Overexpression of COX-2 and clinicopathological features of gastric cancer: a meta-analysis
Introduction
According to the latest global cancer statistics, gastric cancer is the fifth most common malignant tumor in the world, ranking behind breast cancer, prostate cancer, lung cancer and colorectal cancer (1). Also, the World Health Organization (WHO) indicates that the annual incidence of gastric cancer is 13.86/100,000 and there are about 1.39 million cases and 1.09 million deaths every year in the world (1). More than 70% of patients are from developing countries (456 thousand male, 221 thousand female), and more than 50% are from East Asia, especially China (2). The number of gastric cancer cases and deaths in China accounted for 42.6% and 45.0% of the global gastric cancer morbidity and deaths, respectively, ranking fifth in morbidity and sixth in mortality among 183 countries in the world.
China has a high incidence of gastric cancer, accounting for about 50% of the world's morbidity and deaths (1,3,4). The occurrence of gastric cancer is a multi-step and multi-stage process based on the activation of multiple proto-oncogene and inactivation of anti-oncogene, the former acts on the cell growth, differentiation and metabolism through its expression products, leading to canceration (5,6). Also, malignant tumors are characterized by metastasis and invasion, both pathways are complex and acts upon the gene regulatory network between oncogene and tumor suppressor gene. Therefore, the invasion and metastasis of gastric cancer are the main factors affecting the life quality and prognosis of the patients. Many researches are concern about the relationship between cyclooxygenase (COX)-2 and invasion and metastasis. COX-2 is a rate-limiting enzyme in the synthesis of inducible prostaglandin (PG). It metabolizes arachidonic acid into a variety of PG products and participates in the maintenance of various physiological and pathological functions of the body (7). COX-2, an inducible enzyme, begins to synthesize and express stimulated by cytokines (EGF, TGF, INR, TNF), growth factors, nitric oxide synthetase, oncogenes, mitogen, bacterial toxins. PGs can enter the cell directly to regulate the transcription of target genes, and then participate in a variety of pathophysiological processes, including inflammatory reaction, tumorigenesis and its development (8). In recent years, it has been found that there are amplification of COX-2 gene and high expression of its protein in many kind of tumor cells and tissues, such as gastric cancer, breast cancer, thyroid cancer and colon cancer (9). Moreover, multiple studies have shown that protein expression of COX-2 gene is closely related to the occurrence of gastric cancer, but the relationship between it and the clinicopathological behavior of gastric cancer is unclear. Therefore, the aim of this study is to investigate the relationship between COX-2 and clinicopathological characteristics of gastric cancer, and the mechanism of invasion and metastasis of gastric cancer is explored, thus providing theoretical basis for further elucidating the pathogenesis of gastric cancer. To this end, Meta-analysis was used to analyze previous literature related to COX-2 and gastric cancer.
Methods
Literature retrieval
The literature about the expression of COX-2 and gastric cancer was searched in Pubmed, Wangfang, VIP, CNKI from the inception to September 2019, with “gastric cancer”, “COX-2”, “cyclooxygenase” as keywords. And then the literature was manually screened by reading the abstract and the full text.
Inclusion and exclusion criteria
Inclusion criteria: (I) the case-control studies published at home and abroad with original data and immunohistochemistry (IHC) detection; (II) studies about the clinicopathological features or prognosis of gastric cancer and COX-2 overexpression, excluding the studies of adenoma of stomach or esophagogastric junction cancer; (III) In the case group, the patients, with definite pathological diagnostic criteria and their complete clinicopathological data, were not treated with radiotherapy and chemotherapy before operation; in the control group, the patients with adjacent tissues or benign diseases tissues; (IV) Chinese literature was published in the Peking University core journals or in the key magazine of China technology; (V) appropriate statistical method, reliable data, clear expression of results, and at least one of the pathological features, such as differentiation, lymph node metastasis and TNM stage, could calculate OR (95% CI).
Exclusion criteria: (I) review, abstract, letter; (II) the same group of people reported repeatedly without good quality, insufficient reporting information and OR (95% CI).
