
Mortality from heart disease following radiotherapy in esophageal carcinoma: a retrospective cohort study in US SEER cancer registry
Introduction
Esophageal carcinoma is an incredibly aggressive malignancy cancer with a stable incidence. Approximately 17,290 new cases were diagnosed in 2018 in the USA alone (1). Because surgery alone could not prolong overall survival (OS), researchers grew more interests in developing neoadjuvant therapy (2). Radiotherapy, including neoadjuvant radiotherapy (NeoRT), has been proven to raise OS rates in esophageal carcinoma patients, especially those in non-metastatic advanced (>T2 or N+) stage (3,4). There has been compelling evidence that radiation therapy (RT) is concerned with a raised mortality risk from heart disease when the heart is located in the RT region in lung cancer, breast cancer, and Hodgkin lymphoma (5-9). Moreover, the radiation intensity is usually too high for the heart in esophageal carcinoma patients.
Radiation is a potent inducer of thrombotic and inflammatory changes, including increased production and release of thromboxane and von Willebrand factor and decreased production of prostacyclin, thrombomodulin, and ADPase. Both in-vitro and in-vivo studies demonstrated that microvascular injury and fibrosis induced by radiation contributed to the worsening of atherosclerosis (10-12). The cardiac diseases induced by radiation were reported to include coronary artery disease, pericarditis, cardiomyopathy, valvular disease, abnormal conduction, and cardiac death (13-15).
The relationship between RT and cardiac death mortality in esophageal carcinoma patients remained unclear. Gayed et al. found cardiac complications after radiotherapy were more common in patients with esophageal cancer than those with lung cancer, although the difference was not significant (16). It was suggested that cardiac death after radiotherapy in patients with esophageal carcinoma could not be ignored.
Because the outcome was improved due to adding RT as a supplementary therapy, the number of patients at risk of RT-associated toxicity is growing. Evaluating the risk of cardiac death and identifying patients based on risk factors can be helpful in screening the patients who should receive treatment to diminish cardiotoxicity. In this research, the database of the Surveillance, Epidemiology, and End Results (SEER) was used to find out the cardiac death rate in patients with esophageal cancer who have received RT.
Furthermore, whether RT toxicity is related to tumor location and radiation sequence remained undiscovered. While in patients with breast cancer and lung cancer, those who had tumors on their left side suffered from a higher risk of cardiovascular diseases after RT compared with those who had tumors on their right side (17,18).
Methods
Source of data and population of the study
Subjects were chosen from the database of SEER Program in the US National Cancer Institute which recently reported the incidence of cancer and consequent mortality of specific causes in 30% of the United States population. Patients who were diagnosed as multiple tumors, identified through autopsy and death certificates, or died of cancer were excluded. Our study included 8,210 cancer survivors who were diagnosed as esophageal cancer from January 1, 1973 to December 31, 2012. Patients identified through autopsy and death certificates, diagnosed with other types of cancer or followed-up less than 3 months were excluded. Cardiac death was defined as death from heart diseases (recode: 50060). The characteristics of the subjects and carcinoma listed below were adjusted in the analysis of multiple variables: ethnicity/race; year of diagnosis; age; sex; disease stage; tumor grade; histology; stage, esophageal subsite, and modality from treatment (chemotherapy and RT).
Statistical analysis
Descriptive data were applied to demonstrate the characteristics of the disease in two groups (RT and non-RT). Chi-squared tests were used to make comparisons between the groups. Cardiac specific survival (CSS) was evaluated with the Kaplan-Meier test (along with log-rank statistics). Cox hazard regression model was applied in the multivariate analysis of survival. Confidence intervals (95% CIs) and hazard ratios (HRs) were calculated. All statistics analyses were performed with SPSS statistics software (Version 25.0.0, IBM Corp in Armonk, New York, USA) and R Version 2.13.2 (website: http://www.r-project.org). P<0.01 or 0.05 indicated significant difference.
Results
Features of the patients and carcinoma
We analyzed 8,210 patients who survived from cancer and they were predominantly Caucasian (84.0%) and males (75.5%). One-third of the subjects had cancers of low differentiation but barely any distant metastasis. The average follow-up was 68 months in patients with irradiation and 78 months in those without irradiation.
