
Neurosurgery for brain metastasis from breast cancer
Introduction
Breast cancer is the most common malignancy among women worldwide, and a recent database review showed that 20% to 30% of patients with breast cancer develop metastasis as the main cause of death (1,2). Approximately 10% to 16% of patients with metastatic breast cancer develop brain metastases (3,4), and this rate is increasing as more people are living longer with a primary diagnosis (5). Most patients with brain metastasis have shorter survival because of progressive systemic disease or uncontrolled neurological disease. The median survival of patients with breast cancer after relapse in the central nervous system ranges from 5 to 14 months (6). Recent advancements in adjuvant treatments such as anti-human epidermal growth factor receptor-2 (anti-HER-2) monoclonal antibody have made extracranial lesions more controllable, thus increasing the likelihood that brain metastasis is the first site of recurrence and that appropriate treatment of brain metastasis will lead to longer survival (7). The treatment of brain metastasis includes corticosteroids, surgery, radiosurgery or radiotherapy, chemotherapy, and immunotherapy. Surgical treatment of brain metastasis has been significantly developed with advancements in supporting neurosurgical tools and technologies. The purpose of this review is to discuss the characteristics and surgical treatment of metastatic brain tumors from breast cancer.
Characteristics of metastatic brain tumors of breast cancer
Imaging modalities are necessary to detect and differentiate cerebral neoplasms from other nonmalignant tumors. Intracranial metastases typically show enhancement with contrast reagent because of destruction of the blood-brain barrier. Metastases generally occur as cortical or subcortical lesions because of hematogenous spread and often start as smaller and solidly enhancing lesions that become ring-enhancing lesions secondary to necrosis (8). Many common malignancies, including breast, colon, renal cell, and thyroid cancers, often develop a single brain metastasis, whereas lung cancer and melanoma are more likely to develop multiple brain tumors (9). Nodular solid enhancement can be found in a variety of pathologies, including metastatic disease, lymphoma, sarcoids, vasculitides such as Behçet’s disease, demyelinating disorders, and bacterial or fungal infections (10). In contrast, the most common etiology of ring-enhanced lesions is high-grade glioma (40%), followed by metastases (30%), abscesses (8%), and demyelinating disease (6%) (11). Standard magnetic resonance imaging (MRI) sequences such as T2-weighted imaging, diffusion-weighted imaging, and contrast-enhanced T1-weighted imaging can distinguish between metastases and other clinical conditions, although differentiating a single metastasis from a glioblastoma remains a top diagnostic challenge. Pope (10) reviewed the neuroimaging features of metastatic brain tumors and found that magnetic resonance spectroscopies and relative cerebral blood volumes seem to help differentiate metastases from glioblastomas.
MRI is one of the most reliable modalities with which to evaluate metastatic brain tumors, although very few studies in the literature have reported the relationships between MRI features and the histology of tumors. Yeh et al. (12) retrospectively analyzed the MRI features of brain metastasis from different subtypes of recurrent breast cancer for subclassification. In that study, the patients were categorized as having luminal type, HER-2-enriched type, or triple-negative breast cancers, and all MRI examinations were performed on a 1.5-Tesla MRI scanner. Both the patients with luminal type cancers and those with HER-2 enriched type cancers showed solid tumors with or without perifocal edema, whereas most patients with triple-negative breast cancers showed distinct features of cystic and necrotic lesions. Brain metastatic lesions frequently show characteristics different from those of the primary tumor histologically and genetically (13-15), indicating that MRI is a desirable modality with which to explore the tumor nature of brain metastasis (12).
Tumor invasion into surrounding central nervous system tissues should be considered when resecting brain tumors. Glioblastoma, one of the primary central nervous system tumors, is difficult to totally remove surgically because tumor cells can infiltrate the surrounding tissue far beyond the tumor core (16). In contrast, metastatic brain tumors are less invasive. Baumert et al. (17) histologically evaluated the invasiveness of metastatic brain tumors and found that breast cancer infiltrated the surrounding tissue up to 1 mm from the tumor core. Therefore, gross total removal of breast cancers can be achieved by resecting the tumor with an additional margin from the tumor border.
Indications for surgical treatment
Surgical resection continues to play an important role in patients with a limited number of brain metastases and a relatively good performance status. In the early 1990s, three randomized trials on single brain metastasis were conducted to evaluate the efficacy of surgical resection followed by whole-brain radiation therapy compared with whole-brain radiation therapy alone, and the data indicated that surgical resection significantly prolonged overall survival in patients without active systemic disease and with a higher Karnofsky performance status (18-20). According to the JCOG0504 trial, surgical resection followed by salvage stereotactic radiosurgery (SRS) has been established as a standard therapy for patients with fewer brain metastases (21). SRS is also the effective alternative to surgical treatment for a single metastasis (22,23), but the higher doses of SRS increase the risk of the late effect of radiation necrosis (24). In addition, brain edema caused by metastatic brain tumors resolves significantly faster after surgical resection than after SRS (25). Moreover, in patients with neurological symptoms caused by brain lesions of >3 cm with a mass effect or associated hydrocephalus, surgical resection can immediately alleviate these symptoms (26). Instead, surgical resection followed by SRS can be considered as standard treatment in patients with a few (three or fewer) brain metastases, mainly with lesions of >3 cm in diameter (26).
The Congress of Neurological Surgeons published guidelines for the surgical treatment of metastatic brain tumors (23,27). In these guidelines, the indication for surgical resection of metastatic brain tumors is considered separately according to whether the patient has a single tumor or multiple tumors. Surgery followed by whole-brain radiation therapy is recommended as the first-line treatment in patients with a single brain metastasis with a favorable performance status and limited extracranial disease. In patients with multiple brain metastases, however, tumor resection is recommended only in patients with symptomatic lesions with a mass effect or hydrocephalus. The Japan Society for Neuro-Oncology also recently disclosed clinical guidelines for metastatic brain tumors (28). For a single brain lesion, surgical treatment is considered equivalent to radiation therapy. Tumor removal is also recommended in patients with two to four brain metastases if they have a higher Karnofsky performance status and the tumor locations are resectable. For patients with five or more brain lesions, the indication for surgical resection is limited to those in whom surgery is expected to provide functional and survival benefits. These guidelines are expected to change with the emergence of new treatment modalities in the near future.
