Assessing the nature of the association of human papillomavirus in oral cancer with and without known risk factors
Introduction
Most oral squamous cell carcinoma (OSCC) cases have known associated risk factors including tobacco, alcohol, areca nut, etc. In addition to this, there has been an ongoing debate as to the etiology of oral cancer in patients without any known risk factors. In such cases, a microbial etiology has been hypothesized. Human papillomavirus (HPV) a proven risk factor for cervical and oropharyngeal cancer (1,2) has been closely associated with oral cancer, although conclusive evidence for causal inference is not established. Significant epidemiological evidence exists associating HPV and oral cancer, especially the high-risk types 16 and 18. Syrjänen et al., in 1983 were the first to associate HPV with squamous cell lesions in various sites of the body including the oral cavity (3). Evidence for the possible association between HPV infection and oral cancer is provided by both in-vitro and in-vivo studies (4). Given that HPV is proven to be a causal factor in oropharyngeal cancer and based on epidemiological association with oral cancer, it is possible that HPV could be a potential risk factor for oral cancer.
Apart from HPV being a potential independent risk factor for oral cancer, it is also possible for a combined synergistic effect wherein the HPV infection predisposes the oral mucosa to the known carcinogen or vice versa. Thus, it is possible that patients with a history of tobacco usage and HPV have the additional risk of oral cancer (5,6). The present study aims to determine the association between HPV and OSCC with no known risk factors. HPV subtypes 16 and 18 were selected for the study due to their close epidemiological association with oral cancer (7). The mere presence of HPV in oral cancer does not constitute a causal role. Thus, in addition to establishing the presence of the virus, it is vital to assess the factors involved in the HPV mediated carcinogenesis. One such factor is p16. In HPV mediated carcinogenesis, HPV E-7 oncoprotein is shown to destabilize pRb which is responsible for suppressing p16 expression. Thus, tumor cells bypass the pRb-dependent cell cycle arrest resulting in marked overexpression of p16 (8,9). Similarly, in HPV mediated carcinogenesis, HPV E6 oncoprotein was found to induce FoxM1B by suppressing p53 resulting in loss of cell cycle regulation (10). Thus, in addition to detecting HPV through PCR, a positive p16 immunostaining and a negative p53 immunostaining would provide additional evidence for an HPV mediated carcinogenesis.
Methods
Ethical approval was obtained from the Dr. D. Y. Patil Vidayepeeth institutional ethics committee (Ref no. DYPDCH/EC-5/OP-35).
Informed consent was not applicable to the present study as the included specimens were from archival collection. Forty-five archival paraffin-embedded blocks of tissue specimens consisting of 30 OSCC cases and 15 oral epithelial dysplasia cases were collected.
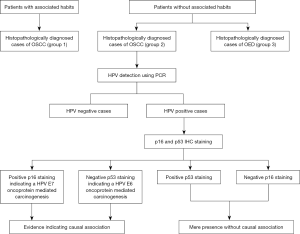
The study samples were divided into the following groups:
- Group 1: 15 histopathologically diagnosed cases of OSCC with a known history of tobacco smoking;
- Group 2: 15 histopathologically diagnosed cases of OSCC with no known risk factors;
- Group 3: 15 histopathologically diagnosed cases of oral epithelial dysplasia (OED) with no known risk factors.
Group 1 consisted of 6 females and 9 males, group 2 comprised 3 females and 12 males group 3 comprised of 4 females and 11 males. The age range of the patients was 30–70 years. Table 1 summarizes the distribution of cases based on the histopathological diagnosis.
Table 1
| Histopathological | Group 1 (%) | Group 2 (%) | Group 3 (%) | Total (%) |
|---|---|---|---|---|
| Well differentiated OSCC | 5 (33.33) | 7 (46.67) | 0 | 12 (26.67) |
| Moderately differentiated OSCC | 9 (60.00) | 6 (40.00) | 0 | 15 (33.33) |
| Poor differentiated OSCC | 1 (6.67) | 2 (13.33) | 0 | 3 (6.67) |
| Mild dysplastic lesion | 0 | 0 | 5 (33.33) | 5 (11.11) |
| Moderate dysplastic lesion | 0 | 0 | 10 (66.66) | 10 (22.22) |
| Total | 15 (100.00) | 15 (100.00) | 15 (100.00) | 45 (100.00) |
OSCC, oral squamous cell carcinoma.
