
Long term results of different radiotherapy techniques and fractions for esophageal squamous cell carcinoma
Introduction
As a common and aggressive neoplasia, esophageal cancer occurs more than 450,000 new cases and 400,000 deaths in 2012 (1). As the major pathologic type of esophageal cancer in East Asia including China, esophageal squamous cell carcinoma (ESCC) has a poor long-term prognosis (2). Surgery and concurrent chemoradiotherapy are both recommended regimens for patients with locally advanced ESCC.
To improve the outcome of radiotherapy, a variety of altered fractionation schedules, such as hyperfractionation and continuous accelerated hyperfractionated fraction (CAHF) have been used for esophageal cancers (3,4). And these have been identified by some reviews and meta-analyses as potentially advantageous compared to conventional fraction (5,6). To avoid severe mucous membrane toxicities after altered fractionated schedules, a variant of accelerated treatment, referred to as ‘concomitant boost’ or ‘late course accelerated hyperfractionated fraction (LCAF)’ technique, was devised (7,8).
Six prospective phase II or III clinical trials had been conducted in our hospital from 2002 to 2014 (9-14). Of these, patients with locally advanced ESCC underwent definitive radiotherapy using conventional two-dimensional (2D) or three-dimensional (3D) radiotherapy techniques including three-dimensional conformal radiotherapy (3DCRT) and intensity-modulated radiotherapy (IMRT).
In the present study, we aim to update the results of previous six prospective trials. All patients were combined to investigate the long-term survival, relapse and metastasis of ESCC.
Methods
Patients
From August 1996 to August 2009, six prospective studies including locally advanced ESCC patients were conducted in our hospital: three randomized phase III clinical trials using 2DRT and three phase II clinical trials using 3DCRT/IMRT. Of three 2DRT trials, one compared late course accelerated hyperfractionated fraction (LCAF) alone against continuous accelerated hyperfractionated fraction (CAHF) alone, whereas the others compared LCAF and LCAF with concurrent chemotherapy (CT). There were two 3D trials using LACF and CAHF radiotherapy alone, respectively. The other one compared LCAF radiotherapy with or without CT. These regimens were summarized in Table 1. Totally, 383 patients received LCAF and 92 patients received CAHF radiotherapy. Overall, 303 patients were treated with 2DRT, and 172 patients were treated with 3DRT. The study protocol was approved by the Ethics Committee of Fudan University Shanghai Cancer Center (ID: 050432-4-1212B). All participants gave informed consent before taking part.
Table 1
| Study | Year of publication | Clinical trial | Technique | Schedule | Radiation dose (Gy) | No. of patients |
|---|---|---|---|---|---|---|
| Wang (4) | 2002 | Phase III | 2DRT | LCAF | 68.4 | 52 |
| 2DRT | CAHF | 66.0 | 49 | |||
| Zhao (5) | 2005 | Phase III | 2DRT | LCAF | 66.0 | 57 |
| 2DRT | LCAF + CT | 68.4 | 54 | |||
| Liu (6) | 2005 | Phase III | 2DRT | LCAF | 68.4 | 44 |
| 2DRT | LCAF + CT | 68.4 | 47 | |||
| Zhao (7) | 2010 | Phase II | 3DCRT | LCAF | 68.4 | 53 |
| Wang (8) | 2012 | Phase II | 3DCRT/IMRT | CAHF | 57–60 | 43 |
| Tang (9) | 2014 | Phase II | 3DCRT/IMRT | LCAF/CF + CT | 61.2/68.4 | 76 |
3DCRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; 2DRT, conventional two-dimensional radiotherapy; LCAF, late course accelerated hyperfractionated fraction; CAHF, continuous accelerated hyperfractionated fraction; CT, chemotherapy
Treatment regimens
All patients included in the study received either LCAF or CAHF radiotherapy. LCAF radiotherapy was administered at 1.8 Gy/Fx in day 1–5 per week during the first two-thirds of the radiotherapy course, representing a total dose 41.4 Gy/23 Fx/4–5 weeks. Then, the course was followed by accelerated hyperfraction using reduced fields twice daily at 1.5 Gy/Fx, with a minimum interval of 6 hours between fractions. The accelerated dose was approximately 27 Gy, thus the total dose was 68.4 Gy/41 Fx/44 days. CAHF radiotherapy was conducted twice daily at 1.5 Gy/Fx with a minimum interval of 6 hours between fractions. The radiotherapy was administered at day 1–5 per week until cumulative dose of 39 Gy in 26 fractions, after of which reduced fields were used. The total dose was 66 Gy in 44 fractions over 4.4 weeks for 2DRT group and 57 to 60 Gy in 38 to 40 fractions over 3.8 to 4.0 weeks in the 3DRT group. The majority of enrolled patients received definitive radiotherapy alone.
