MicroRNA expression integrated analysis and identification of novel biomarkers in small cell lung cancer: a meta-analysis
Introduction
Recent studies showed that lung cancer was still the most common tumor in China and even the world, accounting for 18.4% of all tumor deaths (1). Small cell lung cancer (SCLC) is a subtype of lung cancer, as accounting for 10% to 15% in lung cancer. As the difficulty in early diagnosis, short treatment time window and lacking specific therapeutic regimens for SCLC, the five-year survival rate of the patient was only about 5% (2,3). Therefore, the key points to improving SCLC survival is deeply understand the tumorigenesis mechanism and exploration of more effective and improved treatment methods.
An increasing number of studies have demonstrated that microRNA was an important tumor suppressor during carcinogenesis as interacted with targeted mRNA. It involved in promoting central biological processes such as immune regulation, chemical resistance and tumor metastasis in various tumors (4,5). In recent years, benefited from high-throughput platforms development, microarray technology has become an effective and promising tool as exploring the role of small regulatory gene molecules in many human diseases (6,7). But the inter-lab results were not entirely consistent due to the small sample effects, non-replication and the lack of crossplatform standardization. To overcome these limitations, a meta-analysis of these independent studies data may be essential for the role of microRNAs in SCLC biologic process (8).
Microarray data of small cell lung cancer were collected from the gene expression omnibus (GEO) and EBI Array Express database. The recent published robust ranking aggregation method, which was specifically designed to compare several different platforms for ranking gene list arrays, was used to identify differential expression of microRNA between SCLC sample datasets and adjacent normal sample/normal sample datasets (9). Then, we predicted microRNA s regulatory genes. Pathway analysis was performed to identify common overlapping gene and predict the physiological effect of significantly deregulated microRNA regulation on the occurrence and development of SCLC.
The study explored the pathogenesis of SCLC by identifying the key microRNAs in carcinogenesis of SCLC. Furthermore, functional analysis and bioinformatics enrichment analysis of target genes were conducted, which may provide a new perspective on the exploration of tumorigenetic mechanism and targeted diagnostic strategies of SCLC.
Methods
Selection of studies and datasets
Selection of studies and datasets for SCLC microRNA microarrays expression profiling studies were performed in NCBI GEO database (www.ncbi.nlm.nih.gov/geo/) and EBI Array Express database (AE) (www.ebi.nc.uk/arrayexpress/). Retrieval using search term TITLE-ABS-KEY((((((Small Cell Lung Carcinoma) OR Small Cell Lung Cancer) OR Oat Cell Lung Cancer) OR Small Cell Cancer Of The Lung) OR Carcinoma, Small Cell Lung) OR Oat Cell Carcinoma of Lung) OR SCLC) AND profil* AND(((((((((((((((((MicroRNAs) OR MicroRNA) OR microRNAs) OR Micro RNA) OR RNA, Micro) OR microRNA) OR Primary MicroRNA) OR MicroRNA, Primary) OR Primary microRNA) OR microRNA, Primary) OR pri-microRNA) OR pri microRNA) OR RNA, Small Temporal) OR Temporal RNA, Small) OR stRNA) OR Small Temporal RNA) OR pre-microRNA) OR pre microRNA). The last search was performed in june 2019. The studies were included based on the following criteria: (I) samples were from humans; (II) focused on the diagnostic potential of microRNAs for SCLC tissue; (III) included microRNA array; and (IV) came from raw data of datasets. The exclusion criteria were as follows: (I) non-English papers; (II) case reports, letters, commentaries, meeting records, or review articles; (III) focused on animal models or cancer cell lines; (IV) cancer samples number fewer than 3 patients; (V) individual preselected candidate genes; (VI) Studies that profiled different histologic subtypes but did not include noncancerous tissue; and (VII) duplicate records. All evaluations were independently performed by two authors (Dandan Han and Lailing Li) to make sure the accurate inclusion of studies. The discrepancies were resolved by discussion with a third author (Xiaolei Zhang). This study was based on published literature. Therefore, all the included studies obtain relative ethics approval. All data sets used in this study are shown in Table 1.
Table 1
| Author and year | Region | Platform | Number of microRNA probes | Tumor type | Tissue | Number of samples | Series GEO accession | Refs. |
|---|---|---|---|---|---|---|---|---|
| Tan et al., 2010 | China | GPL8716 | 565 | SCLC | Lung | 27 pair | GSE15008 | (10) |
| Jin et al., 2015 | China | GPL19622 | 746 | SCLC | Lung | 16 TU, 3N | GSE74190 | (11) |
| Ohba et al., 2013 | Japan | GPL9948 | 601 | SCLC | Lung | 35 TU, 8 N | GSE19945 | (12) |
| Yoshimoto et al., 2016 | Japan | GPL16670 | 1,226 | SCLC | Lung | 9 TU, 6 N | GSE77380 | (13) |
SCLC, small cell lung cancer; TU, tumor samples; N, noncancerous samples; pairs, TU and N samples from the same patient.
Integrated analysis of gene expression datasets
Datasets was downloaded from gene expression omnibus. According to the platform annotation file, Probes IDs were annotated to gene symbol. If the Probes ID was not matched the gene symbol, the Probes ID was deleted. For different probes mapped to the same microRNA, the final expression value of the microRNA took the average expression value of different probes. All microRNA names were standardized according to miRBase version 22 (14). The raw microarray data were pre-processed individually for quality control (including background adjustment and normalization) by robust multi-array average (RMA). We used the computer of R version 3.4.4 software Bioconductor package to calculate and generate the relevant data (15,16).