Literature quality evaluation
After reading the full text and according to the Newcastle-Ottawa scale (10), the literature quality was evaluated. Literature awarded less than 6 stars as low quality literature, and 6 stars or more as high quality one. The latter one was included in this study. According to the uniform quality standard, two assessors evaluated the literatures independently to extract data, and then the collecting data was cross-checked. Discrepancies were resolved by discussion or assistance of the third researcher.
Data extraction
The unified data extraction table was adopted, in which the relevant information included as following: (I) general data: title, first author, year of publication; (II) characteristics: gender, age, COX-2 expression, differentiation, infiltration depth, lymph node metastasis, tumor size and TNM stage; (III) methodological information: detection method of COX-2.
Statistical method
Meta-analysis was performed by Stata 15.0. OR (95% CI) was calculated as effect size. Q test was used to test the heterogeneity of the research results. I2 ≥50% or P≤0.05 suggested heterogeneity, so the fixed effect model (FEM) was used. I2 <50% and P>0.05 suggested no heterogeneity, so the random effect model (REM) was used. Z test was used to test the significance of the pooled OR. This Meta-analysis used the symmetry of funnel plot to evaluate publication bias. Standard error of log (OR) and OR were used to draw funnel plot. The asymmetric plot indicated publication bias. Egger’s Test was used to test the publication bias.
Results
Literature retrieval result
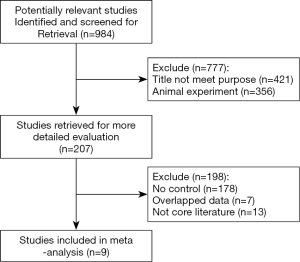
According to the inclusion and exclusion criteria and quality evaluation, a total of 9 literature was included after researching and cross-checking. The specific screening process was shown in Figure 1. The basic characteristics of the literature were shown in Table 1. There were 1,289 gastric cancer patients, and the positive expression rate was 68.1% (n=878).
Table 1
| First author | Year | Cox-2 expression, n (%) | Cox-2 Detection method | Clinical characteristics | NOS score |
|---|---|---|---|---|---|
| Okano | 2004 | 54.8% | IHC | Infiltration depth, lymph node metastasis | 8 |
| Ji-Ren Yu | 2005 | 57.9% | IHC | Infiltration depth, lymph node metastasis | 7 |
| Joo | 2006 | 60.5% | IHC | Gender, Infiltration depth, lymph node metastasis, TNM stage, tumor size | 8 |
| Park | 2009 | 81.0% | IHC | Gender, age, differentiation degree, lymph node metastasis, TNM stage | 8 |
| Jun-Shan Zhai | 2016 | 62.7% | IHC | Gender, infiltration depth, lymph node metastasis, TNM stage | 7 |
| Xiao-Lan Lv | 2018 | 60.0% | IHC | Gender, age, differentiation degree, infiltration depth, lymph node metastasis, TNM stage, tumor size | 7 |
| Ye Liu | 2018 | 72.9% | IHC | Gender, age, differentiation degree, tumor size | 6 |
| Jian-Jun Zhang | 2018 | 63.8% | IHC | Gender, age, lymph node metastasis, tumor size | 7 |
| Ying-Hui Zhang | 2018 | 66.1% | IHC | Gender, infiltration depth, lymph node metastasis, TNM stage, tumor size | 7 |
Relationship between COX-2 overexpression and clinicopathological features of gastric cancer
COX-2 overexpression and infiltration depth
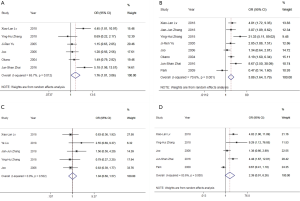
A total of 6 literature was included, including 431 extraserous patients and 284 intraserous patients. The two groups had statistical heterogeneity (P=0.012), so the REM was used (Table 2). The result showed that there was significant difference between the two groups. Compared with the control group (invasion intraserous group), OR=1.76 (95% CI: 1.01−3.06, P=0.045) and the forest plot (Figure 2A) showed that with the increase of COX-2 expression, the infiltration depth was deeper.