Surgical strategy for metastatic brain tumors
Complete removal of metastatic brain tumors, termed gross total resection (GTR), is the ideal goal in surgical treatment. According to the latest guidelines published by the Congress of Neurological Surgeons, GTR is recommended over subtotal resection to improve overall survival and prolong the time to recurrence (23). However, recurrence affects about 20% of patients even after treatment with GTR followed by SRS (29). In contrast to diffusely invading tumors such as gliomas, metastatic brain tumors are more often well demarcated masses surrounded by gliotic tissue (26). Several reports have shown that supramarginal resection achieved by additional 5-mm surrounding tissue resection from the tumor edge improved the local control rate compared with conventional GTR (30-32). Even for brain metastasis in eloquent areas, supramarginal resection can be achieved with awake surgery in many cases (33). However, supramarginal resection cannot prevent temporary deficits such as supplementary motor area syndrome even with intraoperative neurophysiological monitoring or awake surgery (34). Therefore, deliberative planning for maximal safe resection with minimal tissue trauma is ideal for both surgeons and patients.
Tumor resection is usually performed either in a piecemeal fashion or en bloc fashion. Piecemeal resection involves debulking the mass and subsequently removing the capsule, which is traditionally performed. Although this technique can achieve GTR, it is associated with a risk of local recurrence and dissemination. Suki et al. (35,36) evaluated the rate of leptomeningeal disease after resection of supra- and infratentorial metastasis and found that only 5.7% of patients who had undergone en bloc resection developed leptomeningeal disease compared with 13.9% of patients who had undergone piecemeal resection. In en bloc resection, the tumor is safely dissected along the brain-tumor interface, avoiding exposure of the tumor itself to the surrounding tissue (37). However, this recurrence-lowering effect of en bloc resection is diminished in the surgical treatment of tumors larger than 9.71 cm3 (38). Additionally, piecemeal resection is inevitable in certain situations, such as tumors that are adherent to or infiltrating eloquent areas (39). Based on these reports, en bloc tumor resection is basically recommended to decrease leptomeningeal disease when resecting a single brain metastasis (23).
Resection of cystic tumors
Cystic brain metastasis of breast cancer is associated with a poor prognosis (40). In the surgical treatment of cystic tumors, entire removal of the cyst wall is necessary to achieve GTR because of the higher risk of leptomeningeal dissemination (41). Cyst puncture is sometimes performed to decompress the tumor during surgery, but the boundary between the tumor and the surrounding brain tissue becomes indistinct by cyst shrinkage. Tomita et al. (42) introduced a technique for visualization of the inner cyst wall by injection of pyoktanin blue solution diluted in 0.3% saline. Although tumor dissemination is a potential concern when performing cyst puncture, solidification with fibrin glue might prevent dissemination and enable easier dissection of the tumor from the surrounding brain tissue (43).
Supporting devices for safe GTR
Microscopic surgery
Operative equipment with which to clearly observe the surgical field is essential in modern neurosurgery. An operating microscope provides detailed views of the neurovascular microstructures, and such microscopes have been routinely adopted worldwide for almost all cranial and spinal surgeries (44-46). Moreover, the microscope can be linked to other image-guiding instruments. Fluorescein or indocyanine green with the dedicated microscope filter can help to increase the extent of resection in patients with cerebral metastasis (47,48).
Neuronavigation
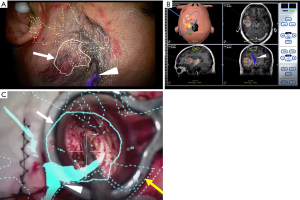
The use of an intraoperative frameless stereotactic navigation device, so-called “neuronavigation”, has been developed as an essential tool for complicated interventions including the surgical treatment of malignant tumors during the past few decades (49,50). A neuronavigation system allows the surgeon to relate the physical location of a tumor with the preoperative images such as computed tomography, MRI, positron emission tomography, and functional MRI (51). This enables an understanding of the surgical target and surrounding brain tissue anatomy and identification of the resection site (Figure 1A,B,C). There are two types of neuronavigation: optical neuronavigation and electromagnetic neuronavigation. The optical system allows the use of a variety of metal tools during surgery. However, the advantage of electromagnetic neuronavigation is elimination of the optical line-of-sight problem (52-54). The usefulness of electromagnetic neuronavigation is especially evident during endoscopic surgery for sellar lesions and ventricular lesions (55-57). The accuracy is high and comparable for both types of neuronavigation (58). One limitation of using a navigation system is that brain shift reduces the accuracy of surgical guidance. Brain shift is caused by cerebrospinal fluid leakage after cutting the dura mater, gravity, and the shift of surrounding brain tissue back to the resection cavity (59-61). Gerard et al. (51) reviewed 26 studies focusing on brain shift in neurosurgical intervention. No universal measurement technique was available to detect brain shift; thus, the degree of maximal brain shift widely ranged from 2.3 to 30.9 mm. In their review, Gerard et al. (51) concluded that one of the causes of brain shift is localization error of the pointer or measuring tool. Registration error immediately after patient-to-image registration reportedly ranges from 1 to 6 mm (62). Several techniques to minimize the influence of brain shift have been reported. Intraoperative MRI, which provides real-time feedback on the extent of resection and residual neoplasm, can overcome the brain shift problem by updating the source images used for neuronavigation (63,64). Additionally, the navigation-guided fence post procedure before cutting of the dura mater is a useful and safe technique to avoid brain shift during tumor resection (65). Several recent reports have indicated that intraoperative ultrasound combined with neuronavigation can improve the accuracy of neuronavigation during the surgery (66,67).
Neurophysiological monitoring
The use of intraoperative neurophysiological monitoring is essential to predict and prevent postoperative neurological deficits. Effective intraoperative mapping and monitoring techniques have developed in the context of glioma surgery (68-71). The purpose of intraoperative monitoring is to reliably identify cortical areas and subcortical pathways including motor, sensory, language, and cognitive functions (72,73), which leads to safe maximal resection of the tumor. A prospective controlled study showed that the use of intraoperative monitoring could achieve an equivalent extent of resection in both eloquent and non-eloquent areas (74). Zhang et al. (71) retrospectively evaluated the long-term functional and survival outcomes of patients with glioma after tumor resection with intraoperative neurophysiologic monitoring and reported that localization of gliomas in eloquent areas should no longer be viewed as a poor prognostic factor. Intraoperative monitoring of the motor systems was recently reported to help reduce surgery-related motor deficits also for surgical resection of metastatic brain tumor (75-77). For metastatic brain tumors, supramarginal resection including additional removal of the adjacent brain tissue is desired to prevent local recurrence (30,32). Therefore, intraoperative neurophysiological monitoring provides important functional information during resection of tumors, especially when the extent of resection reaches an eloquent area (77).