To obtain the lists of statistically significant expressed microRNAs in each data set, the Bayesian statistical analysis was employed to compare the expression of SCLC samples compared with adjacent normal samples/normal tissue samples via the limma package in Bioconductor of R language (17). In the four included studies, 27 patients were treated with paracancer tissue as the control group, and 60 SCLC patients were treated with healthy lung tissue as the control group (8). Differentially expressed microRNAs were screened with the thresholds of Benjamini-Hochberg adjusted P<0.05 and |log2 (fold change)|>1. Up- and down-regulated probes were determined according to their fold differences. To assess the correlations between the results of each individual studies, exclude the effect of microRNA spectral analysis technique or sample size on this study, DIANA (Divisive Analysis) of cluster packet was applicated to do hierarchical clustering analysis of the Differentially expressed microRNAs. Spearman rank correlation and average linkage method were adopted.
Meta-analysis of microRNA expression
Gene integration of differentially expressed microRNAs identified from four studies was performed using RobustRankAggreg7 implemented as an R package (18). The lists of extracted up- and down-regulated microRNAs were prioritized based on their Benjamini-Hochberg adjusted P values (less than 0.05 was considered to be statistically significant). RRA method was an aggregate probability model with robust to noise, which was convenient to calculate the significance probability of all elements in the final ranking. The stability of p value evaluated and a cross validation algorithm was used. All the microRNA gene lists of the selected articles were ordered according to P value without considering the change of folding information (P<0.05). Assign a P value to each element in the list, indicating how high the element ranks compared to an empty model that expects random ordering. The analysis was repeated 10,000 times, each time excluding a random list of genes. in the end, an average of the assumed values for each microRNA round is obtained.
Bioinformatic analysis
Four individual databases (TargetScan, miRTarBase, MicroRNADB and DIANA) were utilized to identify the predicted targets of the meta-signature microRNA. Target prediction algorithms as follow, TargetScan v6.1 (http://www.targetscan.org/), MicroRNADB-2016 (http://www.mirdb.org/), DIANA-microT-CDS v5.0 (http://diana.imisathena-innovation.gr/) and miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/). Mammalian conservative microRNAs were used for the prediction of target genes. Only genes with target sites in 3'UTR were used. In order to reduce the predicted false positive rate and improve the persuasiveness, consensus targets for the further study were then defined as genes predicted by at least two of three algorithms (TargetScan, MicroRNADB and DIANA) plus validated targets from miRTarBase (19-22).
To access the pathways of SCLC-related different express microRNA found in the meta-analysis, Database for Annotation, Visualization and Integrated Discovery (DAVID) (http://www.david.ncifcrf.gov.) was applicated to do Enrichment analyses (23). Pathway identification and enrichment analysis for the consensus targets of each microRNA in SCLC were investigated using gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG). There were three categories derived from GO analysis, which were biological processes (BP), cellular components (CC) and molecular functions (MF). The significance of GO and KEGG pathway correlation was expressed as P value (P<0.05) (24-26).
Results
Search strategy and study inclusion/exclusion criteria
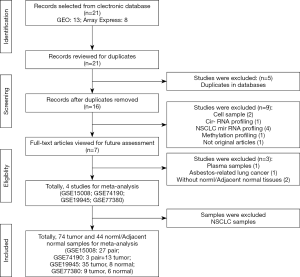
According to the search criteria, we initially retrieved 21 SCLC related expression analysis datasets from the public database (GEO and AE). After considering all the collected studies carefully, 5 duplicated data sets and 12 unqualified data sets were removed (Figure 1), four primary data sets (GSE15008, GSE74190, GSE19945, GSE77380) satisfied the required inclusion criteria, as a training cohort in further meta-analysis (10,11,27,28). A flowchart of the selection process in the study was provided in Figure 1.
Four articles were published between 2010 and 2016. The number of patients investigated ranged from 6 to 35 (median 22) across the studies. All studies used the microarray platform research scheme and the average number of microRNAs assayed was 785 (ranging from 565 to 1,226). The studies included were focused on SCLC patients only, not other specific histological subtype. Our collected dataset included a total of 74 tumor and 44 noncancerous samples. Four microarray data sets were used to the microRNA expression profiles of SCLC tissues relative to their controls. Table 1 presented the details of the studies included in the meta-analysis.
Selection of dysregulated microRNAs from the datasets
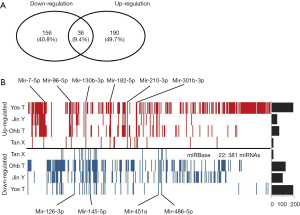
In this study, 382 distinct microRNAs were identified as differentially expressed microRNAs in the four studies, after harmonization of annotations. Among them, 226 microRNAs showed up-regulation in the list of all identified microRNAs, 192 microRNAs showed down-regulation, and 36 microRNAs showed discordant alteration, both up-regulated and down-regulated in different studies. We found that the microRNA expression profile in different studies was generally different, the average number of up-regulated microRNAs in the selected four study was 81 (ranging from 17 to 191), and it was 74 (ranging from 17 to 145) for down-regulation (Figure 2).