Radiotherapy technique
All patients in current study received involved-field radiotherapy either 2DRT or 3DRT. No prophylactic radiation was conducted to cover the supraclavicular region or other lymph node regions. For cervical esophageal cancer, two anterior oblique fields with wedge filters were used in the 2DRT group. As to thoracic tumor, a three-field approach including one anterior and two posterior oblique portals was employed. In order to cover subclinical lesions, the width of the fields was adjusted to at least 2 to 3 cm margins off the tumor margin. To cover clinical tumors, the lengths of the fields were 3 to 5 cm off the ends of the lesion. The prescribed dose was adjusted based on the isodose line that covered the organs at risk. In the 3DCRT/IMRT group, the gross tumor volume (GTV) was defined as any visible primary tumor and metastatic lymph nodes in a CT scan or barium esophagram. The following radiographic criteria were used to identify metastatic nodes: ≥1 cm on the shortest axis in the intra-thoracic and intra-abdominal regions; and nodes alongside the recurrent nerve with their shortest axis ≥0.5 cm. The clinical target volume (CTV) consisted of CTV1 and CTV2. CTV1 was defined as tumor CTV plus 3 cm in longitudinal direction, while CTV2 was defined as the lymph node GTV plus 1 cm also in longitudinal direction without lateral margins. The planning target volume (PTV) was defined by adding a 1 cm margin around either CTV1 or CTV2.
Evaluation of toxicity
The Common Terminology Criteria of Adverse Events (CTCAE) was consulted to estimate radiation related toxicities. In the first 90 days of treatment course, occurred reactions were defined as acute radiation toxicities, while those occurring outside 90 days of treatment were defined as late radiation toxicities.
Statistical analysis
The survival time was defined as the period from the end of initial treatment to death or the last follow-up evaluation, and the local control time was defined to progression or death for any reason. χ2 test was used to estimate the statistic significance between groups. The Kaplan-Meier method and log-rank were used to compare overall survival and local control curves. Statistical analysis was conducted with SPSS (Version 22.0).
Results
Patients and overall survival
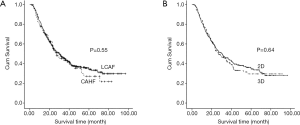
At our hospital, six randomized phase II-III clinical trials for locally advanced ESCC patients were conducted from 2002 to 2014 (Table 1). Basic characteristics of enrolled patients are presented in Table 2. The median follow-up time was 57.5 months (20.2 to 96.9 months). For all 475 patients, the overall median survival time was 30 months. The 1-, 3- and 5-year estimated overall survival rates and local control rates were 78%, 44% and 33% and 84%, 58% and 46%, respectively. Comparison of survival times between different radiotherapy techniques and fractions is shown in Figure 1. No significant difference was observed in both LACF vs. CAHF and 2DRT vs. 3DRT groups (P=0.55 and 0.64, respectively).