Leading-edge surgical instruments and techniques
Endoscope and exoscope
During the past two decades, endoscopic surgery has dramatically increased, especially in surgery for intraventricular lesions and in transsphenoidal surgery. Additionally, the visualization of deep structures is often better with an angled endoscope than a microscope (78). An endoscope has several characteristics that complement those of a microscope, making an endoscope a useful adjunct to microsurgery with a microscope (79,80). Recently, exoscope systems such as the video telescope operating monitor (VITOM; Karl Storz GmbH & Co., Tuttlingen, Germany) and ORBEYE (Sony Olympus Medical Solutions, Tokyo, Japan) were introduced as an alternative to a microscope and an endoscope. An exoscope enables surgeons to stand upright in a comfortable head-up position during surgery regardless of patient positioning or anatomy and provides outstanding image quality in a display (81-84). Moreover, development of three-dimensional technology in the exoscope provides a high perception of depth and surgical dissection techniques comparable with those of a microscope (85-88). Several studies have shown the effectiveness of an exoscope for surgical resection of metastatic brain tumors (89,90). In the future, all surgeries will be performed with a microscope, endoscope, exoscope, or a combination of these modalities according to the tumor site.
Tubular retractor
During surgical treatment of deep-seated lesions, obtaining a safe corridor into the tumor and visualizing the interface between the tumor and surrounding structures are important (91). Various kinds of brain retraction systems combined with a microscope or endoscope have been introduced to achieve these goals. The self-retaining retraction system was first introduced by Greenberg (92) in 1981. This system is widely used in brain surgery, although it is associated with a risk of brain infarction and brain damage due to excessive brain retraction pressure (93-95). Many recent reports have indicated the effectiveness of tubular retractors such as the ViewSite (Vycor Medical Inc., Boca Raton, FL, USA) (Figure 1C) (55,96-101). The ViewSite tubular retractor has a plastic body with a tapered end, which allows adjacent tissue to be visualized. Additionally, the ViewSite tubular retractor can be held with a self-retracting arm to prevent shifting of the operative field (101). Moreover, an endoscope and modified surgical instruments for endoscopic surgery can overcome the disadvantage of limited working space by the ViewSite retractor itself (55). The use of tubular retractors with an exoscope has recently shown promising results in the surgical resection of metastatic brain tumors (89,90,102).
Photodynamic detection
Increasing attention has recently been given to 5-aminolevulinic acid (5-ALA) (103-107), a precursor molecule in the heme biosynthetic pathway. Previous studies have demonstrated that both primary and metastatic brain tumors preferentially take up exogenous 5-ALA and store it as protoporphyrin IX (108,109). Several studies have demonstrated the usefulness of 5-ALA for surgical resection of metastatic brain tumors, including breast cancer (110-113). Marbacher et al. (113) assessed the frequency of positive 5-ALA fluorescence in a cohort of patients with metastases and found that 71% of the metastatic brain tumors from breast cancer were 5-ALA fluorescence-positive. Another study showed that the fluorescence intensity of 5-ALA was high in both the sentinel lymph node and primary lesion of breast cancer; thus, 5-ALA shows promise in the detection of metastatic tumors from breast cancer (114). Moreover, the combination of fluorescence and intraoperative monitoring has been shown to be effective with respect to resection radicality and functional preservation (115).
Future directions
High-intensity focused ultrasound (HIFU)
HIFU was recently proposed as a type of thermal therapy. HIFU has been successfully applied to the treatment of essential tremor (116). Modern HIFU treatment systems, called MRI-guided focused ultrasonography (MRgFUS) units, have evolved to include intraprocedural anatomy- and temperature-sensitive MRI guidance and hemispherical multi-element phased-array transducers, leading to accurate coagulation against the lesion (117). In the field of neurology, MRgFUS has been approved by the US Food and Drug Administration (FDA) for the treatment of essential tremor, chronic neuropathic pain, parkinsonism, and Parkinson’s disease. MacDonald et al. (72) reported the clinical application of MRgFUS in three patients with glioblastoma, which was the first time that an ultrasound beam was focused in a brain tumor through an intact skull. Additionally, Coluccia et al. (118) reported the effectiveness and safety of MRgFUS for recurrent glioblastoma. Regarding the application of MRgFUS to metastatic brain tumors, two clinical trials (NCT 00147056 and NCT 01473485: clinicaltrials.gov) are currently ongoing to verify the safety and efficacy of MRgFUS against brain tumors, whereas the reporting of another study’s findings is pending (NCT01698437). Moreover, HIFU has been used for palliation in patients with bone metastasis and in the treatment of breast cancer (119). MRgFUS can temporarily permeabilize the blood-brain barrier by its non-thermal effects on the targeted tissue (120-122), leading to prospective treatments of brain tumors (including breast cancer metastasis) such as targeted agents, nanoparticles, and immunotherapies (123-126).
Laser interstitial thermal therapy (LITT)
LITT is another thermal therapy for intracranial lesions and epilepsy, and it was approved as an ablation therapy by the FDA in 2007 (127). The mechanism of LITT involves the release of thermal energy caused by light absorption and scatter, which raises the temperature to 50 to 100 °C and results in coagulation necrosis (128). LITT can be used to both achieve a pathological diagnosis and perform ablative therapy (129). Additionally, a major benefit of LITT is the shorter recovery time and hospitalization period, especially in asymptomatic patients. In contrast, a drawback of LITT is the risk of significant postablation edema, especially in patients with tumors of >9 cm3 (130-132). LITT is reportedly as effective as conventional surgical resection for recurrent irradiated brain metastasis (129). Clinical trials involving LITT showed improved survival in patients with recurrent metastatic brain tumors although the varied pathology of the metastatic lesions limited the interpretation (133). Because insufficient evidence is available to make a recommendation regarding the use of LITT at this time (134), further prospective studies are needed to demonstrate the utility of LITT.