Cluster analysis of datasets
Hierarchical clustering analysis results showed as follows: The most similar findings were carried out by Tan X and Jin Y’ studies, conducted by the workgroup from the same country. The worst similarity come from Yoshimoto T’ study, which has the smallest sample size. Sample size may be one explanation for the heterogeneity of the results. In addition, in terms of control selection, paracancerous tissue was used as the control group in Tan X’ study, while healthy lung tissue was used as the control group in the other three studies. The results showed that there was no significant difference between Tan X’ study and the other three groups, excluding the differences between the studies for the follow-up study (data not shown, available upon request).
Meta-analysis of deregulated microRNAs in small cell lung cancer
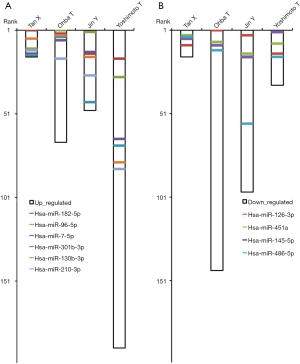
By applying robust rank aggregation, our study identified a statistically significant meta-signature of 6 up-regulated (hsa-miR-182-5p, hsa-miR-96-5p, hsa-miR-7-5p, hsa-miR-301b-3p, hsa-miR-130b-3p, hsa-miR-210-3p) and 4 down-regulated (hsa-miR-126-3p, hsa-miR-451a, hsa-miR-145-5p, hsa-miR-486-5p) microRNAs in SCLC samples compared to noncancerous lung tissue. All the 10 meta-signature microRNAs were statistically significant after Bonferroni correction. Throughout our study, the ranking of meta-signification differs considerably in different studies (Figure 3). There is no microRNAs showed different regulated directions, but passed the identification threshold.
The Meta-signature microRNA genes identified in this study were located at 8 different chromosome locations according to miRBase. It’s worth noting that Chromosome 7, chromosome 9, and chromosome 22 all harbored 2 different microRNAs; meanwhile, chromosomes 15,19,11,17,5, and 8 severally harbored one microRNAs, miR-7-5p genes were located on three different chromosomal locations 9,15,19 (Table 2). In terms of homology, majority of the meta-signature microRNAs from the broadly conserved seed families (conserved across most bonyfish and vertebrates). Four meta-signature microRNAs belong to the cluster of two microRNAs, hsa-miR-182-5p, hsa-miR-96-5p and hsa-miR-301b-3p, hsa-miR-130b-3p belong to miR-182/96/183 and miR-130-3p/301-3p/454-3p microRNA Cluster respectively. A microrna cluster was defined to located at a distance less than 50 kb and in the same direction as the transcription, not separated by a microRNA or transcription unit in the opposite direction.
Table 2
| microRNA | Chromosome | Score | Adjusted P value | Studies | Family seed + m8 | microRNA cluster | miRBase release |
|---|---|---|---|---|---|---|---|
| Up-regulated | |||||||
| hsa-miR-182-5p | Chr7: 129,770,447-129,770,470(-) | 9.77E−08 | 0.000199 | 1, 2, 3, 4 | miR-182-5p UUGGCAA | miR-182/96/183 | 18 (11/2011) |
| hsa-miR-96-5p | Chr7: 129,774,739-129,774,761(-) | 6.40E−07 | 0.001307 | 1, 2, 3, 4 | miR-96-5p/1271-5p UUGGCAC | miR-182/96/183 | 18 (11/2011) |
| hsa-miR-7-5p | chr9: 83,969,812-83,969,834(-); chr15: 88,611,856-88,611,878(+); chr19: 4,770,700-4,770,722 (+) | 4.37E−06 | 0.008914 | 1, 2, 3, 4 | miR-7-5p GGAAGAC | 18 (11/2011) | |
| hsa-miR-301b-3p | chr22: 21,653,025-21,653,047(+) | 7.23E−06 | 0.014764 | 1, 2, 3, 4 | miR-130-3p/301-3p/454-3p AGUGCAA | miR-130-3p/301-3p/454-3p | 21 (06/2014) |
| hsa-miR-130b-3p | chr22: 21,653,354-21,653,375(+) | 9.42E−06 | 0.019242 | 1, 2, 3, 4 | miR-130-3p/301-3p/454-3p AGUGCAA | miR-130-3p/301-3p/454-3p | 18 (11/2011) |
| hsa-miR-210-3p | chr11: 568,112 -568,133(-) | 1.64E−05 | 0.033494 | 1, 2, 3, 4 | mir-210-3p UGUGCGU | – | 20 (06/2013) |
| Down-regulated | |||||||
| hsa-miR-126-3p | chr9: 136,670,653-136,670,674(+) | 6.40E−07 | 0.001307 | 1, 2, 3, 4 | miR-126-3p CGUACCG | – | 18 (11/2011) |
| hsa-miR-451a | chr17: 28861369-28861440(-) | 1.56E−06 | 0.003191 | 1, 2, 3, 4 | mir-451 AACCGUU | miR-4732/334/451 | 18 (11/2011) |
| hsa-miR-145-5p | chr5: 149,430,661-149,430,683(+) | 1.56E−06 | 0.003191 | 1, 2, 3, 4 | mir-145-5p UCCAGUU | mir-143/145 | 18 (11/2011) |
| hsa-miR-486-5p | chr8: 41,660,444-41,660,465(+); chr8: 41,660,484-41,660,505(-) | 2.04E−05 | 0.041581 | 1, 2, 3, 4 | miR-486-5p CCUGUAC | hsa-miR-486-5p/3017 | 20 (06/2013) |
Bioinformatics analysis
MicroRNA target prediction
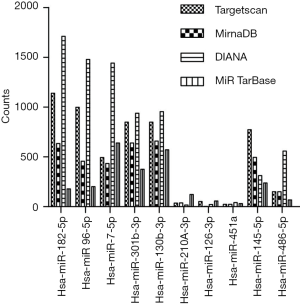
The distribution of target counts was presented in on Figure 4. The targets of hsa-miR-182-5p, hsa-miR-96-5p, hsa-miR-7-5p showed highest number, while hsa-miR-210-3p, hsa-miR-126-3p, and hsa-miR-451a were the microRNAs with smallest number of targets.
