
Progesterone receptor inhibits the proliferation and invasion of endometrial cancer cells by up regulating Krüppel-like factor 9
Introduction
Endometrial cancer (EC) is one of the most common genital malignancies in women (1). With the development of society and economy and the change of life style, the incidence is increasing rapidly worldwide (2). The prognosis of EC, even in the early stage, is poor, let alone those patients with either advanced stage at diagnosis or recurrent disease following treatment (3). The molecular mechanisms of development of EC are not fully understood. Although many oncogenes and tumor suppressors have been identified as key players underlying tumorigenesis of EC (4-6), however, almost no commonly-accepted biomarkers have been established to facilitate the comprehensive management of EC patients. Therefore, understanding the molecular events underlying endometrial carcinogenesis and identifying new biomarkers and therapeutic targets will be important.
Krüppel-like factor 9 (KLF9), also known as basic transcription element binding protein 1 (BTEB1), belongs to large KLF transcription factor family, which consists of 17 members (7). KLF9 is expressed in various tissues, most abundantly in the brain, kidney, lung, and testis. KLF9 is also involved in various biological processes such as cell proliferation, differentiation, and neural development (8,9). Recently, KLF9 has emerged as a regulator of oncogenesis and reported to play critical roles in several types of tumor. For example, KLF9 was found to be down-regulated in human colorectal cancer, breast cancer, ovarian cancer, glioblastoma, and hepatocellular cancer and was considered as a tumor suppressor (10-13). The downregulation of KLF9 is associated with tumorigenesis, metastasis, and apoptosis resistance (14,15). However, the possibility that KLF9 is a suppressor of endometrial tumorigenesis in the colon has not been evaluated.
In the present study, we illustrated that KLF9 was downregulated in EC tissues, and its expression was associated with EC cell proliferation and metastasis. We also showed that KLF9 reduced EC cell proliferation and invasion by inhibiting β-catenin signaling activity. Furthermore, we demonstrated that decreased progesterone receptor (PR) was responsible for the downregulation of KLF9 in EC cells.
Methods
Cell culture
Four EC cell lines (RL-952, Ishikawa, HEC-1A and KLE) were purchased from the American Type Culture Collection (ATCC). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) or DMEM/F12 supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin (Sigma-Aldrich Co, St Louis, MO, USA) and 100 U/mL streptomycin (Sigma-Aldrich) in humidified air at 37 °C with 5% CO2.
Tissue samples collection
A total of 52 EC specimens were obtained from the Department of Obstetrics and Gynecology, Shanghai General Hospital of Nanjing Medical University and the Department of Obstetrics and Gynecology, Shanghai First Maternity and Infant Hospital, School of Medicine, Tongji University. The clinical data of the patients were collected from the medical records, including gender, age, pathological subtype, lymph node metastasis, etc. The study protocol was approved by Research Ethics Committee of Nanjing Medical University.
RNA extraction and quantitative real-time PCR assay
Total RNA was extracted from 52 EC specimens using Trizol reagent (Invitrogen). The primers of KLF9 were as follows: 5'-AAC TGC TTT TCC CCA GTG TG-3' (forward) and 5'-TCC CAT CTC AAA GCC CAT TA-3' (reverse). The following protocol was described previously (16).
Western blot assay
Anti-KLF9 antibody (ab26074, Abcam, Cambridge, MA, USA), anti-β-catenin antibody (ab32572), anti-CCND1 antibody (ab16663), anti-C-myc antibody (ab32072) and anti-GAPDH antibody (ab181602) were all purchased from Abcam Co., Ltd. The detailed protocol was described previously (17).