Oncolytic virus therapy and gene therapy
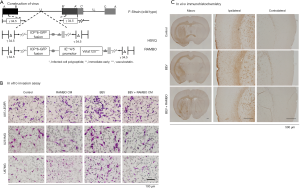
Oncolytic virus therapy has been described as a prospective treatment option that selectively targets cancer. Various types of oncolytic viruses have been engineered to increase the effectiveness of this treatment and have been shown to improve the therapeutic effect in preclinical research (135,136). We have also evaluated combination therapy with genetically engineered oncolytic viruses and systemic treatments such as molecular targeting drugs in mouse glioma models (Figure 2A,B,C) (137-139). Administration of talimogene laherparepvec into the tumor improved the durable response rates in a randomized phase III clinical trial (140), for which the FDA approved the use of this oncolytic virus for patients with recurrent melanoma. Moreover, phase I and II trials of HF10 in patients with malignant tumors, including recurrent metastatic breast carcinoma, have been successfully conducted (141). Although no oncolytic viruses have been approved for the treatment of brain tumors, we are now starting a phase I/II study evaluating the safety and effectiveness of Ad-SGE-REIC in patients with recurrent malignant glioma as gene therapy. Several recent reports have shown the effectiveness of oncolytic viruses against brain metastasis in preclinical models (142-144). Therefore, oncolytic viruses and gene therapy can be a clinically applicable therapeutic platform to target metastatic brain tumors from breast cancer.
Conclusions
The incidence of metastatic brain tumors from breast cancer has increased because of recent advancement in systemic treatment. Neuroimaging of metastatic brain tumors can estimate the molecular subtypes of breast cancer, which predicts the aggressiveness of the tumor. Surgical resection continues to play an important role in patients with a limited number of brain metastases and a relatively good performance status. En bloc tumor resection is basically recommended to prevent leptomeningeal disease. We predict that recent advancements in supporting neurosurgical tools and technologies will greatly improve the local control rate of brain metastasis. Many preclinical reports have described thermal therapy, oncolytic viral therapy, and gene therapy. In the near future, novel treatment modalities will emerge and evolve into standard treatments.
Acknowledgments
We thank Angela Morben, DVM, ELS, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tadahiko Shien and Kaori Terata) for the series “Loco-regional therapy for metastatic breast cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.68). The series “Loco-regional therapy for metastatic breast cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- NCGA. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330-7. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004;22:2865-72. [Crossref] [PubMed]
- Weil RJ, Palmieri DC, Bronder JL, et al. Breast cancer metastasis to the central nervous system. Am J Pathol 2005;167:913-20. [Crossref] [PubMed]
- Nolan C, Deangelis LM. Overview of metastatic disease of the central nervous system. Handb Clin Neurol 2018;149:3-23. [Crossref] [PubMed]
- Xiong Y, Cao H, Zhang Y, et al. Nomogram-predicted survival of breast cancer brain metastasis: a SEER-based population study. World Neurosurg 2019;128:e823-34. [Crossref] [PubMed]
- Olson EM, Abdel-Rasoul M, Maly J, et al. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol 2013;24:1526-33. [Crossref] [PubMed]
- Smirniotopoulos JG, Murphy FM, Rushing EJ, et al. Patterns of contrast enhancement in the brain and meninges. Radiographics 2007;27:525-51. [Crossref] [PubMed]
- Barajas RF Jr, Cha S. Imaging diagnosis of brain metastasis. Prog Neurol Surg 2012;25:55-73. [Crossref] [PubMed]
- Pope WB. Brain metastases: neuroimaging. Handb Clin Neurol 2018;149:89-112. [Crossref] [PubMed]
- Schwartz KM, Erickson BJ, Lucchinetti C. Pattern of T2 hypointensity associated with ring-enhancing brain lesions can help to differentiate pathology. Neuroradiology 2006;48:143-9. [Crossref] [PubMed]
- Yeh RH, Yu JC, Chu CH, et al. Distinct MR imaging features of triple-negative breast cancer with brain metastasis. J Neuroimaging 2015;25:474-81. [Crossref] [PubMed]
- Broom RJ, Tang PA, Simmons C, et al. Changes in estrogen receptor, progesterone receptor and Her-2/neu status with time: discordance rates between primary and metastatic breast cancer. Anticancer Res 2009;29:1557-62. [PubMed]
- Kalikaki A, Koutsopoulos A, Trypaki M, et al. Comparison of EGFR and K-RAS gene status between primary tumours and corresponding metastases in NSCLC. Br J Cancer 2008;99:923-9. [Crossref] [PubMed]
- Wang B, Guan ZZ, Liu DG, et al. Discordance of estrogen receptor (ER), progestin receptor (PR), and HER-2 receptor statuses between primary and metastatic focuses of breast cancer. Ai Zheng 2004;23:1710-3. [PubMed]
- Ichikawa T, Otani Y, Kurozumi K, et al. Phenotypic Transition as a Survival Strategy of Glioma. Neurol Med Chir (Tokyo) 2016;56:387-95. [Crossref] [PubMed]
- Baumert BG, Rutten I, Dehing-Oberije C, et al. A pathology-based substrate for target definition in radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys 2006;66:187-94. [Crossref] [PubMed]
- Mintz AH, Kestle J, Rathbone MP, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 1996;78:1470-6. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 1993;33:583-90. [Crossref] [PubMed]
- Kayama T, Sato S, Sakurada K, et al. Effects of surgery with salvage stereotactic radiosurgery versus surgery with whole-brain radiation therapy in patients with one to four brain metastases (JCOG0504): a phase III, noninferiority, randomized controlled trial. J Clin Oncol 2018;JCO2018786186. [Epub ahead of print]. [PubMed]
- Caruso JP, Moosa S, Fezeu F, et al. A cost comparative study of Gamma Knife radiosurgery versus open surgery for intracranial pathology. J Clin Neurosci 2015;22:184-8. [Crossref] [PubMed]
- Nahed BV, Alvarez-Breckenridge C, Brastianos PK, et al. Congress of neurological surgeons systematic review and evidence-based guidelines on the role of surgery in the management of adults with metastatic brain tumors. Neurosurgery 2019;84:E152-5. [Crossref] [PubMed]
- Blonigen BJ, Steinmetz RD, Levin L, et al. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2010;77:996-1001. [Crossref] [PubMed]
- Shimony N, Shofty B, Harosh CB, et al. Surgical resection of cerebral metastases leads to faster resolution of peritumoral edema than stereotactic radiosurgery: a volumetric analysis. Ann Surg Oncol 2017;24:1392-98. [Crossref] [PubMed]
- Carapella CM, Gorgoglione N, Oppido PA. The role of surgical resection in patients with brain metastases. Curr Opin Oncol 2018;30:390-95. [PubMed]
- Ammirati M, Nahed BV, Andrews D, et al. Congress of neurological surgeons systematic review and evidence-based guidelines on treatment options for adults with multiple metastatic brain tumors. Neurosurgery 2019;84:E180-2. [Crossref] [PubMed]
- Neuro-Oncology TJSf. Practical Guidelines for Neuro-Oncology 2019. Tokyo: Neuro-Oncology TJSf, 2019.