As shown in Table 1, 43% patients had not received radiotherapy and 57% received radiotherapy, including 3,941 patients who were treated with chemoradiotherapy. The average age was 65 years in non-RT group and 64 in RT group. Compared with the patients in non-RT group, those who received radiotherapy were more prone to receive chemotherapy (84.3% vs. 13.3%, P≤0.0001). However, distribution of lower or middle thoracic esophageal carcinoma and superjacent tumor in two groups were almost identical, suggesting that in this population carcinoma location scarcely had influence on whether radiotherapy was given (56.9% and 43.1% vs. 57.2% and 42.8%, P=0.855). Additionally, the number of patients with adenocarcinoma in the non-RT group was increased compared with the RT group.
Table 1
| Factor | No radiation | Radiation | |||
|---|---|---|---|---|---|
| N | (%) | N | (%) | ||
| Entire cohort | 3,534 | 43 | 4,676 | 57 | |
| Mean age | 65 | 64 | |||
| <40 | 137 | 3.9 | 189 | 4 | |
| 40–49 | 187 | 5.3 | 287 | 6.1 | |
| 50–59 | 778 | 22 | 1,082 | 23.1 | |
| 60–69 | 1,128 | 31.9 | 1,569 | 33.6 | |
| 70–79 | 913 | 25.8 | 1,093 | 23.4 | |
| ≥80 | 391 | 11.1 | 456 | 9.8 | |
| Chemo | |||||
| No | 3,064 | 86.7 | 735 | 15.7 | |
| Yes | 470 | 13.3 | 3,941 | 84.3 | |
| AJCC stage | |||||
| Local | 1,194 | 33.8 | 452 | 9.7 | |
| Regional | 408 | 11.5 | 1,720 | 36.8 | |
| Distant | 148 | 4.2 | 227 | 4.9 | |
| Unknow | 1,784 | 50.5 | 2,277 | 48.7 | |
| Surgery | |||||
| Unperformed | 882 | 25 | 1,953 | 41.8 | |
| Performed | 1,893 | 53.6 | 1,694 | 36.2 | |
| Unknow | 759 | 21.5 | 1,029 | 22 | |
| Year of diagnosis | |||||
| 1973–1999 | 857 | 24.3 | 1,192 | 25.5 | |
| 2000–2013 | 2,677 | 75.7 | 3,484 | 74.5 | |
| Subsite | |||||
| Cervical | 29 | 0.8 | 162 | 3.5 | |
| Upper thoracic | 107 | 3 | 307 | 6.6 | |
| Middle thoracic | 467 | 13.2 | 940 | 20.1 | |
| Lower thoracic | 2,269 | 64.2 | 2,671 | 57.1 | |
| Unknow | 662 | 18.7 | 596 | 12.7 | |
| Race | |||||
| Black | 278 | 7.9 | 567 | 12.1 | |
| Other | 206 | 5.8 | 264 | 5.6 | |
| White | 3,050 | 86.3 | 3,845 | 82.2 | |
| Grade | |||||
| G1 | 388 | 11 | 271 | 5.8 | |
| G2 | 1,145 | 32.4 | 1,703 | 36.4 | |
| G3/G4 | 901 | 25.5 | 1,763 | 37.7 | |
| Unknow | 1,100 | 31.1 | 939 | 20.1 | |
| Sex | |||||
| Female | 813 | 23 | 1,193 | 25.5 | |
| Male | 2,721 | 77 | 3,483 | 74.5 | |
| Histology | |||||
| Adenocarcinoma | 2,281 | 64.5 | 2,182 | 46.7 | |
| Squamous cell | 811 | 22.9 | 2,180 | 46.6 | |
| Other | 442 | 12.5 | 314 | 6.7 | |
Survival analysis and independent prognostic factors of cardiac death
Multivariate analysis with Cox regression was performed (Table 2) and the following factors were found to have a negative influence on CSS: radiotherapy, early year of diagnosis, distant metastasis, black race, refusing chemotherapy, and elder age. Histological features and tumor grade were not independent predictors for survival. Age (P<0.001; HR, 14.297; 95% CI: 9.174–22.283) and radiation (P<0.001; HR, 1.952; 95% CI: 1.684–2.263) were found to be the most significant independent prognostic metrics.