DNA extraction from paraffin-embedded tissue
Sample block was trimmed to remove excess paraffin. Tissue sections were prepared to a thickness of 5–10 µm and were immediately transferred to 1.5 or 2 mL microcentrifuge tube to which 1 mL xylene was added. At room temperature, the sample was centrifuged for 2 minutes. Supernatant resulting from the centrifugation was pipetted leaving the pellet behind. To the pellet, 1 mL ethanol (96–100%) was added and were mixed at room temperature using vortexing and centrifugation for 2 min. At 37 °C, the samples were incubated for 10 minutes. 180 µL Buffer ATL was used to resuspend the pellet, following which 20 µL proteinase K was added and were vortexed and incubated for 1 hour at 56 °C. Carefully without wetting the rim, the entire lysate was transferred to the QIAamp MinElute column (in a 2 mL collection tube). After closing the lid, centrifugation was carried out for 1 minute at 6,000 ×g (8,000 rpm). After opening the QIAamp MinElute column, 500 µL Buffer AW1 was carefully added without wetting the rim. After closing the lid, centrifugation was carried out for 1 minute at 6,000 ×g (8,000 rpm). QIAamp MinElute column was placed in a clean 2 mL collection tube, following which the collection tube containing the flow-through was discarded. Without wetting the rim, the QIAamp MinElute column was carefully opened and 500 µL Buffer AW2 was added, following which the lid was closed and centrifugation was carried out for 1 minute at 6,000 ×g (8,000 rpm). QIAamp MinElute column was placed in a clean 2 mL collection tube, following which the collection tube containing the flow-through was discarded. To dry the membrane completely, centrifugation was carried out at 20,000 ×g; 14,000 rpm for 3 min. A clean 1.5 mL microcentrifuge tube was used to place the QIAamp MinElute column, following which the collection tube containing the flow-through was discarded. After opening the lid of the QIAamp MinElute column, 20–100 µL Buffer ATE was applied to the center of the membrane. After closing the lid, it was incubated for 1 minute at room temperature (15–25 °C). Centrifugation was carried out for 1 minute at 20,000 ×g; 14,000 rpm.
Polymerase chain reaction (PCR)
To determine the absence of PCR inhibitors and the integrity of the DNA, a 165 bp segment of a human β2-microglobin gene was amplified. HPV16/18 DNA was analyzed only for those samples that were positive for the β2-microglobin sequence. Primer pairs located in the E6 gene open reading frame (ORF) which are specific for HPV subtype were used. 1.0 µg of DNA in a total volume of 25 µL (1× Tris buffer, 1.5 mM MgCl2, 1 µM of each primer, 200 µM each of dATP, dCTP, dGTP, dTTP and 1 U Taq) polymerase was used to perform the amplification reactions. In the DNA thermocycler (Eppendorf) 35 amplification cycles were performed with the following profile:
- Denaturation 1 minute at 95 °C;
- Annealing 1 minute at 54 °C;
- Elongation 2 minute at 72 °C.
The last cycle was followed by a final extension of 72 °C for 7 minute. A negative control (Sterile water instead of DNA) and positive control (HPV16 positive Cervical and HPV 18 positive uterine carcinoma) was used during each PCR experiments. 1.5% agarose gel electrophoresis and ethidium bromide staining were used to analyze the PCR products. SPSS 17.0 version was used to analyze the resulting data.