Table 2
| Pathological features | n | case | OR | 95% CI | P | I2 (%) | P for heterogeneity | Model | P for publication bias |
|---|---|---|---|---|---|---|---|---|---|
| Gender | 7 | Man: 620 | 1.08 | 0.79–1.46 | 0.642 | 0.00 | 0.892 | FEM | 0.305 |
| Woman: 318 | |||||||||
| Age | 4 | ≥60: 235 | 1.27 | 0.85–1.89 | 0.246 | 0.00 | 0.410 | FEM | 0.492 |
| <60: 439 | |||||||||
| Differentiation degree | 3 | Medium and high: 210 | 0.77 | 0.14–4.23 | 0.761 | 88.70 | 0.000 | REM | 0.559 |
| Low: 309 | |||||||||
| Infiltration depth | 6 | Outside serous: 431 | 1.76 | 1.01–3.06 | 0.045 | 65.70 | 0.012 | REM | 0.308 |
| Inside serous: 284 | |||||||||
| Lymph node metastasis | 8 | Yes: 969 | 3.08 | 1.64–5.79 | 0.000 | 70.60 | 0.001 | REM | 0.097 |
| No: 282 | |||||||||
| Tumor size | 5 | >5 cm: 178 | 1.04 | 0.68–1.57 | 0.860 | 0.00 | 0.502 | FEM | 0.106 |
| ≤5 cm: 220 | |||||||||
| TNM stage | 5 | I-II: 511 | 2.39 | 0.91–6.26 | 0.075 | 83.60 | 0.000 | REM | 0.025 |
| III-IV: 310 |
COX-2 overexpression and lymph node metastasis
A total of 9 literature (11-19) was included, including 969 patients with lymph node metastasis and 282 without lymph node metastasis. The two groups had statistical heterogeneity (P=0.001), so the REM was used (Table 2). Compared with the control group (without lymph node metastasis), OR=3.08 (95% CI: 1.64–5.79, P<0.001) and the forest plot (Figure 2B) showed that with the increase of COX-2 expression, the risk of lymph node metastasis increased.
COX-2 overexpression and tumor size
A total of 5 literature was included, including 178 patients with tumor size >5 cm and 220 with tumor size ≤5 cm. The two groups had no statistical heterogeneity (P=0.502), so the FEM was used (Table 2). Compared with the control group (tumor size ≤5 cm), OR=1.04 (95% CI: 0.68–1.57, P>0.05) and the forest plot (Figure 2C) showed that COX-2 overexpression was not related to tumor size of gastric cancer.
COX-2 overexpression and TNM stage
A total of 5 literature was included, including 511 patients in T1+T2 and 310 in T3+T4. The two groups had statistical heterogeneity (P=0.000), so the REM was used (Table 2). Compared with the control group (patients in T1+T2), OR=2.39 (95% CI: 0.91–6.26, P=0.075) and the forest plot (Figure 2D) showed that COX-2 overexpression was not related to TNM stage of gastric cancer.
COX-2 overexpression and gender, age and differentiation
Based on whether having heterogeneity, the corresponding statistical model was selected. The differentiation had heterogeneity (P<0.05), so the REM was used (Table 2). Table 2 showed that there was no correlation between COX-2 overexpression and gender, age and differentiation (P>0.05).
Publication bias
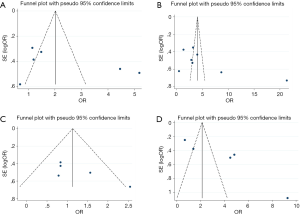
The included literature was analyzed by funnel plot, and then the symmetry of the funnel plot was analyzed by Egger’s Test. The specific results were shown in Table 2, and part of the funnel plot was shown in Figure 3. The results showed that there was no publication bias in gender, age, differentiation, infiltration depth, lymph node metastasis and tumor size, but there was partial publication bias in TNM stage.