Construction of overexpression and knockdown model
The overexpression and short-hairpin RNA (shRNA)-mediated knockdown lentivirus plasmids and packaging vectors were prepared as previously described (18). For overexpression model, cells were infected with lentivirus expressing KLF9 (OE-KLF9)/negative control (OE-Control), β-catenin (OE-β-catenin)/negative control (OE-β-Control), PR (OE-PR)/negative control (OE-PR-Control). Full-length of cDNA was inserted into the lentivirus pLenti-EF1a-EGFP-P2A-Puro-CMV-MCS vector (Obio Technology, Co., Ltd, Shanghai, China). shRNA targeting KLF9 (sh-KLF9) or scrambled shRNA (sh-KLF9-Control) were cloned into pLKD-CMV-G&PR-U6-shRNA (Obio Technology). The sequences used for sh-KLF9 was 5'-GCT TGT TGG ACC TGA ACA AGT-3'. All of these plasmids were confirmed by DNA sequencing. Co-transfection of OE-KLF9 together with OE-β-catenin or OE-β-Control, co-transfection of OE-PR or OE-PR-Control together with sh-KLF9 or sh-KLF9-Control were performed as described previously (19).
Cell proliferation, clone formation and transwell assays
Cell Counting Kit-8 (CCK8), clone formation and transwell assays were performed in to detect the malignant behaviors of KLF9, including its effects on cell proliferation and invasion, as described previously (20).
Tumor formation assay in xenograft model
For the subcutaneous xenograft model, 5-week-old female athymic BALB/c mice were injected subcutaneously with 2×107 tumor cells in 100 mL phosphate buffer saline (PBS). Tumor volumes were examined every 2 days when the implantations were starting to grow bigger. Sixteen days after injection, the mice were killed and the primary tumors were excised.
Luciferase reporter assay
For TOPFLASH assay: cells were seeded in 12-well plates and co-transfected with TOPFLASH reporter and KLF9 expression vector or control vector on the following day. For KLF9-luciferase assay: cells were seeded in 12-well plates and co-transfected with KLF9-promoter reporter, pRL-SV40 Renilla luciferase reporter vector, and PR expression plasmid or control plasmid. Firefly luciferase activity, normalized to Renilla luciferase activity, was measured 48 h after transfection by the Dual Luciferase Assay System (Promega, Madison, USA).
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed using the ChIP assay kit according to the instruction (Millipore, USA). Anti-PR was obtained from Abcam (ab2765, Abcam). The input genomic DNA and the immunoprecipitated DNA was amplified by PCR. The PCR products were subjected to gel electrophoresis.
Immunohistochemistry (IHC) analysis
Fifty-two EC tumor samples were immunostained with KLF9 and PR antibodies. The IHC procedure and scoring of protein expression were performed as previously described (21). Tissue microarray sections were incubated with primary polyclonal rabbit anti-KLF9 antibody (ab26074, Abcam) and monoclonal mouse anti-PR antibody (ab2765, Abcam) overnight at 4 °C, followed by incubation with biotinylated anti-rabbit and anti-mouse secondary antibody at 37 °C for 30 min respectively. Sections were then incubated with a streptavidin-horseradish peroxidase complex, colorized with 3,3-diaminobenzidine (DAB) chromogen solution and counterstained with hematoxylin. Results were analyzed as previously described. Briefly, the percentage of KLF9 and PR positive cells was scored as follow: 0 for 0%, 1 for 1–33%, 2 for 34–66% and 3 for 67–100%. The intensity of KLF9 and PR staining was also scored as follows: 0 for negative staining, 1 for yellow color staining, 2 for light brown color staining and 3 for brown color staining. The product of the staining intensity and percentage gave rise to the IHC score (IHS). The final results of IHC analysis were defined using a two level system following the IHS: <4 indicates low expression, while 4–12 indicates high expression. Negative controls were employed by replacement of the primary antibody with PBS.
Statistical analysis
All experiments were repeated three times. All statistical analyses were performed using SPSS 20.0 software (IBM, SPSS, USA). The statistical differences between groups were determined by t-test or χ2 test. P<0.05 was considered to be statistically significant.