Enrichment analysis
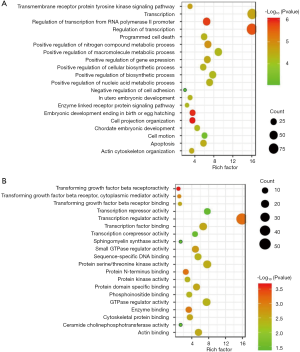
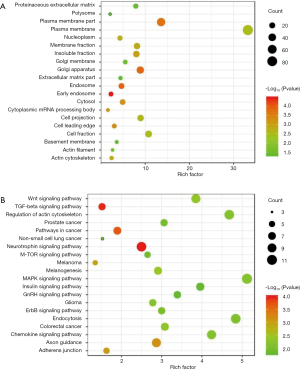
To explore which GO and KEGG pathways of meta-signature microRNAs were involved in SCLC, the predicted gene targets were analyzed by DAVID web tool. The GO ontology contains three terms: biological process (BP), molecular function (MF) and cellular component (CC). The results demonstrated that the most significant GO terms for meta-signature microRNAs were “chordate embryonic development (BP)”, “transforming growth factor beta receptor activity (MF)” and “early endosome (CC)”. The top 20 terms in the GO ontology analysis were shown in Figures 5,6A. Furthermore, the KEGG pathway analysis indicated that “Neurotrophin signaling pathway”, “TGF-beta signaling pathway”, “Pathways in cancer “and “Axon guidance “pathways played an essential role in SCLC pathogenesis. Figure 6B showed the top 20 items in KEGG pathway analysis.
Discussion
Since the inception of epigenetic in 1940s, microRNAs as the important epigenetic regulators were widely studied (12,13). The development of massive parallel sequencing technology allowed a more detailed analysis of mutations that occur in tumors. SCLC is a neuroendocrine tumor within the range of lung cancers. Many studies have reported that multiple microRNAs played a regulatory role in evolution of SCLC (29-31), and SCLC exhibited an extremely high mutation rate compared to other tumors have been reported (32,33). However, SCLC specimens was difficult to obtain since the large majority of SCLC patients were diagnosed in advanced stage and not allowed undergo surgical resection. In different SCLC microarray studies, common causes of biological inconsistencies were relatively small sample sizes and noise of microarray data. In addition, the integration of raw data sets was very complex due to the number of mutant microRNAs and the technical platforms used. Fortunately, with the continuous improvement of online network technology platforms recent years, the availability of many published experimental data sets and the emergence of different statistical methods for analyzing them made it possible to use different research data to address the same research issues. In our study, we pooled analyzed four prioritized microRNA lists from four published studies comprising 118 lung cancer and noncancerous tissue samples. To get the differentially expressed microRNAs rank lists of these four alternatives, SAM algorithm which was applicable to samples with biological duplication. It has been reported that SAM algorithm was more stable and the results selected were more accurate compared with other algorithms. To get the meta-signature microRNAs of all these differentially expressed microRNAs, we adopt a novel robust rank aggregation (RRA) method which was proposed for the biological environment of the inherent noise of gene sequences.
In our research, we identified 6 up-regulated (hsa-miR-182-5p, hsa-miR-96-5p, hsa-miR-7-5p, hsa-miR-301b-3p, hsa-miR-130b-3p, hsa-miR-210-3p) and 4 down-regulated (hsa-miR-126-3p, hsa-miR-451a, hsa-miR-145-5p, hsa-miR-486-5p) microRNAs in SCLC samples compared to noncancerous lung tissue. We suggested that these meta-signature microRNA s were key regulatory molecular of the carcinogenic process of SCLC. This was supported by the functional and pathway enrichment analysis. Functional enrichment analysis indicated that these microRNAs were primarily involved in the biological processes associated with cell activity, including chordate embryonic development, early endosome, and transforming growth factor beta receptor activity. Pathway enrichment analysis indicates that differentially expressed microRNAs were strong impact on neurotrophin signaling pathway, TGF-beta signaling pathway, pathways in cancer, axon guidance and adherents junction, which was associated with the pathogenesis and progression of SCLC. The neurotrophin signaling pathway axonal involved 10 target genes (BCL2, SHC1, FRS2, FOXO3, IRS1, MAP3K3, SORT1, YWHAG, KRAS, CRKL), the TGF-beta signaling pathway involved 6 target genes (SKP1, SMAD2, SMAD3, SMAD5, TGFBR2, ZFYVE9), the pathways in cancer involved 7 target genes (SMAD2, CDK4, FGF9, FOXO1, PTEN, PIK3R1, STK4), axon guidance involved 9 target genes (EPHA3, RASA1, ARHGEF12, EFNB2, GNAI3, NTN4, PAK1, PPP3R1, KRAS) and adherents junction involves 5 target genes (SMAD2, SMAD3, ACTB, TGFBR2, YES1) with SCLC. In general, these genes have directed or indirect interregulatory networks. However, the specific role of these genes associated with pathways has not been elucidated clearly. Therefore, meta-analysis significant differentially expressed microRNAs may be a potential biomarker of SCLC that may contribute to elucidate the tumorigenesis and development of SCLC, however, further studies are needed to confirm this.