Table 2
| Parameters | n (%) |
|---|---|
| Sex | |
| Male | 340 (71.6) |
| Female | 135 (28.4) |
| Age (yr) | |
| Median (range) | 57.8 (39–76) |
| Lesion location | |
| Cervical | 32 (6.7) |
| Upper thoracic | 150 (31.6) |
| Middle thoracic | 271 (57.1) |
| Low thoracic | 22 (4.6) |
| Length (cm) | |
| Median (range) | 6.3 (2−10) |
| TNM (6th) | |
| I | 8 (1.7) |
| II | 236 (49.7) |
| III | 197 (41.5) |
| IV | 34 (7.2) |
Patterns of failure and treatment toxicity
The incidence of local/regional failure was 28% and distant failure was 22%. The overall failure frequency was 49%. The detailed incidences of Grade 3 or higher acute and late treatment-related toxicities were presented in Table 3. In total, the frequencies of ≥ Grade 3 acute pneumonitis and esophagitis were 52/475 (11%) and 80/475 (17%), respectively. The frequency of Grade III acute pneumonitis was 17% (16/92) in the CAHF group and 9.4% (36/383) in the LCAF group, respectively. However, the difference between the two groups was not statistically significant (P=0.08). The rates of Grade III and IV acute esophagitis was 47% (43/92) in the CAHF group and 9.6% (37/383) in the LCAF group, respectively. The difference was statistically significant (P<0.01) (Table 3). There were 15 patients who died of perforation/fistula that were considered as serious adverse events during radiotherapy. Patterns of failure and toxicity based on different radiotherapy techniques and fractions were also summarized in Table 3.
Table 3
| Pattern | n (%) | LCAF | CAHF | P | 2DRT | 3DRT | P |
|---|---|---|---|---|---|---|---|
| Local/regional failure | 151/475 (32%) | 120/383 (31%) | 31/92 (34%) | NS | 104/303 (34%) | 47/172 (27%) | NS |
| Metastasis | 107/475 (23%) | 83/383 (22%) | 24/92 (26%) | NS | 71/303 (23%) | 36/172 (21%) | NS |
| Failure | 234/475 (49%) | 191/383 (50%) | 43/92 (47%) | NS | 163/303 (54%) | 71/172 (41%) | NS |
| Acute esophagitis (≥ grade III) | 80/475 (17%) | 37/383 (9.6%) | 43/92 (47%) | <0.01 | 66/303 (22%) | 14/172 (8.1%) | <0.01 |
| Acute pneumonitis (≥ grade III) | 52/475 (11%) | 36/383 (9.4%) | 16/92 (17%) | 0.08 | 45/303 (15%) | 7/172 (4.1%) | <0.01 |
| Perforation (fistula) | 15/475 (3.2%) | 12/383 (3.1%) | 3/92 (3.2%) | NS | 8/303 (2.6%) | 7/172 (4.1%) | NS |
Metastatic sites in detail
Lymph node metastases occupied the first area of metastatic esophageal squamous cell carcinoma with high frequency (n=111, 23%). Among them, 41 patients had supraclavicular lymph nodes metastases. A total of 107 patients were considered to have metastatic disease at distant organs: lung (n=39, 8.2%), liver (n=35, 7.4%), bone (n=21, 4.4%), brain (n=8, 1.7%), and pleura (n=4, 1%). Metastatic sites based on different radiotherapy techniques and fractions were summarized in Table 4. No significant difference was observed in both LACF vs. CAHF and 2DRT vs. 3DRT groups (P>0.05).