Table 2
| Variable | Hazard ratio | 95% CI | P |
|---|---|---|---|
| Radiation: yes/no | 1.952 | 1.684–2.263 | <0.001 |
| Year: <2000/>2000 | 1.359 | 1.082–1.705 | 0.008 |
| Grade | |||
| G1 | 1 | [Reference] | |
| G2 | 0.926 | 0.764–1.121 | 0.429 |
| G3/G4 | 0.914 | 0.754–1.108 | 0.36 |
| Stage | |||
| I | 1 | [Reference] | |
| II | 1.044 | 0.819–1.33 | 0.73 |
| III | 1.079 | 0.826–1.41 | 0.576 |
| IV | 1.812 | 1.318–2.492 | <0.001 |
| Chemotherapy: yes/no | 0.687 | 0.597–0.79 | <0.001 |
| Race: black/white | 1.452 | 1.246–1.693 | <0.001 |
| Sex: male/female | 0.703 | 0.625–0.792 | <0.001 |
| Hist: squamous/adenoid | 1.016 | 0.889–1.162 | 0.814 |
| Subsite | |||
| Cervical | 1.123 | 0.976–1.292 | 0.105 |
| Upper thoracic | 1 | [Reference] | |
| Middle thoracic | 0.925 | 0.714–1.198 | 0.556 |
| Lower thoracic | 1.018 | 0.902–1.149 | 0.769 |
| Age | |||
| <40 | 1 | [Reference] | |
| 40–49 | 1.823 | 1.077–2.083 | 0.025 |
| 50–59 | 2.976 | 1.915–3.465 | <0.001 |
| 60–69 | 4.946 | 3.206–7.632 | <0.001 |
| 70–79 | 8.174 | 5.295–12.619 | <0.001 |
| ≥80 | 14.297 | 9.174–22.283 | <0.001 |
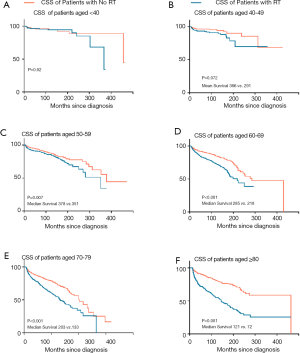
Given the significant influence of age, Kaplan-Meier method for CSS was performed by comparing radiation with non-radiation groups to evaluate the effect of radiation on cardiac death in the subgroup of age (Figure 1). RT was closely associated with declined CSS in patients over 50 years old. Five-year survival rate gap was 7.4%, 8.4%, 12.6% and 13.9% between irradiated and non-irradiated patients aged 50–59, 60–69, 70–79 and over 80 years old, respectively. Patients older than 70 years old appeared to be more easily affected by this risk factor compared with younger patients.
Influence of cancer sites and the duration after radiotherapy on the cardiac mortality ratios
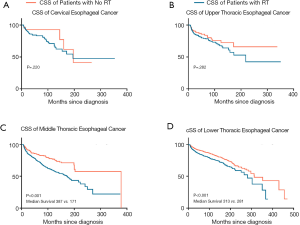
CSS was higher if the tumors in non-irradiated patients were located in the middle (HR, 1.872; 95% CI: 1.464–2.395; P<0.001) and lower thoracic segments of the esophagus (HR, 1.539; 95% CI: 1.464–1.772; P<0.001) (Figure 2). In non-irradiated patients with upper thoracic (P=0.181; 95% CI: 0.853–2.318; HR, 1.406) or cervical esophagus cancer (P=0.273; 95% CI: 0.663–4.282; HR, 1.685), the superiority of CSS was not statistically significant.
In order to evaluate whether the post-radiation cardiac death changed over time, we analyzed patients diagnosed before 1995 to make sure that the follow-up time exceeded 20 years. Among patients with middle or lower thoracic esophagus carcinoma, the HR of post-radiation cardiac death was the highest (HR, 2.21 and 1.84, respectively) during the first year since diagnosis. From the second year to the second decade since diagnosis, the HR declined steadily by time, but maintained at 1.46 and 1.15, respectively (Table 3). Thus, we found proof of actual hazard, even in the first year after irradiation.