It was noted that the chromosomal location regions of the metacentric signature microRNA are often associated with cancer. 6 microRNAs were over-expressed as a proto-oncogene in many types of tumor, and promote tumor proliferation, migration and invasion. Mir-96-5p and mir-182-5p were members of the mir-183 family (mir-183, mir-96 and mir-182). They were located on human chromosome 7q32.2 and transcribe in the same direction. Copy number gain in 7p32.2 observed in SCLC tumors, which contains MAD1L1, a mitotic checkpoint gene (34,35). Studies found that mir-183 and mir-96 were significantly over-expressed in lung cancer cell lines, human clinical lung cancer tissues and clinical serum samples, and their expression level was negatively correlated with overall survival (36,37). Mir-183 family played important regulatory roles in cell growth, colony formation capacity and cell cycle progression, through the signal pathway of Wnt/grain-catenin TGF habitat (transforming growth factor habitat) (38). Furthermore, it could play a role in promoting cancer through targeted tumor negative regulatory factors: cell programmed death factor 4 (PDCD4) (39). Most of the confirmed mir-210 targets were related to angiogenesis, apoptosis, cell cycle regulation and x chromosome inactivation, of which the most significant is related to mitochondrial metabolism regulation (40). The effect involved vascular endothelial growth factor, HPV16E6/E7 Protein, fibroblast growth factor receptor and other target molecules (41). Mir-210 could also maintain cell iron homeostasis by inhibiting the expression of iron sulfur cluster assembly protein (ISCU) and transferritin receptor 1, ensuring the survival and proliferation of cancer cells and promoting the survival of tumor cells (42). In addition, reports have shown that mir-130 family affects cancer progression (43). Chang et al. reported that mir-130b increased proliferation and emt-induced metastasis through PTEN/p-akt/hif-1 signaling in hepatocellular carcinoma (43,44). MiR-7 has been described as an intrinsic tumor suppressor gene in many human cancers including lung cancer (45). However, many studies suggested miR-7 as an ontogeny to promote the tumor progression in SCLC. Chou and colleagues reported that stimulating the expression of mir-7 through the Ras/ERK/Myc pathway can play a biological role in promoting the proliferation anchoring the growth of unrelated cells and tumor formation of lung cancer cells (46). In addition, it has been shown that miR-7 enhanced the chemotherapy resistance potential of lung cancer cells by repressing MRP1/ABCC1 and Ras/MAPK pathway (47,48). Our observation that the level of miR-7 was up regulated in SCLC support the notion that miR-7 expression plays a role in oncogenesis of lung cancer.
Similarly, 4 down-regulated microRNAs we identified play role of tumor inhibition in a variety of tumors. The direction of regulation was as same as our research results. Mir-451 was located in the human genome 17q11. In hepatocellular carcinoma human and colorectal cancer, studies have shown that mir-451 could directly inhibit cell proliferation by down-regulating the PI3k/Akt pathway (49,50). In colorectal cancer, mir-451 inhibited tumor growth and invasion by inhibiting macrophage migration inhibitor (MIF) of target gene (51). In addition, Mir-451 could also act as a tumor suppressor gene for human non-small cell lung cancer (NSCLC) (52). In the study of chemosensitivity, up-regulation of mir-451a was associated with a better response to cisplatin in patients with lung cancer (53). MiR-145 is located within a 4.09 kb region on human chromosome 5 (5q32–33). Mir-145 has been reported to have a strong inhibitory effect on the proliferation of cancer cells in multiple studies, and is involved in the inhibition of metastasis of various solid tumors, including cervical cancer, colorectal cancer and hepatocellular carcinoma (54-57). Moreover, lower miR-145 level was observed in NSCLC tissue (58,59). Mechanism study have shown that miR-145 functioned as EMT-suppressor in NSCLC by targeting MTDH, Yin Q also found that the over-expression of mir-145 could inhibit TGF- β-induced EMT in non-small cell lung cancer cells (60). MiR-126-3p located in the host gene of epidermal growth factor domain 7 (EGFL7) of human chromosome 9q34.3, was also involved in different rearrangements in various cancers (61). One of the most notable targets in lung cancer is EGFL7 (62,63). The functions of miR-126-3p ranged from angiogenesis to cell proliferation regulation (64). In adults, microRNA-126 was highly expressed in vascularized tissues such as the heart and lungs. MicroRNA-126 showed low expression in most tumor tissues, such as gastric cancer, breast cancer, colon cancer and lung cancer (64,65). MiR-486-5p mimics inhibited the progression of colorectal cancer by inhibiting the activation of AKT signaling pathway via targeting PIK3R1 (66). The mechanism of mir-486-5p in lung cancer has not been extensively studied, but study have shown that mir-486-5p could target CD40, the cell surface receptor for ductal adenocarcinoma (67).