Results
KLF9 is down-regulated in EC tissue samples
The KLF9 expression was detected in 52 paired EC tissue samples and corresponding noncancerous tissue samples by real time PCR test. Figure 1A showed that the expression levels of KLF9 were significantly decreased in EC tissues than those in noncancerous tissues (P<0.01). Moreover, the detailed relationships between KLF9 expression status and clinicopathological variables were shown in Table 1. Noticeably, lower KLF9 expression was significantly correlated with myometrial invasion (P=0.000), lymph node metastasis (P=0.002), and distant metastasis (P=0.026).
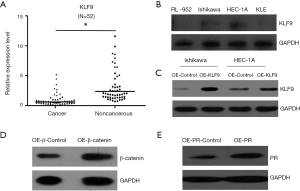
Table 1
| Clinical parameter | No. | KLF9 expression | χ2 | P value | |
|---|---|---|---|---|---|
| Low (%) | High (%) | ||||
| FIGO stages | |||||
| I/II | 25 | 14 (56.00) | 11 (44.00) | 0.693 | 0.405 |
| III/IV | 27 | 12 (44.44) | 15 (55.56) | ||
| Grade | |||||
| G1 | 22 | 10 (45.45) | 12 (54.55) | 0.315 | 0.575 |
| G2-G3 | 30 | 16 (53.33) | 14 (46.67) | ||
| Myometrial invasion | |||||
| Negative | 29 | 6 (20.69) | 23 (79.31) | 22.531 | 0.000* |
| Positive | 23 | 20 (86.96) | 3 (13.04) | ||
| Lymph node metastasis | |||||
| Negative | 29 | 9 (31.03) | 20 (68.97) | 9.433 | 0.002* |
| Positive | 23 | 17 (73.91) | 6 (26.09) | ||
| Distant metastasis | |||||
| Negative | 28 | 10 (35.71) | 18 (64.29) | 4.952 | 0.026* |
| Positive | 24 | 16 (66.67) | 8 (33.33) | ||
*, P<0.05. KLF9, Krüppel-like factor 9; EC, endometrial cancer.
Construction of overexpression model in cell lines
As is shown in Figure 1B, differential expression of KLF9 was detected in EC cell lines. Then we chose Ishikawa and HEC-1A cells to construct OE-KLF9 model and the result demonstrated the effectiveness of OE-KLF9 model in Ishikawa and HEC-1A cells (Figure 1C). Similarly, OE-β-catenin and OE-PR models were also successfully prepared in Ishikawa cell (Figure 1D,E).
OE-KLF9 inhibits EC cell development in vitro and in vivo
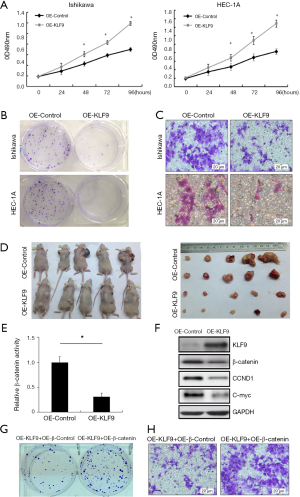
In vitro, CCK8, clone formation and transwell assays remarkably illustrated that OE-KLF9 inhibits EC cell proliferation and invasion (Figure 2A,B,C). Furthermore, the role of KLF9 in tumor development was also explored in vivo. As shown in Figure 2D, compared with OE-Control group, the OE-KLF9 group developed significantly smaller xenograft tumors.