Immunohistochemistry (IHC)
The cases which were positive for HPV in the PCR, were subjected to immunostaining for p16 and p53. IHC was performed on formalin-fixed paraffin-embedded sections of 3 µm thickness. The sections were deparaffinized and rehydrated by giving them 3 changes of xylene and various grade of alcohol. For antigen retrieval sections were boiled in 0.01 citrate buffer (pH, 6.0) for 10 minutes. The sections were incubated with p16INK4a primary mouse monoclonal antibody (Biogenex Lot no. AM5400315) and anti-p53(Clone D0-7, 1:200 dilution, DAKO A/S, CA, USA). The sections were then washed with Tris buffer and sliders were further incubated with poly horseradish peroxidase for 30 minutes. The slides were then reacted with diaminobenzene hydrochloride for 5 minutes and finally counterstained with Mayer’s hematoxylin. For, p16 expression results were considered positive only with 70–75% nuclear and cytoplasmic staining (9). For p53 quantification was done as (+) mild, (++) moderate and (+++) intense expression of nuclear stain. Figure 1 summarizes the workflow of the study.
Results
PCR
In group 1, only 1 case (6.67%) was positive for HPV 16, whereas, in group 2, 3 cases (20%) were positive for HPV 16. There was no statistically significant difference in the prevalence of HPV 16 between group 1 and 2 (Table 2). There were no HPV positive cases in group 3. HPV 18 was negative in all the 3 groups. Table 3 provides data on site-specificity of HPV in OSCC. Table 4 provides data on OSCC grade based specificity of HPV in OSCC.
Table 2
| Group | n | HPV |
|---|---|---|
| Group 1 | 15 | 1 (6.67) |
| Group 2 | 15 | 3 (20.0)* |
| Group 3 | 15 | 0#& |
*: compare with group 1, Z=1.10, P>0.05; #: compared with group 1, Z=1.04, P>0.05; &: compared with group 1, Z=1.94, P>0.05. HPV, human papilloma virus.
Table 3
| Site | Presence of HPV | ||
|---|---|---|---|
| Group 1 (n=15) | Group 2 (n=15) | Group 3 (n=15) | |
| Buccal mucosa | 1 (6.67%) | 0 | 0 |
| Gingiva | 0 | 2 (13.33%) | 0 |
| Tongue | 0 | 1 (6.67%) | 0 |
HPV, human papillomavirus; OSCC, oral squamous cell carcinoma.
Table 4
| Histopathological grade | Presence of HPV | ||
|---|---|---|---|
| Group 1 (n=15) | Group 2 (n=15) | Group 3 (n=15) | |
| Well differentiated OSCC | 0 | 2 (13.33) | 0 |
| Moderately differentiated OSCC | 1 (6.67) | 1 (6.67) | 0 |
OSCC, oral squamous cell carcinoma; HPV, human papillomavirus.
IHC
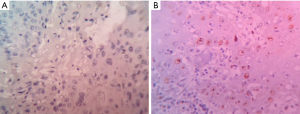
All the HPV positive cases subjected to immunostaining were negative for p16 and positive for p53 (Figure 2A,B).
Discussion
The OSCC incidence and mortality rate has shown a steady increase, with 6.6/100,000 and 3.1/100,000 being the age-standardized incidence and mortality in men and 2.9/100,000 and 1.4/100,000 being the age-standardized incidence and mortality in women (11). The increasing prevalence of oral cancer is presumed to be the result of the increasing use of known risk factors such as alcohol, tobacco, areca nut, etc. (7). Despite most of the oral cancer cases being associated with these well-established risk factors, there are reported cases wherein the associated risk factor is not identified. Recent studies have explored the role of potential risk factors such as diet, immunity, infection in oral cancer, with special emphasis given to HPV 16 and 18 (12,13). Syrjänen et al. observation of the cytopathic effects of HPV (koilocytosis) in oral lesions under light microscopy was the first substantial evidence for a potential link between OSCC and HPV (3). The presence of HPV DNA in oral potentially malignant lesions (4/5 leukoplakias) and malignancies (3/6 carcinomas) was later confirmed using in situ hybridization, based on which Loning et al suggested a potential causal association between HPV and oral cancer (14). Syrjänen et al. (3) findings were substantiated by a number of studies including that of Wilczynski et al. who reported altered cytologic features in HPV infection with an additional feature of lack of keratin (15). Münger et al. reported that oral epithelial cells can attain immortality if they are infected with HPV 16 infection and are expressing E6 and E7 genes (16).