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998;280:1485-9. [Crossref] [PubMed]
- Kamp MA, Dibue M, Niemann L, et al. Proof of principle: supramarginal resection of cerebral metastases in eloquent brain areas. Acta Neurochir (Wien) 2012;154:1981-6. [Crossref] [PubMed]
- Pessina F, Navarria P, Cozzi L, et al. Role of surgical resection in patients with single large brain metastases: feasibility, morbidity, and local control evaluation. World Neurosurg 2016;94:6-12. [Crossref] [PubMed]
- Yoo H, Kim YZ, Nam BH, et al. Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg 2009;110:730-6. [Crossref] [PubMed]
- Chua TH, See AAQ, Ang BT, et al. Awake craniotomy for resection of brain metastases: a systematic review. World Neurosurg 2018;120:e1128-35. [Crossref] [PubMed]
- Kamp MA, Rapp M, Slotty PJ, et al. Incidence of local in-brain progression after supramarginal resection of cerebral metastases. Acta Neurochir (Wien) 2015;157:905-10; discussion 910-1. [Crossref] [PubMed]
- Suki D, Abouassi H, Patel AJ, et al. Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg 2008;108:248-57. [Crossref] [PubMed]
- Suki D, Hatiboglu MA, Patel AJ, et al. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery 2009;64:664-74; discussion 674-6. [Crossref] [PubMed]
- Patel AJ, Suki D, Hatiboglu MA, et al. Impact of surgical methodology on the complication rate and functional outcome of patients with a single brain metastasis. J Neurosurg 2015;122:1132-43. [Crossref] [PubMed]
- Patel AJ, Suki D, Hatiboglu MA, et al. Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurosurg 2010;113:181-9. [Crossref] [PubMed]
- Mut M. Surgical treatment of brain metastasis: a review. Clin Neurol Neurosurg 2012;114:1-8. [Crossref] [PubMed]
- Sun B, Huang Z, Wu S, et al. Cystic brain metastasis is associated with poor prognosis in patients with advanced breast cancer. Oncotarget 2016;7:74006-14. [Crossref] [PubMed]
- Press RH, Zhang C, Chowdhary M, et al. Hemorrhagic and cystic brain metastases are associated with an increased risk of leptomeningeal dissemination after surgical resection and adjuvant stereotactic radiosurgery. Neurosurgery 2019;85:632-41. [Crossref] [PubMed]
- Tomita Y, Sasaki T, Tanabe T, et al. Pyoktanin blue injection for resection of cystic brain tumor: a case report. No Shinkei Geka 2013;41:687-91. [PubMed]
- Hayashi N, Sasaki T, Tomura N, et al. Removal of a malignant cystic brain tumor utilizing pyoktanin blue and fibrin glue: technical note. Surg Neurol Int 2017;8:24. [Crossref] [PubMed]
- Jannetta PJ. The surgical binocular microscope in neurological surgery. Am Surg 1968;34:31-4. [PubMed]
- Yaşargil MG. A legacy of microneurosurgery: memoirs, lessons, and axioms. Neurosurgery 1999;45:1025-92. [Crossref] [PubMed]
- Yaşargil MG, Krayenbühl H. The use of the binocular microscope in neurosurgery. Bibl Ophthalmol 1970;81:62-5. [PubMed]
- Höhne J, Hohenberger C, Proescholdt M, et al. Fluorescein sodium-guided resection of cerebral metastases-an update. Acta Neurochir (Wien) 2017;159:363-7. [Crossref] [PubMed]
- Kim EH, Cho JM, Chang JH, et al. Application of intraoperative indocyanine green videoangiography to brain tumor surgery. Acta Neurochir (Wien) 2011;153:1487-95; discussion 1494-5. [Crossref] [PubMed]
- Roberts DW, Strohbehn JW, Hatch JF, et al. A frameless stereotaxic integration of computerized tomographic imaging and the operating microscope. J Neurosurg 1986;65:545-9. [Crossref] [PubMed]
- Yamada SM, Masahira N, Ikawa N, et al. Preoperative surgical approach planning for metastatic pituitary stalk tumor using multimodal fusion imaging in a neuronavigation system--case report. Neurol Med Chir (Tokyo) 2010;50:259-63. [Crossref] [PubMed]
- Gerard IJ, Kersten-Oertel M, Petrecca K, et al. Brain shift in neuronavigation of brain tumors: A review. Med Image Anal 2017;35:403-20. [Crossref] [PubMed]
- Komune N, Matsushima K, Matsuo S, et al. The accuracy of an electromagnetic navigation system in lateral skull base approaches. Laryngoscope 2017;127:450-59. [Crossref] [PubMed]
- Kurozumi K, Kameda M, Ishida J, et al. Simultaneous combination of electromagnetic navigation with visual evoked potential in endoscopic transsphenoidal surgery: clinical experience and technical considerations. Acta Neurochir (Wien) 2017;159:1043-48. [Crossref] [PubMed]
- Suess O, Kombos T, Kurth R, et al. Intracranial image-guided neurosurgery: experience with a new electromagnetic navigation system. Acta Neurochir (Wien) 2001;143:927-34. [Crossref] [PubMed]
- Matsumoto Y, Kurozumi K, Shimazu Y, et al. Endoscope-assisted resection of cavernous angioma at the foramen of Monro: a case report. Springerplus 2016;5:1820. [Crossref] [PubMed]
- Tomita Y, Kurozumi K, Inagaki K, et al. Delayed postoperative hyponatremia after endoscopic transsphenoidal surgery for pituitary adenoma. Acta Neurochir (Wien) 2019;161:707-15. [Crossref] [PubMed]
- Tomita Y, Kurozumi K, Terasaka T, et al. A Case of an Adrenocorticotropic Hormone-Producing Pituitary Adenoma Removed via Electromagnetic-Guided Neuroendoscopy. No Shinkei Geka 2016;44:473-9. [PubMed]
- Sieśkiewicz A, Łysoń T, Mariak Z, et al. Neuronavigation in transnasal endoscopic paranasal sinuses and cranial base surgery: comparison of the optical and electromagnetic systems. Otolaryngol Pol 2009;63:256-60. [PubMed]