Table 4
| Sites | n (%) | LCAF | CAHF | P | 2DRT | 3DRT | P |
|---|---|---|---|---|---|---|---|
| Lung | 39/475 (8.2%) | 30/383 (7.8%) | 9/92 (9.8%) | NS | 23/303 (7.6%) | 16/172 (9.3%) | NS |
| Liver | 35/475 (7.4%) | 29/383 (7.6%) | 6/92 (6.5%) | NS | 25/303 (8.3%) | 10/172 (5.8%) | NS |
| Bone | 21/475 (4.4%) | 15/383 (3.9%) | 6/92 (6.5%) | NS | 15/303 (4.9%) | 6/172 (3.5%) | NS |
| Brain | 8/475 (1.7%) | 6/383 (1.6%) | 2/92 (2.2%) | NS | 5/303 (1.7%) | 3/172 (1.7%) | NS |
| Pleura | 4/475 (1%) | 3/383 (0.7%) | 1/92 (1.1%) | NS | 3/303 (1.0%) | 1/172 (0.6%) | NS |
| Lymph nodes [Supraclavicular] | 111/475 (23%) [41/475 (8.6%)] | 69/383 (18%) | 22/92 (24%) | NS | 71/303 (23%) | 40/172 (23%) | NS |
Discussion
We conducted a retrospective study using data from our prospective clinical trials to delineate the prognosis of radiotherapy including the 2D vs. 3D radiotherapy techniques and LCAF vs. CAHF. All prospective studies included in the study were performed at our cancer center, and the detailed patient characteristics and toxicity data were collected. There were 303 patients treated with 2D radiotherapy and 172 patients with 3D radiotherapy. Our previously published study demonstrated that no significant difference existed in overall survival and in local control rates between the 2D and 3D radiotherapy groups (15). In the current study, significant fewer incidences of acute esophagitis and pneumonitis were observed in 3DRT comparison of 2DRT. Due to better conformity of target volume and tumor, better protection of normal tissues can be conducted in 3DRT (16,17).
CAHF was based on a dual premise that aggressive shortening of the overall treatment time and a reduction in the radiation dose per fraction (18). The schedule was conducted at the beginning of radiotherapy course. However, LCAF was administered at conventional fraction during the first two-thirds of the radiotherapy course and then was followed by accelerated hyperfraction. In theory, the prevalence of early side effects was different based on different fractions. In the current study, fewer acute radiation esophagitis was observed in LCAF comparison of CAHF. The result was comparable to that in our previous report (9). From the radiobiologic point of view, the esophagus mucous membrane is early response tissue. In the treatment schedule, there is a higher ETD and higher accumulated weekly dose in CAHF than LACF, which may be the reason of higher incidence of acute radiation esophagitis.
Despite the improvements noted with multimodality treatment in esophageal cancer, cure rates are consistently dismal. The overall 5-year survival rate was 33%, and local control rate was 46%. The survival rate was slightly higher than reported rates: 20–30% in many studies (19,20). The majority of prior studies concentrated on patients with stage II and III ESCC and that may be the reason for the difference. In RTOG 94-05, the rate of local/regional failure and persistent local disease was 50–55%. Our result was similar with previous research (21).
In our study, local control was defined as the primary site of tumor. The incidence of local/regional failure in the study was 28%and distant failure was 22%. In RTOG 94-05, the rates of local failure were 9% and 12% in high dose and standard dose groups, respectively. Regional failure rates were 7% in both groups. The frequency of local/regional failure in RTOG 94-05 was 16–19%. Distant failure rates in RTOG 94-05 were 9% and 16%, respectively (22). Our results were consistent with the RTOG 94-05 results. No significant difference about local control and metastasis between LCAF and CAHF.
Regarding metastatic sites, additional published research analyzed data from the SEER database where the primary histology was adenocarcinoma (23-25). In our results, the first metastasis was lung, which was not same with the published result that reported the liver (26). Liver metastasis was second metastatic site in the current study. In fact, esophageal squamous cell carcinoma and adenocarcinoma show different metastatic characteristics. In esophageal adenocarcinoma, liver metastases represents the most frequent metastatic site and the second most frequent site is the lung (26). Anatomically, we know that the blood supply of different esophageal sites is different. The blood supply of lower thoracic esophagus mainly arises from the portal vein. Thus, liver was the most common site of metastases. In non-Eastern populations, the main histology of esophageal cancer is adenocarcinoma at the gastroesophageal junction. Venous drainage from the upper and middle thoracic esophagus was through the azygos and hemiazygos veins (24). In the patients in our study, the major site for ESCC was the upper and middle thoracic esophagus. This finding may be the reason why the liver was not the most common metastasis site. Research indicates that survival time is different according to metastasis sites in esophageal squamous cell carcinoma. Distant lymph node metastases were associated with better survival compared to liver, bone, or lung metastases. Overall survival was worse for bone metastases (median, 4 months) when compared to that of metastases to other sites (26).