In conclusion, the present study identified meta-signature microRNA in SCLC samples compared with healthy lung tissues, as comprehensive statistics analysis of four microRNA expression data. And the screening of meta-signature microRNA target genes was conducted using the TargetScan database. Bioinformatics analyses were conducted to explore associated functions and pathways. Our study used a microarray dataset to predict a meta-analysis of SCLC at the microRNA and gene levels. Biomarkers that have previously been identified, including miR-182-5p, miR-96-5p, and miR-126-3p, hsa-miR-7-5p, and several novel biomarkers in SCLC, including hsa-miR-301b-3p, hsa-miR-130b-3p, hsa-miR-210-3p, hsa-miR-451a, hsa-miR-145-5p, hsa-miR-486-5p, were identified. So far it has been shown to be associated with tumorigenesis, However, the specific function of these biomarkers in SCLC remains to be elucidated. It needs more research and exploration, such as identification of microRNA meta-signature with clinical data, such as patient survival, chemotherapy resistance, metastasis and results.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.04.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine Tumors of the Lung: Current Challenges and Advances in the Diagnosis and Management of Well-Differentiated Disease. J Thorac Oncol 2017;12:425-36. [Crossref] [PubMed]
- Galka-Marciniak P, Urbanek-Trzeciak MO, Nawrocka PM, et al. Somatic Mutations in microRNA Genes in Lung Cancer-Potential Functional Consequences of Non-Coding Sequence Variants. Cancers 2019;11:793. [Crossref] [PubMed]
- Zhou X, Zhang Z, Lian X. Regulatory Network Analysis to Reveal Important microRNA s and Genes in Non-Small Cell Lung Cancer. Cell J 2020;21:459-66. [PubMed]
- Wu KL, Tsai YM, Lien CT, et al. The roles of MicroRNA in lung cancer. Int J Mol Sci 2019;20:1611. [Crossref] [PubMed]
- Markou A, Zavridou M, Lianidou ES. microRNA -21 as a novel therapeutic target in lung cancer. Lung Cancer 2016;7:19-27. [PubMed]
- Alanni R, Hou J, Azzawi H, et al. A novel gene selection algorithm for cancer classification using microarray datasets. BMC Med Genomics 2019;12:10. [Crossref] [PubMed]
- Kolde R, Laur S, Adler P, et al. Robust rank aggregation for gene list integration and meta-analysis. Bioinformatics 2012;28:573-80. [Crossref] [PubMed]
- Van Peer G, Lefever S, Anckaert J, et al. miRBase Tracker: keeping track of microRNA annotation changes. Database (Oxford) 2014; [Crossref] [PubMed]
- Yoshimoto T, Motoi N, Yamamoto N, et al. Pulmonary Carcinoids and Low-Grade Gastrointestinal Neuroendocrine Tumors Show Common MicroRNA Expression Profiles, Different from Adenocarcinomas and Small Cell Carcinomas. Neuroendocrinology 2018;106:47-57. [Crossref] [PubMed]
- Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 2017;16:203-22. [Crossref] [PubMed]
- Wang X, Kang DD, Shen K, et al. An R package suite for microarray meta-analysis in quality control, differentially expressed gene analysis and pathway enrichment detection. Bioinformatics 2012;28:2534-6. [Crossref] [PubMed]
- Guan YJ, Ma JY, Song W. Identification of circRNA-microRNA-mRNA regulatory network in gastric cancer by analysis of microarray data. Cancer Cell Int 2019;19:183. [Crossref] [PubMed]
- Liu W, Ouyang S, Zhou Z, et al. Identification of genes associated with cancer progression and prognosis in lung adenocarcinoma: Analyses based on microarray from Oncomine and The Cancer. Mol Genet Genomic Med 2019;7:e00528. [PubMed]
- Klein O, Kanter F, Kulbe H, et al. MALDI-Imaging for Classification of Epithelial Ovarian Cancer Histotypes from a Tissue Microarray Using Machine Learning Methods. Proteomics Clin Appl 2019;13:e1700181. [Crossref] [PubMed]
- Li P, Piao Y, Shon HS, et al. Comparing the normalization methods for the differential analysis of Illumina high-throughput RNA-Seq data. BMC Bioinformatics 2015;16:347. [Crossref] [PubMed]
- Võsa U, Kolde R, Vilo J, et al. Comprehensive meta-analysis of microRNA expression using a robust rank aggregation approach. Methods Mol Biol 2014;1182:361-73. [Crossref] [PubMed]
- Vergoulis T, Vlachos IS, Alexiou P, et al. TarBase 6.0: capturing the exponential growth of microRNA targets with experimental support. Nucleic Acids Res 2012;40:D222-9. [Crossref] [PubMed]
- McGeary SE, Lin KS, Shi CY, et al. The biochemical basis of microRNA targeting efficacy. Science 2019; [Crossref] [PubMed]