- Dorward NL, Alberti O, Velani B, et al. Postimaging brain distortion: magnitude, correlates, and impact on neuronavigation. J Neurosurg 1998;88:656-62. [Crossref] [PubMed]
- Hill DL, Maurer CR Jr, Maciunas RJ, et al. Measurement of intraoperative brain surface deformation under a craniotomy. Neurosurgery 1998;43:514-26; discussion 527-8. [Crossref] [PubMed]
- Roberts DW, Hartov A, Kennedy FE, et al. Intraoperative brain shift and deformation: a quantitative analysis of cortical displacement in 28 cases. Neurosurgery 1998;43:749-58; discussion 758-60. [Crossref] [PubMed]
- Stieglitz LH, Fichtner J, Andres R, et al. The silent loss of neuronavigation accuracy: a systematic retrospective analysis of factors influencing the mismatch of frameless stereotactic systems in cranial neurosurgery. Neurosurgery 2013;72:796-807. [Crossref] [PubMed]
- Barbosa BJ, Mariano ED, Batista CM, et al. Intraoperative assistive technologies and extent of resection in glioma surgery: a systematic review of prospective controlled studies. Neurosurg Rev 2015;38:217-26; discussion 226-7. [Crossref] [PubMed]
- Kubben PL, ter Meulen KJ, Schijns OE, et al. Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol 2011;12:1062-70. [Crossref] [PubMed]
- Kajiwara K, Yoshikawa K, Ideguchi M, et al. Navigation-guided fence-post tube technique for resection of a brain tumor: technical note. Minim Invasive Neurosurg 2010;53:86-90. [Crossref] [PubMed]
- Ganau M, Ligarotti GK, Apostolopoulos V. Real-time intraoperative ultrasound in brain surgery: neuronavigation and use of contrast-enhanced image fusion. Quant Imaging Med Surg 2019;9:350-58. [Crossref] [PubMed]
- Moiraghi A, Prada F, Delaidelli A, et al. Navigated intraoperative 2-dimensional ultrasound in high-grade glioma surgery: impact on extent of resection and patient outcome. Oper Neurosurg (Hagerstown) 2020;18:363-73. [PubMed]
- Duffau H. Contribution of cortical and subcortical electrostimulation in brain glioma surgery: methodological and functional considerations. Neurophysiol Clin 2007;37:373-82. [Crossref] [PubMed]
- Neuloh G, Pechstein U, Schramm J. Motor tract monitoring during insular glioma surgery. J Neurosurg 2007;106:582-92. [Crossref] [PubMed]
- Szelényi A, Bello L, Duffau H, et al. Intraoperative electrical stimulation in awake craniotomy: methodological aspects of current practice. Neurosurg Focus 2010;28:E7. [Crossref] [PubMed]
- Zhang N, Yu Z, Hameed NUF, et al. Long-term functional and oncologic outcomes of glioma surgery with and without intraoperative neurophysiologic monitoring: a retrospective cohort study in a single center. World Neurosurg 2018;119:e94-105. [Crossref] [PubMed]
- MacDonald DB. Intraoperative neurophysiology of the motor system in children. Childs Nerv Syst 2010;26:595-6. [Crossref] [PubMed]
- Sanai N, Berger MS. Intraoperative stimulation techniques for functional pathway preservation and glioma resection. Neurosurg Focus 2010;28:E1. [Crossref] [PubMed]
- Kombos T, Picht T, Derdilopoulos A, et al. Impact of intraoperative neurophysiological monitoring on surgery of high-grade gliomas. J Clin Neurophysiol 2009;26:422-5. [Crossref] [PubMed]
- Krieg SM, Schaffner M, Shiban E, et al. Reliability of intraoperative neurophysiological monitoring using motor evoked potentials during resection of metastases in motor-eloquent brain regions: clinical article. J Neurosurg 2013;118:1269-78. [Crossref] [PubMed]
- Obermueller T, Schaeffner M, Shiban E, et al. Intraoperative neuromonitoring for function-guided resection differs for supratentorial motor eloquent gliomas and metastases. BMC Neurol 2015;15:211. [Crossref] [PubMed]
- Sanmillan JL, Fernandez-Coello A, Fernandez-Conejero I, et al. Functional approach using intraoperative brain mapping and neurophysiological monitoring for the surgical treatment of brain metastases in the central region. J Neurosurg 2017;126:698-707. [Crossref] [PubMed]
- Akiyama O, Matsushima K, Gungor A, et al. Microsurgical and endoscopic approaches to the pulvinar. J Neurosurg 2017;127:630-45. [Crossref] [PubMed]
- Abolfotoh M, Bi WL, Hong CK, et al. The combined microscopic-endoscopic technique for radical resection of cerebellopontine angle tumors. J Neurosurg 2015;123:1301-11. [Crossref] [PubMed]
- Ichikawa T, Otani Y, Ishida J, et al. Hybrid microscopic-endoscopic surgery for craniopharyngioma in neurosurgical suite: technical notes. World Neurosurg 2016;85:340-8.e1. [Crossref] [PubMed]
- Mamelak AN, Danielpour M, Black KL, et al. A high-definition exoscope system for neurosurgery and other microsurgical disciplines: preliminary report. Surg Innov 2008;15:38-46. [Crossref] [PubMed]
- Mamelak AN, Drazin D, Shirzadi A, et al. Infratentorial supracerebellar resection of a pineal tumor using a high definition video exoscope (VITOM(R)). J Clin Neurosci 2012;19:306-9. [Crossref] [PubMed]
- Mamelak AN, Nobuto T, Berci G. Initial clinical experience with a high-definition exoscope system for microneurosurgery. Neurosurgery 2010;67:476-83. [Crossref] [PubMed]
- Murai Y, Sato S, Yui K, et al. Preliminary clinical microneurosurgical experience with the 4K3-dimensional microvideoscope (ORBEYE) system for microneurological surgery: observation study. Oper Neurosurg (Hagerstown) 2019;16:707-16. [Crossref] [PubMed]
- Beez T, Munoz-Bendix C, Beseoglu K, et al. First clinical applications of a high-definition three-dimensional exoscope in pediatric neurosurgery. Cureus 2018;10:e2108. [PubMed]