KLF9 inhibits β-catenin expression
It was reported that KLF9 could inhibit β-catenin activity and β-catenin signaling is a well-known oncogenic pathway in cancer development (22-24), we highly wondered that whether KLF9 could inhibit cell proliferation and invasion by regulating β-catenin pathway in EC. We firstly conducted TOPFlash luciferase reporter assay in EC cells. Cells were transfected with the TOPFlash reporter, which directly detected β-catenin activity, together with OE-KLF9 or OE-Control. As shown in Figure 2E, OE-KLF9 decreased TOPFlash reporter activity. Furthermore, as the Wnt/β-catenin pathway readouts and β-catenin response elements, the expression of C-Myc and CCND1 were detected. Indeed, similarly to TOPFlash activity, C-Myc and CCND1 expression were significantly decreased in OE-KLF9 cells (Figure 2F). Then we addressed whether β-catenin pathway was involved in KLF9-mediated attenuated proliferation and invasion. EC cells were co-transfected with OE-KLF9 together with OE-β-catenin or OE-β-Control. Clone formation assay and transwell assay were conducted again to test cell proliferation and invasion ability. The results showed that OE-β-catenin partly restrained the effect of OE-KLF9 and restored cell proliferation and invasion ability (Figure 2G,H).
PR could bind to KLF9 promoter to positively regulate KLF9 expression
PR is a well-known transcription factor, regulating target gene expression by binding to a specific sequence within the promoter (25). Bioinformatics analysis revealed that KLF9 promoter contains potential PR binding sequence (Figure 3A). Therefore, we proposed whether PR could activate KLF9 expression. Firstly, we constructed luciferase reporter vector containing KLF9 promoter sequence. Then Ishikawa cells were co-transfected with reporter plasmid and OE-PR or OE-PR-Control. Figure 3B showed that OE-PR significantly increased the KLF9 reporter gene activity. Consistently, PCR and western blot assays further proved that the cellular mRNA and protein levels of KLF9 were elevated by OE-PR (Figure 3C,D). Then ChIP assay was performed to determine whether PR could regulate KLF9 by binding to its promoter. As indicated in Figure 3E, the KLF9 promoter region was specifically immunoprecipitated with anti-PR antibody, indicating that PR bound to KLF9 promoter. These findings strongly suggest that PR could activate KLF9 expression by directly binding to its promoter. The notion was further supported by the positive correlation between KLF9 and PR expression (P=0.031) detected by IHC analysis in 52 EC tissue samples (Figure 3F, Table 2).
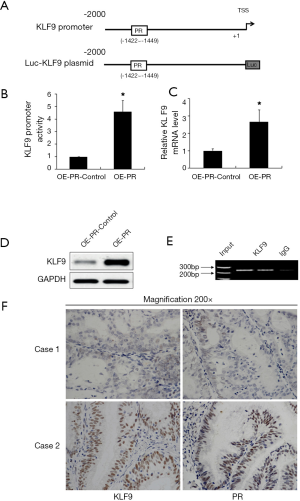
Table 2
| Parameter | No. | PR expression | P value | |
|---|---|---|---|---|
| Low (%) | High (%) | |||
| KLF9 | ||||
| Low | 28 | 22 (78.57) | 6 (21.43) | 0.031* |
| High | 24 | 12 (50.00) | 12 (50.00) | |
*, P<0.05. KLF9, Krüppel-like factor 9; PR, progesterone receptor; IHC, immunohistochemistry; EC, endometrial cancer.
KLF9 mediated PR-induced tumor suppressor in EC
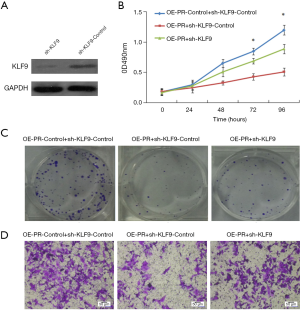
We next investigated whether KLF9 is functionally important in PR-induced phenotypic alterations in EC. sh-KLF9 model was confirmed by western blot assay in Ishikawa cell line (Figure 4A). Then Ishikawa cell was co-transfected with OE-PR together with sh-KLF9 or sh-KLF9-Control. The CCK8 assay and clone formation assay showed that sh-KLF9 partly abrogated the reduction of proliferation ability caused by ectopic expression of PR (Figure 4B,C). The cell invasion assay demonstrated that sh-KLF9 partly restrained the effect of OE-PR (Figure 4D). These results remarkably indicated that KLF9 was an important downstream target gene of PR and involved in PR-induced tumor suppressor effect in EC.