The above-mentioned studies support the hypothesis that HPV may not be specific to the anogenital epithelium and could be closely associated with oral epithelium as well (17). The prevalence of HPV in normal oral mucosa, oral potentially malignant disorders and oral malignancies varies widely from 0 to 70%, 0 to 85%, and 0 to 100%, respectively (18). The variations could, in turn, be the result of using diagnostic modalities with varying sensitivity and specificity. Based on the data from the above-mentioned studies, we hypothesized that although HPV could be present in both oral cancer cases with and without risk factors, a causal association is more likely in oral cancer without risk factors.
The HPV prevalence in all three groups of the present study sample was low, which is in contrary to several studies which have isolated HPV in OSCC at a relatively larger proportion. Ha et al. detected HPV in 86.2% of hyperplasia, 100% of dysplasia and 94.7% OSCC cases (19). Bouda et al. found HPV-16 DNA in 65% of dysplasia and 35% OSCC cases (20). Sugiyama et al. detected HPV in 74% of OSCC samples (21,22). The absence of HPV 16 and 18 in all the cases of OED in the present study is in contrast to several other studies including that of McCord et al. and Mravak-Stipetić et al. (23,24). Both these found a close association between HPV and OED with McCord et al. (23) observing diffuse loss of squamous differentiation and a high proliferation index in cases of severe epithelial dysplasia/carcinoma in situ indicating a close association to HPV and Mravak-Stipetić et al. (24) detecting HPV in 17.7% of oral dysplastic lesions.
The nature of association of the HPV with oral cancer was assessed by subjecting HPV positive cases to p53 and p16 immunostaining. In an HPV mediated carcinogenesis, the destabilization of the pRB by the HPV E-7 oncoprotein results in increased expression of p16 (8,9). In the present study, all the cases positive for HPV in the PCR were negative for the p16, thus excluding an E-7 oncoprotein mediated carcinogenesis. An alternated pathway of HPV induced carcinogenesis is through E-6 oncoprotein mediated suppression of p53. In the present study, all the HPV positive cases were positive for p53. Thus, even among the minor proportion of HPV 16 positive cases, the immunostaining (p53 positive and p16 negative) infers that the association may not be of causal nature.
Conclusions
Among cases with no known risk factors only 3 OSCC cases were positive for HPV 16. None of the OED cases with no known risk factors were positive for HPV 16 or 18. Among the cases with known risk factors, only 1 OSCC case was positive for HPV 16. There was no statistically significant difference between the groups. Based on the results HPV 16 can only be weakly associated with oral cancer cases with and without known risk factors. The PCR results in combination with the absence of p16 and the presence of p53 immunostaining indicate that even the minimal HPV presence may not be of causal nature in the present study sample
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Oral Pre-cancer and Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.81). The series “Oral Pre-cancer and Cancer” was commissioned by the editorial office without any funding or sponsorship. SP served as an unpaid Guest Editor of the series and serves as an unpaid Editorial Board Member of Translational Cancer Research from Jul 2018 to Jun 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was obtained from the Dr. D. Y. Patil Vidayepeeth institutional ethics committee (Ref no. DYPDCH/EC-5/OP-35). Informed consent was not applicable to the present study as the included specimens were from archival collection.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Andrews E, Seaman W. Oropharyngeal carcinoma in non-smokers and non-drinkers: A role for HPV. Oral Oncology 2009;45:486-91. [Crossref] [PubMed]