- Oertel JM, Burkhardt BW. Vitom-3D for exoscopic neurosurgery: initial experience in cranial and spinal procedures. World Neurosurg 2017;105:153-62. [Crossref] [PubMed]
- Rossini Z, Cardia A, Milani D, et al. VITOM 3D: preliminary experience in cranial surgery. World Neurosurg 2017;107:663-68. [Crossref] [PubMed]
- Sack J, Steinberg JA, Rennert RC, et al. Initial experience using a high-definition 3-dimensional exoscope system for microneurosurgery. Oper Neurosurg (Hagerstown) 2018;14:395-401. [Crossref] [PubMed]
- Bakhsheshian J, Strickland BA, Jackson C, et al. Multicenter investigation of channel-based subcortical trans-sulcal exoscopic resection of metastatic brain tumors: a retrospective case series. Oper Neurosurg (Hagerstown) 2019;16:159-66. [Crossref] [PubMed]
- Gassie K, Wijesekera O, Chaichana KL. Minimally invasive tubular retractor-assisted biopsy and resection of subcortical intra-axial gliomas and other neoplasms. J Neurosurg Sci 2018;62:682-89. [Crossref] [PubMed]
- Kassam AB, Engh JA, Mintz AH, et al. Completely endoscopic resection of intraparenchymal brain tumors. J Neurosurg 2009;110:116-23. [Crossref] [PubMed]
- Greenberg IM. Self-retaining retractor and handrest system for neurosurgery. Neurosurgery 1981;8:205-8. [Crossref] [PubMed]
- Andrews RJ, Bringas JR. A review of brain retraction and recommendations for minimizing intraoperative brain injury. Neurosurgery 1993;33:1052-63; discussion 1063-4. [PubMed]
- Harada S, Nakamura T. Retraction induced brain edema. Acta Neurochir Suppl (Wien) 1994;60:449-51. [PubMed]
- Rosenørn J, Diemer NH. Reduction of regional cerebral blood flow during brain retraction pressure in the rat. J Neurosurg 1982;56:826-9. [Crossref] [PubMed]
- Hong CS, Prevedello DM, Elder JB. Comparison of endoscope- versus microscope-assisted resection of deep-seated intracranial lesions using a minimally invasive port retractor system. J Neurosurg 2016;124:799-810. [Crossref] [PubMed]
- Bander ED, Jones SH, Kovanlikaya I, et al. Utility of tubular retractors to minimize surgical brain injury in the removal of deep intraparenchymal lesions: a quantitative analysis of FLAIR hyperintensity and apparent diffusion coefficient maps. J Neurosurg 2016;124:1053-60. [Crossref] [PubMed]
- Fahim DK, Relyea K, Nayar VV, et al. Transtubular microendoscopic approach for resection of a choroidal arteriovenous malformation. J Neurosurg Pediatr 2009;3:101-4. [Crossref] [PubMed]
- Greenfield JP, Cobb WS, Tsouris AJ, et al. Stereotactic minimally invasive tubular retractor system for deep brain lesions. Neurosurgery 2008;63:334-9; discussion 339-40. [PubMed]
- Otani Y, Kurozumi K, Ishida J, et al. Combination of the tubular retractor and brain spatulas provides an adequate operative field in surgery for deep-seated lesions: Case series and technical note. Surg Neurol Int 2018;9:220. [Crossref] [PubMed]
- Recinos PF, Raza SM, Jallo GI, et al. Use of a minimally invasive tubular retraction system for deep-seated tumors in pediatric patients. J Neurosurg Pediatr 2011;7:516-21. [Crossref] [PubMed]
- Day JD. Transsulcal Parafascicular Surgery Using Brain Path(R) for Subcortical Lesions. Neurosurgery 2017;64:151-56. [Crossref] [PubMed]
- Pichlmeier U, Bink A, Schackert G, et al. Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol 2008;10:1025-34. [Crossref] [PubMed]
- Smith LG, Nakano I. Fluorescence-guided brain tumor surgery. World Neurosurg 2012;78:559-64. [Crossref] [PubMed]
- Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006;7:392-401. [Crossref] [PubMed]
- Stummer W, Tonn JC, Mehdorn HM, et al. Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. Clinical article. J Neurosurg 2011;114:613-23. [Crossref] [PubMed]
- Widhalm G, Wolfsberger S, Minchev G, et al. 5-Aminolevulinic acid is a promising marker for detection of anaplastic foci in diffusely infiltrating gliomas with nonsignificant contrast enhancement. Cancer 2010;116:1545-52. [Crossref] [PubMed]
- Kamp MA, Grosser P, Felsberg J, et al. 5-aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: a retrospective study. Acta Neurochir (Wien) 2012;154:223-8; discussion 228. [Crossref] [PubMed]
- Stummer W, Stepp H, Moller G, et al. Technical principles for protoporphyrin-IX-fluorescence guided microsurgical resection of malignant glioma tissue. Acta Neurochir (Wien) 1998;140:995-1000. [Crossref] [PubMed]
- Coburger J, Engelke J, Scheuerle A, et al. Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: a prospective study based on histopathological assessment. Neurosurg Focus 2014;36:E3. [Crossref] [PubMed]
- Ferraro N, Barbarite E, Albert TR, et al. The role of 5-aminolevulinic acid in brain tumor surgery: a systematic review. Neurosurg Rev 2016;39:545-55. [Crossref] [PubMed]
- Kamp MA, Munoz-Bendix C, Mijderwijk HJ, et al. Is 5-ALA fluorescence of cerebral metastases a prognostic factor for local recurrence and overall survival? J Neurooncol 2019;141:547-53. [Crossref] [PubMed]
- Marbacher S, Klinger E, Schwyzer L, et al. Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus 2014;36:E10. [Crossref] [PubMed]
- Frei KA, Bonel HM, Frick H, et al. Photodynamic detection of diseased axillary sentinel lymph node after oral application of aminolevulinic acid in patients with breast cancer. Br J Cancer 2004;90:805-9. [Crossref] [PubMed]