Discussion
Recently, the role of KLF9 in regulating tumorigenesis and progression has been recognized in several cancers. However, the detailed function of KLF9 in human EC is not fully understood. In this present study, we firstly investigated KLF9 expression in EC tissues and cell lines. We found that KLF9 was decreased in EC tissues, and reduced KLF9 expression was associated with highly metastatic capacity of EC cells. We also showed that KLF9 could reduce EC cell proliferation and invasion by inhibiting β-catenin signaling activity. Besides, we demonstrated that decreased PR was associated with the downregulation of KLF9 in EC cells.
The development and progression of EC is influenced by many factors. For example, FKBP51 is significantly decreased in endometrial carcinoma and could reduce cell proliferation and increase the sensitivity of progesterone treatment by inhibiting the Akt pathway (26). The increased expression of Notch2 induced by abnormal miR-181c is closely related to the recurrence of EC (27). Our results indicate a new tumor suppressor of EC. We showed that KLF9 is significantly decreased in EC tissues, and could inhibit cell proliferation, metastasis, and tumorigenesis in vitro and in vivo. Our findings provide a new potential biomarker and target for the diagnosis and treatment of EC.
To explore the mechanisms of KLF9 in EC, we firstly performed in vitro experiment and found that over-expression of KLF9 inhibited Wnt/β-catenin signaling. Wnt/β-catenin pathway, also known as the canonical Wnt pathway, serve critical roles in cell growth, differentiation, migration, polarity, and death (28). In recent years, dysregulation of Wnt/β-catenin signaling pathway has been recognized as one of the hallmarks of several cancers (29,30), making it become an important target for the development of novel therapeutics for various tumors (31). Accumulation of β-catenin in the cytoplasm followed by its translocation and activation in the nucleus is a central event in the progression of canonical WNT/β-catenin signaling. The abnormal expression or mutations of β-catenin are main reasons leading to dysregulation of Wnt/β-catenin pathway. Our results showed that KLF9 could downregulate β-catenin expression and inhibit β-catenin activity, thus inhibiting the activation of Wnt/β-catenin signaling pathway. However, the involved downstream genes of Wnt/β-catenin pathway are unclear, which needs further researching.
Progesterone functions as a tumor suppressor in the endometrium. A majority of endometrial tumors arise in the setting of excess estrogen unopposed by progesterone (32). Not surprisingly, the expression of PR is gradually lost with progression of EC, thus abrogating the tumor suppressive properties of progesterone. Several mechanisms elucidating PR mediated tumor suppressor have been proposed, including cell cycle arrest, inhibiting proliferation, and activating apoptosis (33,34). We demonstrated that PR could also act through upregulating KLF9, thus inhibiting the initiation and progression of EC.
The present study aimed at disclosing the role of KLF9 in EC, to lay the foundation for the development of more effective therapies. Collectively, we showed that KLF9 could inhibit endometrial carcinogenesis and progression and may serve as a potential target for antineoplastic therapies. We proposed that the axis PR/KLF9/β-catenin could be considered as a novel therapeutic application for EC patients.
