev 2015;16:2245-50. [Crossref] [PubMed]
- Maiorino L, Egeblad M. Tumours pick the path to cancer inflammation. Nat Cell Biol 2019;21:1055-7. [Crossref] [PubMed]
- Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol 2014;12:58. [Crossref] [PubMed]
- Yoo EJ, Park JC, Kim EH, et al. Prognostic value of neutrophil-to-lymphocyte ratio in patients treated with concurrent chemoradiotherapy for locally advanced oesophageal cancer. Dig Liver Dis 2014;46:846-53. [Crossref] [PubMed]
- Sharaiha RZ, Halazun KJ, Mirza F, et al. Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 2011;18:3362-9. [Crossref] [PubMed]
- Rashid F, Waraich N, Bhatti I, et al. A pre-operative elevated neutrophil: lymphocyte ratio does not predict survival from oesophageal cancer resection. World J Surg Oncol 2010;8:1. [Crossref] [PubMed]
- Dutta S, Crumley AB, Fullarton GM, et al. Comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative resection of oesophageal cancer. World J Surg 2011;35:1861-6. [Crossref] [PubMed]
- Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493-503. [Crossref] [PubMed]
- Ethier JL, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res 2017;19:2. [Crossref] [PubMed]
- De Cock JM, Shibue T, Dongre A, et al. Inflammation Triggers Zeb1-Dependent Escape from Tumor Latency. Cancer Res 2016;76:6778-84. [Crossref] [PubMed]