- Anders G, Mackowiak SD, Jens M, et al. doRiNA: a database of RNA interactions in posttranscriptional regulation. Nucleic Acids Res 2012;40:D180-6. [Crossref] [PubMed]
- Maragkakis M, Alexiou P, Papadopoulos GL, et al. Accurate microRNA target prediction correlates with protein repression levels. BMC Bioinformatics 2009;10:295. [Crossref] [PubMed]
- Huang da W. Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protocols 2009;4:44-57. [Crossref] [PubMed]
- Bracken CP, Scott HS, Goodall GJ. A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet 2016;17:719-32. [Crossref] [PubMed]
- Shi W, Yang J, Li S, et al. Potential involvement of miR-375 in the premalignant progression of oral squamous cell carcinoma mediated via transcription factor KLF5. Oncotarget 2015;6:40172-85. [Crossref] [PubMed]
- Fukumoto I, Hanazawa T, Kinoshita T, et al. MicroRNA expression signature of oral squamous cell carcinoma: functional role of microRNA-26a/b in the modulation of novel cancer pathways. Br J Cancer 2015;112:891-900. [Crossref] [PubMed]
- Tan X, Qin W, Zhang L, et al. A 5-microRNA signature for lung squamous cell carcinoma diagnosis and hsa-miR-31 for prognosis. Clin Cancer Res 2011;17:6802-11. [Crossref] [PubMed]
- Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer 2015;15:321-33. [Crossref] [PubMed]
- Hu Y, Wang L, Gu J, et al. Identification of microRNA differentially expressed in three subtypes of non-small cell lung cancer and in silico functional analysis. Oncotarget 2017;8:74554-66. [Crossref] [PubMed]
- Du L, Schageman JJ, Irnov GL, et al. MicroRNA expression distinguishes SCLC from NSCLC lung tumor cells and suggests a possible pathological relationship between SCLCs and NSCLCs. J Exp Clin Cancer Res 2010;29:75. [Crossref] [PubMed]
- Sun Y, Zhou Y, Bai Y, et al. A long non-coding RNA HOTTIP expression is associated with disease progression and predicts outcome in small cell lung cancer patients. Mol Cancer 2017;16:162. [Crossref] [PubMed]
- Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104-10. [Crossref] [PubMed]
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. [Crossref] [PubMed]
- Polley E, Kunkel M, Evans D, et al. Small Cell Lung Cancer Screen of Oncology Drugs, Investigational Agents, and Gene and microRNA Expression. J Natl Cancer Inst 2016;108. [PubMed]
- Coe BP, Lee EH, Chi B, et al. Gain of a region on 7p22.3, containing MAD1L1, is the most frequent event in small-cell lung cancer cell lines. Genes Chromosomes Cancer 2006;45:11-9. [Crossref] [PubMed]
- Zhu W, Liu X, He J, et al. Overexpression of members of the microRNA-183 family is a risk factor for lung cancer: a case control study. BMC Cancer 2011;11:393. [Crossref] [PubMed]
- Xu F, Zhang H, Su Y, et al. Up-regulation of microRNA-183-3p is a potent prognostic marker for lung adenocarcinoma of female non-smokers. Clin Transl Oncol 2014;16:980-5. [Crossref] [PubMed]
- Chen D, Li SG, Chen JY, et al. MiR-183 maintains canonical Wnt signaling activity and regulates growth and apoptosis in bladder cancer via targeting AXIN2. Eur Rev Med Pharmacol Sci 2018;22:4828-36. [PubMed]
- Zhu W, Zhou K, Zha Y, et al. Diagnostic value of serum miR-182, miR-183, miR-210, and miR-126 levels in patients with early-stage non-small cell lung cancer. PLoS One 2016;11:e0153046. [Crossref] [PubMed]
- Yang X, Shi L, Yi C, et al. MiR-210-3p inhibits the tumor growth and metastasis of bladder cancer via targeting fibroblast growth factor receptor-like 1. Am J Cancer Res 2017;7:1738-53. [PubMed]
- Greither T, Grochola LF, Udelnow A, et al. Elevated expression of microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated with poorer survival. Int J Cancer 2010;126:73-80. [Crossref] [PubMed]
- Mattoo AR, FitzGerald DJ. Combination treatments with ABT-263 and an immunotoxin produce synergistic killing of ABT-263-resistant small cell lung cancer cell lines. Int J Cancer 2013;132:978-87. [Crossref] [PubMed]
- Li BL, Lu C, Lu W, et al. MiR-130b is an EMT-related microRNA that targets DICER1 for aggression in endometrial cancer. Med Oncol 2013;30:484. [Crossref] [PubMed]
- Chang RM, Xu JF, Fang F, et al. MicroRNA-130b promotes proliferation and EMT-induced metastasis via PTEN/p-AKT/HIF-1α signaling. Tumour Biol 2016;37:10609-19. [Crossref] [PubMed]
- Zhang X, Yang D, Wei Y. Overexpressed CDR1as functions as an oncogene to promote the tumor progression via miR-7 in non-small-cell lung cancer. Onco Targets Ther 2018;11:3979-87. [Crossref] [PubMed]