In the current study, the incidence of brain metastasis was 1.1%, which was consistent with published research (27). Weinberg et al. presented a case series of 1,512 esophageal cancer patients treated from 1993 through 2001 with an incidence of brain metastasis of 1.7% (28). However, Smith and Miller reported a 13% incidence of brain metastasis in a small cohort of 53 patients. In addition, all patients with brain metastasis in that report were identified by histology to have adenocarcinoma (29). Perhaps differences in the incidence of brain metastasis are histology dependent.
LCAF aim to decrease tumor proliferation by shortening the overall treatment time, which may result in fewer late side effects to the mucus membranes and skins.
Conclusions
In conclusion, our findings provide evidence regarding prognosis of ESCC patients undergoing radiotherapy based on different radiotherapy techniques and fractions. About half of our patients suffered local/regional failure following radiotherapy. Fewer incidences of acute toxicities were observed in 3DRT treatment compared with 2DRT. Comparison with LCAF, more incidences of severe acute esophagitis were observed in CAHF.
Acknowledgments
The manuscript was edited for proper English language, grammar, punctuation, spelling, and overall style by one or more of the highly qualified native English speaking editors at American Journal Experts.
Funding: This study was financially sponsored by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.47). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Ethics Committee of Fudan University Shanghai Cancer Center (ID: 050432-4-1212B). All participants gave informed consent before taking part. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381-7. [Crossref] [PubMed]
- Jeremic B, Shibamoto Y, Acimovic L, et al. Accelerated hyperfractionated radiation therapy and concurrent 5-fluorouracil/cisplatin chemotherapy for locoregional squamous cell carcinoma of the thoracic esophagus: a phase II study. Int J Radiat Oncol Biol Phys 1998;40:1061-6. [Crossref] [PubMed]
- Ma JB, Song YP, Yu JM, et al. Linear correlation between patient survival and decreased percentage of tumor [(18)F]fluorodeoxyglucose uptake for late-course accelerated hyperfractionated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;82:1535-40. [Crossref] [PubMed]
- Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 2006;368:843-54. [Crossref] [PubMed]
- Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys 2014;89:13-20. [Crossref] [PubMed]
- Li C, Wang X, Wang X, et al. A multicenter phase III study comparing Simultaneous Integrated Boost (SIB) radiotherapy concurrent and consolidated with S-1 versus SIB alone in elderly patients with esophageal and esophagogastric cancer - the 3JECROG P-01 study protocol. BMC Cancer 2019;19:397. [Crossref] [PubMed]
- Li M, Fu C, Zhang W, et al. Phase I study of concurrent selective lymph node late-course accelerated hyperfractionated radiotherapy and S-1 plus cisplatin for locally advanced oesophageal squamous cell carcinoma. Br J Radiol 2016;89:20150476. [Crossref] [PubMed]
- Wang Y, Shi XH, He SQ, et al. Comparison between continuous accelerated hyperfractionated and late-course accelerated hyperfractionated radiotherapy for esophageal carcinoma. Int J Radiat Oncol Biol Phys 2002;54:131-6. [Crossref] [PubMed]
- Zhao KL, Shi XH, Jiang GL, et al. Late course accelerated hyperfractionated radiotherapy plus concurrent chemotherapy for squamous cell carcinoma of the esophagus: a phase III randomized study. Int J Radiat Oncol Biol Phys 2005;62:1014-20. [Crossref] [PubMed]
- Liu G, Wang Y, Guo XM, et al. Late course accelerated hyperfractionated irradiation combined with intraluminal hyperthermia for esophageal carcinoma. Chinese J Radiat Oncol 2005;14:259-61.
- Zhao KL, Ma JB, Liu G, et al. Three-dimensional conformal radiation therapy for esophageal squamous cell carcinoma: is elective nodal irradiation necessary? Int J Radiat Oncol Biol Phys 2010;76:446-51. [Crossref] [PubMed]
- Wang JH, Li XL, Chen Y, et al. Patterns of failure associated with involved field after definitive radiotherapy for squamous cell carcinoma of the esophagus. China Oncol 2012:601-4.