- Feigl GC, Ritz R, Moraes M, et al. Resection of malignant brain tumors in eloquent cortical areas: a new multimodal approach combining 5-aminolevulinic acid and intraoperative monitoring. J Neurosurg 2010;113:352-7. [Crossref] [PubMed]
- Elias WJ, Huss D, Voss T, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med 2013;369:640-8. [Crossref] [PubMed]
- Hynynen K, McDannold N, Clement G, et al. Pre-clinical testing of a phased array ultrasound system for MRI-guided noninvasive surgery of the brain--a primate study. Eur J Radiol 2006;59:149-56. [Crossref] [PubMed]
- Coluccia D, Fandino J, Schwyzer L, et al. First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J Ther Ultrasound 2014;2:17. [Crossref] [PubMed]
- Kennedy JE, Ter Haar GR, Cranston D. High intensity focused ultrasound: surgery of the future? Br J Radiol 2003;76:590-9. [Crossref] [PubMed]
- Berghoff AS, Preusser M. Role of the blood-brain barrier in metastatic disease of the central nervous system. Handb Clin Neurol 2018;149:57-66. [Crossref] [PubMed]
- Hersh DS, Eisenberg HM. Current and future uses of transcranial focused ultrasound in neurosurgery. J Neurosurg Sci 2018;62:203-13. [PubMed]
- Hersh DS, Kim AJ, Winkles JA, et al. Emerging Applications of Therapeutic Ultrasound in Neuro-oncology: Moving Beyond Tumor Ablation. Neurosurgery 2016;79:643-54. [Crossref] [PubMed]
- Alkins R, Burgess A, Kerbel R, et al. Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro Oncol 2016;18:974-81. [Crossref] [PubMed]
- Kobus T, Zervantonakis IK, Zhang Y, et al. Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated blood-brain barrier disruption. J Control Release 2016;238:281-88. [Crossref] [PubMed]
- Nance E, Timbie K, Miller GW, et al. Non-invasive delivery of stealth, brain-penetrating nanoparticles across the blood-brain barrier using MRI-guided focused ultrasound. J Control Release 2014;189:123-32. [Crossref] [PubMed]
- Sta Maria NS, Barnes SR, Weist MR, et al. Low dose focused ultrasound induces enhanced tumor accumulation of natural killer cells. PLoS One 2015;10:e0142767. [Crossref] [PubMed]
- Kang JY, Wu C, Tracy J, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia 2016;57:325-34. [Crossref] [PubMed]
- Franck P, Henderson PW, Rothaus KO. Basics of lasers: history, physics, and clinical applications. Clin Plast Surg 2016;43:505-13. [Crossref] [PubMed]
- Hong CS, Deng D, Vera A, et al. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J Neurooncol 2019;142:309-17. [Crossref] [PubMed]
- Ashraf O, Patel NV, Hanft S, et al. Laser-Induced Thermal Therapy in Neuro-Oncology: A Review. World Neurosurg 2018;112:166-77. [Crossref] [PubMed]
- Jethwa PR, Barrese JC, Gowda A, et al. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: initial experience. Neurosurgery. 2012;71:133-44; 144-5.
- Patel NV, Jethwa PR, Shetty A, et al. Does the real-time thermal damage estimate allow for estimation of tumor control after MRI-guided laser-induced thermal therapy? Initial experience with recurrent intracranial ependymomas. J Neurosurg Pediatr 2015;15:363-71. [Crossref] [PubMed]
- Missios S, Bekelis K, Barnett GH. Renaissance of laser interstitial thermal ablation. Neurosurg Focus 2015;38:E13. [Crossref] [PubMed]
- Elder JB, Nahed BV, Linskey ME, et al. Congress of neurological surgeons systematic review and evidence-based guidelines on the role of emerging and investigational therapties for the treatment of adults with metastatic brain tumors. Neurosurgery 2019;84:E201-3. [Crossref] [PubMed]
- Hua L, Wakimoto H. Oncolytic herpes simplex virus therapy for malignant glioma: current approaches to successful clinical application. Expert Opin Biol Ther 2019;19:845-54. [Crossref] [PubMed]
- Oka T, Kurozumi K, Shimazu Y, et al. A super gene expression system enhances the anti-glioma effects of adenovirus-mediated REIC/Dkk-3 gene therapy. Sci Rep 2016;6:33319. [Crossref] [PubMed]
- Fujii K, Kurozumi K, Ichikawa T, et al. The integrin inhibitor cilengitide enhances the anti-glioma efficacy of vasculostatin-expressing oncolytic virus. Cancer Gene Ther 2013;20:437-44. [Crossref] [PubMed]
- Shimazu Y, Kurozumi K, Ichikawa T, et al. Integrin antagonist augments the therapeutic effect of adenovirus-mediated REIC/Dkk-3 gene therapy for malignant glioma. Gene Ther 2015;22:146-54. [Crossref] [PubMed]
- Tomita Y, Kurozumi K, Yoo JY, et al. Oncolytic herpes virus armed with vasculostatin in combination with bevacizumab abrogates glioma invasion via the CCN1 and AKT signaling pathways. Mol Cancer Ther 2019;18:1418-29. [Crossref] [PubMed]
- Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015;33:2780-8. [Crossref] [PubMed]
- Eissa IR, Naoe Y, Bustos-Villalobos I, et al. Genomic signature of the natural oncolytic herpes simplex virus HF10 and its therapeutic role in preclinical and clinical trials. Front Oncol 2017;7:149. [Crossref] [PubMed]
- Chen X, Han J, Chu J, et al. A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget 2016;7:27764-77. [Crossref] [PubMed]
- Du W, Seah I, Bougazzoul O, et al. Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. Proc Natl Acad Sci U S A 2017;114:E6157-65. [Crossref] [PubMed]
- Kuruppu D, Tanabe KK. HSV-1 as a novel therapy for breast cancer meningeal metastases. Cancer Gene Ther 2015;22:506-8. [Crossref] [PubMed]