Table 3
| Time (months) | Middle thoracic | Lower thoracic | |||||
|---|---|---|---|---|---|---|---|
| No RT | RT | HR | No RT | RT | HR | ||
| 12 | 0.033 | 0.073 | 2.212 | 0.026 | 0.048 | 1.846 | |
| 24 | 0.089 | 0.149 | 1.674 | 0.051 | 0.090 | 1.765 | |
| 36 | 0.132 | 0.132 | 1.402 | 0.074 | 0.122 | 1.649 | |
| 60 | 0.155 | 0.262 | 1.690 | 0.074 | 0.122 | 1.649 | |
| 120 | 0.232 | 0.412 | 1.776 | 0.163 | 0.237 | 1.454 | |
| 240 | 0.425 | 0.621 | 1.461 | 0.356 | 0.411 | 1.154 | |
Influence of radiation time on survival
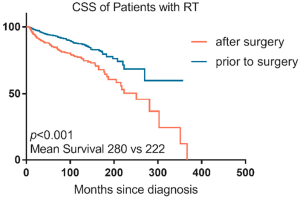
A total of 3,587 patients received both surgery and RT among which 1,935 patients’ radiation sequences were lost. The ratio of patients receiving preoperative or postoperative RT was 4:1 (1,343 vs. 309). Patients in preoperative RT group had an increased survival rate compared with those in postoperative RT group (P<0.001; 95% CI: 0.37–0.62; HR, 0.48). The 10- and 5-year survival rates of CSS were 87% and 93%, separately, in pre-operative RT group comparing 85% and 77% in post-operative RT groups (Figure S1). In subgroup analysis of subsite, the prolonged CSS of preoperative RT against postoperative RT was seen in the middle (248 vs. 150 months in average survival; P<0.001), and lower thoracic segments of the esophagus (283 vs. 239 months in average survival; P<0.001) (data were not given). CSS did not increase in upper thoracic esophagus cancer group (205 vs. 153 months in average survival; P=0.400) or cervical esophagus cancer group (152 vs. 197 months in average survival; P=0.958) (data were not given).
Discussion
The database of SEER Program in the US National Cancer Institute included approximately 10–14% of the whole US population. The database was applied to figure out whether the risk of cardiac death increased in esophageal carcinoma patients who received radiotherapy. In this study, this risk was found dominantly increased in patients aged over 50 years old and those who had tumors located in middle or lower thoracic esophagus.
Darby et al. reported that there was an increased comparative risk of ischemic heart disorder disease of about 7.4% per 1 Gy average heart dose in patients with breast cancer (19). It was reported that higher RT dose was the most significant factor influencing the prognosis (20). Although RT dose was not defined in previous studies, over V30 in pericardium was found to cause cardiac complications.
Most studies proposed that radiation-related cardiac diseases are the adverse reaction which usually occurred in the advanced stage. In atomic bomb survivors, the incidence of heart disorders did not increase significantly until 12 years after exposure (21). In women with breast cancer, the cardiac disease occurs chiefly after the first decade following radiation and the risks would increase in the next 12 years (7). But patients with esophageal carcinoma are not the same as patients with breast carcinoma and those who survived from atomic bomb, because the doses of radiation their heart received are a bit higher. In this study, the risk of cardiac death was the highest in the first year after radiotherapy and slowly decreased over time but would not disappear. This finding was consistent with the research which proved that patients with esophageal cancer would suffer from toxicity in the first 2 years following radiation treatment (20).
Additionally, preoperative RT was better than post-operative treatment in decreasing cardiac toxicity. Similar phenomenon was found in rectum cancer that preoperative CRT had a higher locoregional control ratio, survival rate and toxicity profile (22). In theory, preoperative RT was advantageous in low dose and promoting tumor downstaging (23). Wojcieszynski et al. reported that postoperative treatments required higher radiation dose, resulting that the heart and lung would receive more radiation. Meanwhile, patients receiving preoperative RT tended to accept additional interventions following surgeries, for example, adjuvant chemotherapy (24).