Sensitivity analysis
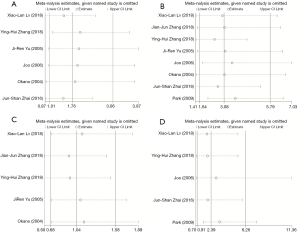
The result of sensitivity analysis was shown in Figure 4. After excluding a single study in order, the Meta-analysis was repeated. The result showed that, after excluding 5 of the literature, the difference of infiltration depth became not statistical significant while only one literature had no effect on the result (18). Lymph node metastasis, tumor size and TNM stage had good stability because the conclusion remained unchanged after excluding one of the literature. On the whole, the Meta-analysis result was stable.
Discussion
Gastric cancer is one of the most common malignant tumors of the digestive system. According to the findings released by the International Cancer Research Center (IARC) in 2012, the number of new case has dropped from 989,600 in 2008 to 951,600 in 2012, and the mortality from 738,000 to 723,100 over the same period. Although the morbidity and mortality have shown a downward trend in most countries and regions of the world (20), over the same period, an increase of 1,422,600 new cases of malignant tumors and 7,571,500 new deaths have occurred (20). COX-2 gene is located long arm of human chromosome 1, about 8 kb and with 9 introns and 10 exons. The mRNA, about 4 kb, encodes an open reading frame of 604 amino acids, consisting of signal peptide of 17 amino acid residues (21). COX-2 is one of the three isomerases of cyclooxygenase, which is the key enzyme to catalyze the conversation of arachidonic acid to PG. COX-2 expression and stability are regulated by many elements of intron and 5'-untranslated region (5'-UTR) and 3'- untranslated region (3'-UTR) in transcription template. The polymorphism of COX-2 gene in the promoter region plays a key role in the regulation of transcription with tissue specificity (22). The gene polymorphism may eliminate or increase binding sites, or change the affinity between binding sites and regulatory factors, thus changing COX-2 expression and affecting the biosynthesis of PGs (23). The occurrence and development of gastric cancer is complex, which provides a opportunity for the effective intervention and prevention. COX-2 has the potential to be an effective target for intervention and prevention of gastric cancer (24). Some clinical studies have shown that nonsteroidal antiinflammatory drugs(NSAIDs) can significantly reduce the incidence of gastric cancer (25), and COX is the main target of NSAIDs. COX has three structural types, among which the role of COX-1 and COX-2 is relatively clear. COX-1 continuously expresses in many tissues while COX-2 is mainly responsible for the inflammatory reaction and tumor development, usually without expression or with expression at a very low level (26,27). Some studies have found that COX-2 is associated with inhibiting apoptosis, promoting tumor neovascularization and increasing metastatic potential (28,29). Also, some researches have shown that COX-2 expression is significantly increased in gastric cancer and precancerous lesion while it is less in non-gastric cancer lesions, and almost no expression in normal gastric mucosa (30). Moreover, the relationship between COX-2 overexpression and clinicopathological features of gastric cancer is not clear. Zhang et al. (18) have believed that COX-2 is not related to infiltration depth of gastric cancer. However, Zhai et al. (11) have suggested that Cox-2 overexpression increases the risk of invasion depth of gastric cancer. Therefore, in order to explore the relationship between COX-2 overexpression and clinicopathological features of gastric cancer, such as lymph node metastasis, infiltration depth, TNM stage, a number of previous studies on the COX-2 expression in gastric cancer detected by IHC were analyzed, thus providing clinical guidance for evidence-based medicine.