- Tang HR, Ma HF, An SM, et al. A Phase II Study of Concurrent Chemoradiotherapy With Paclitaxel and Cisplatin for Inoperable Esophageal Squamous Cell Carcinoma. Am J Clin Oncol 2016;39:350-4. [Crossref] [PubMed]
- Deng JY, Wang C, Shi XH, et al. Reduced toxicity with three-dimensional conformal radiotherapy or intensity-modulated radiotherapy compared with conventional two-dimensional radiotherapy for esophageal squamous cell carcinoma: a secondary analysis of data from four prospective clinical trials. Dis Esophagus 2016;29:1121-7. [Crossref] [PubMed]
- Gupta T, Kannan S, Ghosh-Laskar S, et al. Systematic review and meta-analyses of intensity-modulated radiation therapy versus conventional two-dimensional and/or or three-dimensional radiotherapy in curative-intent management of head and neck squamous cell carcinoma. PLoS One 2018;13:e0200137. [Crossref] [PubMed]
- You R, Cao YS, Huang PY, et al. The Changing Therapeutic Role of Chemo-radiotherapy for Loco-regionally Advanced Nasopharyngeal Carcinoma from Two/Three-Dimensional Radiotherapy to Intensity-Modulated Radiotherapy: A Network Meta-Analysis. Theranostics 2017;7:4825-35. [Crossref] [PubMed]
- Saunders MI, Rojas AM, Parmar MK, et al. Mature results of a randomized trial of accelerated hyperfractionated versus conventional radiotherapy in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2010;77:3-8. [Crossref] [PubMed]
- Toh Y, Numasaki H, Tachimori Y, et al. Current status of radiotherapy for patients with thoracic esophageal cancer in Japan, based on the Comprehensive Registry of Esophageal Cancer in Japan from 2009 to 2011 by the Japan Esophageal Society. Esophagus 2020;17:25-32. [Crossref] [PubMed]
- Chen J, Lin Y, Cai W, et al. A new clinical staging system for esophageal cancer to predict survival after definitive chemoradiation or radiotherapy. Dis Esophagus 2018;31: [Crossref] [PubMed]
- Wang X, Liu X, Li D, et al. Concurrent Selective Lymph Node Radiotherapy and S-1 Plus Cisplatin for Esophageal Squamous Cell Carcinoma: A Phase II Study. Ann Surg Oncol 2019;26:1886-92. [Crossref] [PubMed]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [Crossref] [PubMed]
- Welch G, Ross HJ, Patel NP, et al. Incidence of brain metastasis from esophageal cancer. Dis Esophagus 2017;30:1-6. [Crossref] [PubMed]
- Ai D, Zhu H, Ren W, et al. Patterns of distant organ metastases in esophageal cancer: a population-based study. J Thorac Dis 2017;9:3023-30. [Crossref] [PubMed]
- Wu SG, Xie WH, Zhang ZQ, et al. Surgery Combined with Radiotherapy Improved Survival in Metastatic Esophageal Cancer in a Surveillance Epidemiology and End Results Population-based Study. Sci Rep 2016;6:28280. [Crossref] [PubMed]
- Wu SG, Zhang WW, He ZY, et al. Sites of metastasis and overall survival in esophageal cancer: a population-based study. Cancer Manag Res 2017;9:781-8. [Crossref] [PubMed]
- Feng W, Zhang P, Zheng X, et al. Incidence and treatment of brain metastasis in patients with esophageal carcinoma. World J Gastroenterol 2015;21:5805-12. [Crossref] [PubMed]
- Weinberg JS, Suki D, Hanbali F, et al. Metastasis of esophageal carcinoma to the brain. Cancer 2003;98:1925-33. [Crossref] [PubMed]
- Smith RS, Miller RC. Incidence of brain metastasis in patients with esophageal carcinoma. World J Gastroenterol 2011;17:2407-10. [Crossref] [PubMed]