Recently, a large number of patients with esophageal cancer have received combined modality strategies with curative intension. Although the survival rate of the patients with different kinds of cancers has been improved, cardiac toxicity induced by anti-neoplastic agents remains a severe issue. In traditional chemotherapy, cardiac conditions after application of anthracyclines have been a critical problem for a long time. However, biological molecules and targeted therapies can result in cardiac dysfunction. Nowadays, immunotherapies have been introduced into tumor treatment strategies. The two most important therapies target the programmed cell death 1 (PD-1) and its ligand PD-L1 as well as cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4). These methods have brought good outcomes in cancer treatment. However, relevant research indicated that PD-1 deletion and CTLA-4 inhibition could contribute to autoimmune myocarditis. Furthermore, PD-L1 and PD-1 can be expressed in human and rodent cardiomyocytes (25). It has been reported that lethal heart failure could be induced by these therapies. Accumulating clinical studies have combined immunotherapy with chemoradiation to treat esophageal cancer. In our research, radiotherapy was shown to be toxic to the heart. According to previous studies, immunotherapy could also cause heart diseases (26). A study has demonstrated that chest radiotherapy combined with immunotherapy will affect survival rates. The possible cause is the increase of T cells infiltrating in heart and lung tissue after the treatment of chest irradiation and anti-PD-1 antibody (26). Radiotherapy combined with chemotherapy also increases the cardiotoxicity. As the most common chemotherapeutic drugs, cisplatinum and 5-FU are both reported to be related to raised risks of cardiovascular diseases. Using the combination of mitomycin, ifosfamide with cisplatin, gemcitabine with paclitaxel, carboplatin with paclitaxel or carboplatin alone may not increase the cardiac toxicity in patients with lung cancer (17).
Although cardiac toxicity is associated with radiation dose, the decrease of the heart dose, even with progressive radiation delivery methods such as VMAT or IMRT, will contribute to a higher dose in lung with a raised risk of fibrosis and pneumonitis. Proton therapy can solve this problem, but it is relatively expensive and not widely available. Therefore, selecting patients who can gain the most benefits from the proton therapy is of vital importance (27).
Computed tomography and myocardial perfusion imaging have been used to evaluate subclinical and early heart diseases. Some studies have demonstrated changes in myocardium metabolism and motion disorders of the myocardium in the areas with higher radiation dose. These changes are consistent with the results that focal ischemia, microvasculature and fibrosis appeared in animal studies and autopsy (20).
Recently, Sharma et al. have discovered a novel tetrapeptide Ac-SDKP which seems to be cardio-protective mainly via preventing the aggressive fibrotic process in cardiac tissues when rat models were exposed to radiation (28). But doubts were raised that fibrosis was not the only mechanism of heart damage induced by radiation in human, which included much more complex factors. Researchers also provided suggestions for reducing the radiation dose in heart: (I) maneuvers to keep the heart away from the area that received radiation (e.g., breath-hold, prone positioning); (II) improvements for highly conformal radiation treatment, such as stereotactic body RT; (III) real-time tissue monitoring, including image guidance, such as magnetic resonance imaging or cone beam computed tomography; (IV) strategies of directly delivering the radiation into the lesions such as brachytherapy; and (V) alternative therapies, such as proton or heavy particle therapy (29).
An advantage of our research is the adequate samples of 8,210 which enable the study to have sufficient resource to conduct stratified multivariate analysis and make the findings more reliable. The limitations are the incapability of using cumulative intensity or doses of radiation and chemotherapy treatments to evaluate the risk. Moreover, we have not distinguished the patients who had heart disease before radiotherapy, which may overestimate the cardiac mortality associated with radiotherapy.
Conclusions
In brief, risks of cardiac death were increased in esophageal cancer survivors who received RT. This risk would increase if patients were older than 70 years old or RT was performed after the surgeries. In patients with esophageal cancer, radiation would cause cardiac death since the first year after diagnosis, which is much earlier compared with breast cancer. Early detection and prevention in radiated patients and use of heart protective agents are necessary.