Acknowledgments
Funding: This research was supported in part by grants from
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.53). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research was carried out in accordance with the Declaration of Helsinki [2013] of the World Medical Association and the study protocol was approved by Research Ethics Committee of Nanjing Medical University. Ethical approval to perform this research was approved by Human Research Ethics Committee of Shanghai General Hospital of Nanjing Medical University (No. 201439), and written informed consent was provided by each patient included in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Winterhoff B, Thomaier L, Mullany S, et al. Molecular characterization of endometrial cancer and therapeutic implications. Curr Opin Obstet Gynecol 2020;32:76-83. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Takahashi A, Matsuura M, Matoda M, et al. Clinicopathological Features of Early and Late Recurrence of Endometrial Carcinoma After Surgical Resection. Int J Gynecol Cancer 2017;27:967-72. [Crossref] [PubMed]
- Kavlashvili T, Jia Y, Dai D, et al. Inverse Relationship between Progesterone Receptor and Myc in Endometrial Cancer. PLoS One 2016;11:e0148912. [Crossref] [PubMed]
- Pan X, Li D, Huo J, et al. LINC01016 promotes the malignant phenotype of endometrial cancer cells by regulating the miR-302a-3p/miR-3130-3p/NFYA/SATB1 axis. Cell Death Dis 2018;9:303. [Crossref] [PubMed]
- Waheed S, Cheng RY, Casablanca Y, et al. Nitric Oxide Donor DETA/NO Inhibits the Growth of Endometrial Cancer Cells by Upregulating the Expression of RASSF1 and CDKN1A. Molecules 2019; [Crossref] [PubMed]
- Li Y, Sun Q, Jiang M, et al. KLF9 suppresses gastric cancer cell invasion and metastasis through transcriptional inhibition of MMP28. FASEB J 2019;33:7915-28. [Crossref] [PubMed]
- Tsukahara T, Yamagishi S, Matsuda Y, et al. Lysophosphatidic acid signaling regulates the KLF9-PPARgamma axis in human induced pluripotent stem cell-derived neurons. Biochem Biophys Res Commun 2017;491:223-7. [Crossref] [PubMed]
- Sporl F, Korge S, Jurchott K, et al. Kruppel-like factor 9 is a circadian transcription factor in human epidermis that controls proliferation of keratinocytes. Proc Natl Acad Sci U S A 2012;109:10903-8. [Crossref] [PubMed]
- Mannava S, Zhuang D, Nair JR, et al. KLF9 is a novel transcriptional regulator of bortezomib- and LBH589-induced apoptosis in multiple myeloma cells. Blood 2012;119:1450-8. [Crossref] [PubMed]
- Zhang QH, Dou HT, Tang YJ, et al. Lentivirus-mediated knockdown of Kruppel-like factor 9 inhibits the growth of ovarian cancer. Arch Gynecol Obstet 2015;291:377-82. [Crossref] [PubMed]
- Ying M, Sang Y, Li Y, et al. Kruppel-like family of transcription factor 9, a differentiation-associated transcription factor, suppresses Notch1 signaling and inhibits glioblastoma-initiating stem cells. Stem Cells 2011;29:20-31. [Crossref] [PubMed]
- Fu DZ, Cheng Y, He H, et al. The fate of Kruppel-like factor 9-positive hepatic carcinoma cells may be determined by the programmed cell death protein 5. Int J Oncol 2014;44:153-60. [Crossref] [PubMed]
- Zhong Z, Zhou F, Wang D, et al. Expression of KLF9 in pancreatic cancer and its effects on the invasion, migration, apoptosis, cell cycle distribution, and proliferation of pancreatic cancer cell lines. Oncol Rep 2018;40:3852-60. [PubMed]
- Bagati A, Moparthy S, Fink EE, et al. KLF9-dependent ROS regulate melanoma progression in stage-specific manner. Oncogene 2019;38:3585-97. [Crossref] [PubMed]