- Chou YT, Lin HH, Lien YC, et al. EGFR promotes lung tumorigenesis by activating miR-7 through a Ras/ERK/Myc pathway that targets the Ets2 transcriptional repressor ERF. Cancer Res 2010;70:8822-31. [Crossref] [PubMed]
- Liu H, Wu X, Huang J, et al. miR-7 modulates chemoresistance of small cell lung cancer by repressing MRP1/ABCC1. Int J Exp Pathol 2015;96:240-7. [Crossref] [PubMed]
- Liu H, Huang J, Peng J, et al. Upregulation of the inwardly rectifying potassium channel Kir2.1 (KCNJ2) modulates multidrug resistance of small-cell lung cancer under the regulation of miR-7 and the Ras/MAPK pathway. Mol Cancer 2015;14:59. [Crossref] [PubMed]
- Mamoori A, Wahab R, Vider J, et al. The tumour suppressor effects and regulation of cancer stem cells by macrophage migration inhibitory factor targeted miR-451 in colon cancer. Gene 2019;697:165-74. [Crossref] [PubMed]
- Li HP, Zeng XC, Zhang B, et al. miR-451 inhibits cell proliferation in human hepatocellular carcinoma through direct suppression of IKK-β. Carcinogenesis 2013;34:2443-51. [Crossref] [PubMed]
- Li Q, Li Y, Zhang D, et al. Downregulation of microRNA 451 improves cell migration, invasion and tube formation in hypoxia treated HUVECs by targeting MIF. Mol Med Rep 2019;20:1167-77. [PubMed]
- Liu Y, Li H, Li LH, et al. Mir-451 inhibits proliferation and migration of non-small cell lung cancer cells via targeting LKB1/AMPK. Eur Rev Med Pharmacol Sci 2019;23:274-80. [PubMed]
- Cheng CC, Chiang YW, Lim KH, et al. STAT3 represses miR-145-5p to exacerbate HER3 expression for surviving EGFR-TKIs in lung cancers. BMC Cancer 2019;19:959. [PubMed]
- Bellissimo T, Tito C, Ganci F, et al. Argonaute 2 drives miR-145-5p-dependent gene expression program in breast cancer cells. Cell Death Dis 2019;10:17. [Crossref] [PubMed]
- Reimondez-Troitiño S, González-Aramundiz JV. Versatile protamine nanocapsules to restore miR-145 levels and interfere tumor growth in colorectal cancer cells. Eur J Pharm Biopharm 2019;142:449-59. [Crossref] [PubMed]
- Fuse M, Nohata N, Kojima S, et al. Restoration of miR-145 expression suppresses cell proliferation, migration and invasion in prostate cancer by targeting FSCN1. Int J Oncol 2011;38:1093-101. [PubMed]
- Wu BL, Xu LY, Du ZP, et al. MicroRNA profile in esophageal squamous cell carcinoma: downregulation of miR-143 and miR-145. World J Gastroenterol 2011;17:79-88. [Crossref] [PubMed]
- Hu H, Xu Z, Li C, et al. MiR-145 and miR-203 represses TGF-β-induced epithelial-mesenchymal transition and invasion by inhibiting SMAD3 in non-small cell lung cancer cells. Lung Cancer 2016;97:87-94. [Crossref] [PubMed]
- Shen H, Shen J, Wang L, et al. Low miR-145 expression level is associated with poor pathological differentiation and poor prognosis in non-small cell lung cancer. Biomed Pharmacother 2015;69:301-5. [Crossref] [PubMed]
- Yin Q, Han Y, Zhu D, et al. miR-145 and miR-497 suppress TGF-β-induced epithelial-mesenchymal transition of non-small cell lung cancer by targeting MTDH. Cancer Cell Int 2018;18:105. [Crossref] [PubMed]
- Harris TA, Yamakuchi M, Ferlito M, et al. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A 2008;105:1516-21. [Crossref] [PubMed]
- Chanyshev MD, Koval OA, Nushtaeva AA, et al. Effect of benzo[a]pyrene on the expression of miR-126, miR-190a and their target genes EGFL7, TP53INP1 and PHLPP1 in primary endometrial cells. J Biochem Mol Toxicol 2019;e22314. [Crossref] [PubMed]
- Tu J, Cheung HH, Lu G, et al. microRNA-126 is a tumor suppressor of granulosa cell tumor mediated by its host gene EGFL7. Front Oncol 2019;9:486. [Crossref] [PubMed]
- Wang CZ, Yuan P, Li Y. MiR-126 regulated breast cancer cell invasion by targeting ADAM9. Int J Clin Exp Pathol 2015;8:6547-53. [PubMed]
- Sun Z, Ou C, Liu J, et al. YAP1-induced MALAT1 promotes epithelial-mesenchymal transition and angiogenesis by sponging miR-126-5p in colorectal cancer. Oncogene 2019;38:2627-44. [Crossref] [PubMed]
- Shi H, Bi H, Sun X, et al. Tubeimoside-1 inhibits the proliferation and metastasis by promoting miR-126-5p expression in non-small cell lung cancer cells. Oncol Lett 2018;16:3126-34. [PubMed]
- Zhang Y, Fu J, Zhang Z, et al. miR-486-5p regulates the migration and invasion of colorectal cancer cells through targeting PIK3R1. Oncol Lett 2018;15:7243-8. [PubMed]
- Wang J, Tian X, Han R, et al. Downregulation of miR-486-5p contributes to tumor progression and metastasis by targeting protumorigenic ARHGAP5 in lung cancer. Oncogene 2014;33:1181-9. [Crossref] [PubMed]