This study was aimed to highlight the importance of cardiac protection in radiotherapy for patients with esophageal cancer.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.21). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Cancer Institute: Surveillance Research Cancer Control and Population Sciences: Joinpoint Regression Program. Available online: http://surveillancecancergov/joinpoint/
- Geh JI, Crellin AM, Glynne-Jones R. Preoperative (neoadjuvant) chemoradiotherapy in oesophageal cancer. Br J Surg 2001;88:338-56. [Crossref] [PubMed]
- Schwer AL, Ballonoff A, McCammon R, et al. Survival effect of neoadjuvant radiotherapy before esophagectomy for patients with esophageal cancer: a surveillance, epidemiology, and end-results study. Int J Radiat Oncol Biol Phys 2009;73:449-55. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Taylor CW, McGale P, Darby SC. Cardiac risks of breast-cancer radiotherapy: a contemporary view. Clin Oncol (R Coll Radiol) 2006;18:236-46. [Crossref] [PubMed]
- Lally BE, Detterbeck FC, Geiger AM, et al. The risk of death from heart disease in patients with nonsmall cell lung cancer who receive postoperative radiotherapy: analysis of the Surveillance, Epidemiology, and End Results database. Cancer 2007;110:911-7. [Crossref] [PubMed]
- Darby SC, McGale P, Taylor CW, et al. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol 2005;6:557-65. [Crossref] [PubMed]
- Haybittle JL, Brinkley D, Houghton J, et al. Postoperative radiotherapy and late mortality: evidence from the Cancer Research Campaign trial for early breast cancer. BMJ 1989;298:1611-4. [Crossref] [PubMed]
- Boivin JF, Hutchison GB, Lubin JH, et al. Coronary artery disease mortality in patients treated for Hodgkin's disease. Cancer 1992;69:1241-7. [Crossref] [PubMed]
- Sporn LA, Rubin P, Marder VJ, et al. Irradiation induces release of von Willebrand protein from endothelial cells in culture. Blood 1984;64:567-70. [Crossref] [PubMed]
- Stewart FA, Heeneman S, Te Poele J, et al. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE-/- mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol 2006;168:649-58. [Crossref] [PubMed]
- Roman MJ, Pickering TG, Schwartz JE, et al. Association of carotid atherosclerosis and left ventricular hypertrophy. J Am Coll Cardiol 1995;25:83-90. [Crossref] [PubMed]
- Adams MJ, Lipshultz SE, Schwartz C, et al. Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol 2003;13:346-56. [Crossref] [PubMed]
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007;99:365-75. [Crossref] [PubMed]
- Carver JR, Shapiro CL, Ng A, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol 2007;25:3991-4008. [Crossref] [PubMed]
- Gayed I, Gohar S, Liao Z, et al. The clinical implications of myocardial perfusion abnormalities in patients with esophageal or lung cancer after chemoradiation therapy. Int J Cardiovasc Imaging 2009;25:487-95. [Crossref] [PubMed]
- Hardy D, Liu CC, Cormier JN, et al. Cardiac toxicity in association with chemotherapy and radiation therapy in a large cohort of older patients with non-small-cell lung cancer. Ann Oncol 2010;21:1825-33. [Crossref] [PubMed]
- Onwudiwe NC, Kwok Y, Onukwugha E, et al. Cardiovascular event-free survival after adjuvant radiation therapy in breast cancer patients stratified by cardiovascular risk. Cancer Med 2014;3:1342-52. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- Beukema JC, van Luijk P, Widder J, et al. Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer? Radiother Oncol 2015;114:85-90. [Crossref] [PubMed]
- Preston DL, Shimizu Y, Pierce DA, et al. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950-1997. Radiat Res 2003;160:381-407. [Crossref] [PubMed]
- Roh MS, Colangelo LH, O'Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124-30. [Crossref] [PubMed]
- Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg 2003;185:538-43. [Crossref] [PubMed]
- Wojcieszynski AP, Berman AT, Wan F, et al. The impact of radiation therapy sequencing on survival and cardiopulmonary mortality in the combined modality treatment of patients with esophageal cancer. Cancer 2013;119:1976-84. [Crossref] [PubMed]
- Varricchi G, Marone G, Mercurio V, et al. Immune Checkpoint Inhibitors and Cardiac Toxicity: An Emerging Issue. Curr Med Chem 2018;25:1327-39. [Crossref] [PubMed]
- Fokas E, Rodel C. Definitive, Preoperative, and Palliative Radiation Therapy of Esophageal Cancer. Viszeralmedizin 2015;31:347-53. [PubMed]
- Langendijk JA, Lambin P, De Ruysscher D, et al. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother Oncol 2013;107:267-73. [Crossref] [PubMed]
- Sharma UC, Sonkawade SD, Spernyak JA, et al. A Small Peptide Ac-SDKP Inhibits Radiation-Induced Cardiomyopathy. Circ Heart Fail 2018;11:e004867. [Crossref] [PubMed]
- Lenihan DJ, Cuculich P. Cardioprotection During Therapeutic Radiation Treatment. Circ Heart Fail 2018;11:e005294. [Crossref] [PubMed]