- Zhang H, Qiu J, Ye C, et al. ROR1 expression correlated with poor clinical outcome in human ovarian cancer. Sci Rep 2014;4:5811. [Crossref] [PubMed]
- Yin Z, Gao M, Chu S, et al. Antitumor activity of a newly developed monoclonal antibody against ROR1 in ovarian cancer cells. Oncotarget 2017;8:94210-22. [Crossref] [PubMed]
- Mao Y, Fan W, Hu H, et al. MAGE-A1 in lung adenocarcinoma as a promising target of chimeric antigen receptor T cells. J Hematol Oncol 2019;12:106. [Crossref] [PubMed]
- Ding J, Xie M, Lian Y, et al. Long noncoding RNA HOXA-AS2 represses P21 and KLF2 expression transcription by binding with EZH2, LSD1 in colorectal cancer. Oncogenesis 2017;6:e288. [Crossref] [PubMed]
- Hu R, Wang MQ, Niu WB, et al. SKA3 promotes cell proliferation and migration in cervical cancer by activating the PI3K/Akt signaling pathway. Cancer Cell Int 2018;18:183. [Crossref] [PubMed]
- Qiu M, Bao W, Wang J, et al. FOXA1 promotes tumor cell proliferation through AR involving the Notch pathway in endometrial cancer. BMC Cancer 2014;14:78. [Crossref] [PubMed]
- Shang S, Hua F, Hu ZW. The regulation of beta-catenin activity and function in cancer: therapeutic opportunities. Oncotarget 2017;8:33972-89. [Crossref] [PubMed]
- Wang ZM, Wan XH, Sang GY, et al. miR-15a-5p suppresses endometrial cancer cell growth via Wnt/beta-catenin signaling pathway by inhibiting WNT3A. Eur Rev Med Pharmacol Sci 2017;21:4810-8. [PubMed]
- Qiao F, Yao F, Chen L, et al. Kruppel-like factor 9 was down-regulated in esophageal squamous cell carcinoma and negatively regulated beta-catenin/TCF signaling. Mol Carcinog 2016;55:280-91. [Crossref] [PubMed]
- Tan S, Bajalovic N, Wong ESP, et al. Ligand-activated progesterone receptor B activates transcription factor EB to promote autophagy in human breast cancer cells. Exp Cell Res 2019;382:111433. [Crossref] [PubMed]
- Pei H, Li L, Fridley BL, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell 2009;16:259-66. [Crossref] [PubMed]
- Devor EJ, Miecznikowski J, Schickling BM, et al. Dysregulation of miR-181c expression influences recurrence of endometrial endometrioid adenocarcinoma by modulating NOTCH2 expression: An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 2017;147:648-53. [Crossref] [PubMed]
- El-Sahli S, Xie Y, Wang L, et al. Wnt Signaling in Cancer Metabolism and Immunity. Cancers (Basel) 2019; [Crossref] [PubMed]
- Kwan HT, Chan DW, Cai PC, et al. AMPK activators suppress cervical cancer cell growth through inhibition of DVL3 mediated Wnt/beta-catenin signaling activity. PLoS One 2013;8:e53597. [Crossref] [PubMed]
- Xu R, Hu J, Zhang T, et al. TRIM29 overexpression is associated with poor prognosis and promotes tumor progression by activating Wnt/beta-catenin pathway in cervical cancer. Oncotarget 2016;7:28579-91. [PubMed]
- Sha YL, Liu S, Yan WW, et al. Wnt/β-catenin signaling as a useful therapeutic target in hepatoblastoma. Biosci Rep 2019; [Crossref] [PubMed]
- Raffone A, Travaglino A, Saccone G, et al. Should progesterone and estrogen receptors be assessed for predicting the response to conservative treatment of endometrial hyperplasia and cancer? A systematic review and meta-analysis. Acta Obstet Gynecol Scand 2019;98:976-87. [Crossref] [PubMed]
- Kong X, Li M, Shao K, et al. Progesterone induces cell apoptosis via the CACNA2D3/Ca2+/p38 MAPK pathway in endometrial cancer. Oncol Rep 2020;43:121-32. [PubMed]
- Crobeddu B, Ferraris E, Kolasa E, et al. Di(2-ethylhexyl) phthalate (DEHP) increases proliferation of epithelial breast cancer cells through progesterone receptor dysregulation. Environ Res 2019;173:165-73. [Crossref] [PubMed]