- D’Costa J, Sarnath D, Dedhia P, et al. Detection of HPV-16 genome in human oral cancers and potentially malignant lesions from India. Oral Oncol 1998;34:413-20. [Crossref] [PubMed]
- Syrjänen KJ, Surjänen SM. Histological evidence for the presence of condylomatous epithelial lesions in association with laryngeal squamous cell carcinoma. ORL J Otorhinolaryngol Relat Spec 1981;43:181-94. [Crossref] [PubMed]
- Cruz IB, Snijders PJ, Steenbergen RD, et al. Age-dependence of human papillomavirus DNA presence in oral squamous cell carcinomas. Eur J Cancer B Oral Oncol 1996;32B:55-62. [Crossref] [PubMed]
- Raj AT, Patil S, Awan KH, et al. Odds Ratio for Oral Cancer is Directly Proportional to the Number of associated Habits. World J Dent 2017;8:351. [Crossref]
- Kumar R, Rai AK, Das D, et al. Alcohol and Tobacco Increases Risk of High-Risk HPV Infection in Head and Neck Cancer Patients: Study from North-East Region of India. PLoS One 2015;10:e0140700. [Crossref] [PubMed]
- Park NH, Min BM, Li SL, et al. Immortalization of normal human oral keratinocytes with type 16 humanpapillomavirus. Carcinogenesis 1991;12:1627-31. [Crossref] [PubMed]
- Ma C, Lewis J Jr. Small biopsy specimen reliably indicate p16 expression status of Oropharyngeal squamous cell carcinoma. Head Neck Pathol 2012;6:208-15. [Crossref] [PubMed]
- Lewis JS Jr, Bishop JA, Chernok RD, et al. Human papilloma testing in head and neck carcinoma. Arch Pathol Lab Med 2018;142:559-97. [Crossref] [PubMed]
- Raj AT, Patil S, Gupta AA, et al. Reviewing the role of human papillomavirus in oral cancer using the Bradford Hill criteria of causation Dis Mon 2019;65:155-63. [Crossref] [PubMed]
- Zhang ZY, Sdek P, Cao J, et al. Human papillomavirus type 16 and 18 DNA in oral squamous cell carcinoma and normal mucosa. Int J Oral Maxillofac Surg 2004;33:71-4. [Crossref] [PubMed]
- Ward MJ, Thirdborough SM, Mellows T, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. British Journal of Cancer 2014;110:489-500. [Crossref] [PubMed]
- Landis SH, Murray T, Bolden S, et al. Cancer statistics, 1999. CA Cancer J Clin 1999;49:8-31. [Crossref] [PubMed]
- Löning T, Ikenberg H, Becker J, et al. Analysis of oral papillomas, leukoplakias, and invasive carcinomas for human papillomavirus type related DNA. J Invest Dermatol 1985;84:417-20. [Crossref] [PubMed]
- Wilczynski SP, Lin BT, Xie Y, et al. Detection of human papillomavirus DNA and oncoprotein overexpression are associated with distinct morphological patterns of tonsillar squamous cell carcinoma. Am J Pathol 1998;152:145-56. [PubMed]
- Münger K, Phelps WC, Bubb V, et al. The E6 and E7 genes of human papillomavirus type 16 are necessary and sufficient for transformation of primary human keratinocytes. J Virol 1989;63:4417-21. [Crossref] [PubMed]
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 2009;45:309-16. [Crossref] [PubMed]
- Kumaraswamy KL, Vidhya M. Human papilloma virus and oral infections: An update. J Cancer Res Ther 2011;7:120-7. [Crossref] [PubMed]
- Ha PK, Califano JA. The Role of Human Papillomavirus in Oral Carcinogenesis. Crit Rev Oral Biol Med 2004;15:188-96. [Crossref] [PubMed]
- Bouda M, Gorgoulis VG, Kastrinakis NG, et al. “High risk” HPV types are frequently detected in potentially malignant and malignant oral lesions, but not in normal oral mucosa. Mod Pathol 2000;13:644-53. [Crossref] [PubMed]
- Sugiyama M, Bhawal UK, Dohmen T. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;95:594-600. [Crossref] [PubMed]
- Ibieta BR, Lizano M, Mendivil MF, et al. Human papilloma virus in oral squamous cell carcinoma in a Mexican population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:311-5. [Crossref] [PubMed]
- McCord C, Xu J, Xu W, et al. Association of high-risk human papillomavirus infection with oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;115:541-9. [Crossref] [PubMed]
- Mravak-Stipetić M, Sabol I, Kranjcic J, et al. Human papillomavirus in the lesions of the oral mucosa according to topography. PLoS One 2013;8:e69736. [Crossref] [PubMed]