Nine literature (11-19) was included in this meta analysis, including 1289 patients with gastric cancer. The overall positive rate of COX-2 was 68.1%. The results showed that COX-2 overexpression was correlated with infiltration depth and lymph node metastasis, which meant that the both had predictive value. Also, COX-2 overexpression was not correlated with TNM stage, differentiation, tumor size, age and gender. From statistical heterogeneity, age, gender and tumor size was homogeneity, so the FEM was selected for statistical analysis, while differentiation, infiltration depth, lymph node metastasis and TNM stage all had great heterogeneity, so the REM was selected. From publication bias, there was no publication bias in infiltration depth, lymph node metastasis, age, gender and differentiation, except TNM stage (P<0.05). The sensitivity analysis showed that the study on infiltration depth had poor stability, so the conclusion of it should be careful. Moreover, the result between COX-2 overexpression and other clinicopathological features of gastric cancer was stable, so basically, the result of Meta-analysis was stable, indicating that the conclusion of this study was reliable. The meta analysis by Zhuo et al. (31) has showed that COX-2 was correlated with infiltration depth and lymph node metastasis, but not with differentiation. The Meta-analysis by Zeng et al. (32) on COX-2 expression and lymph node metastasis of gastric cancer has showed that COX-2 overexpression was associated with lymph node metastasis, which promotes lymph node metastasis of gastric cancer. These conclusions are consistent with that in this study. As this study was more cautious in the inclusion of literature, excluding the literature of non-core journals, and including several latest studies, the conclusion of this study is persuasive.
However, this study also has some limitations: (I) there are differences in the scoring criteria of IHC for COX-2 expression; (II) there is high heterogeneity in several factors related to COX-2 overexpression; (III) although all the researches were use IHC, the manufacturer, dilution concentration and judgment criteria of the antibody were not completely consistent, which may also affect the result of Meta-analysis.
In conclusion, this study indicates that COX-2 high expression in gastric cancer could increase infiltration depth and risk of lymph node metastasis. The result of Song et al. (33) show that COX-2 high expression is correlated with poor prognosis of gastric cancer. Therefore, COX-2-targeted therapy combined with radiotherapy and chemotherapy may achieve certain efficacy in the comprehensive treatment of gastric cancer. Certainly, because of the limitations, more clinical data should be collected and the prognosis need to be observed. Further study on the effect of COX-2 overexpression on gastric cancer is required to provide a more sufficient theoretical basis for the application of anti-COX-2 therapy in gastric cancer.
Acknowledgments
Thank you Guangzhou Yujia Biotechnology Co., Ltd. for your help in data analysis.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.52). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 working in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Woo Y, Son T, Song K, et al. A Novel Prediction Model of Prognosis After Gastrectomy for Gastric Carcinoma: Development and Validation Using Asian Databases. Ann Surg 2016;264:114-20. [Crossref] [PubMed]
- Lin J X, Yoon C, Desiderio J, et al. Development and validation of a staging system for gastric adenocarcinoma after neoadjuvant chemotherapy and gastrectomy with D2 lymphadenectomy. Br J Surg 2019;106:1187-96. [Crossref] [PubMed]
- Sheng WQ, Huang D, Ying JM, et al. HER2 status in gastric cancers: a retrospective analysis from four Chinese representative clinical centers and assessment of its prognostic significance. Ann Oncol 2013;24:2360-4. [Crossref] [PubMed]
- Zhang F, Li K, Yao X, et al. A miR-567-PIK3AP1-PI3K/AKT-c-Myc feedback loop regulates tumour growth and chemoresistance in gastric cancer. EBioMedicine 2019;44:311-321. [Crossref] [PubMed]
- Jiang D, Li H, Xiang H, et al. Long Chain Non-Coding RNA (lncRNA) HOTAIR Knockdown Increases miR-454-3p to Suppress Gastric Cancer Growth by Targeting STAT3/Cyclin D1. Med Sci Monit 2019;25:1537-48. [Crossref] [PubMed]
- Cacina C, Kaşarci G, Bektaş K, et al. The COX2 genetic variants in oral squamous cell carcinoma in Turkish population. Cell Mol Biol (Noisy-le-grand) 2018;64:96-100. [Crossref] [PubMed]
- Luo H, Chen Z, Jin H, et al. Cyclooxygenase-2 up-regulates vascular endothelial growth factor via a protein kinase C pathway in non-small cell lung cancer. J Exp Clin Cancer Res 2011;30:6. [Crossref] [PubMed]
- Sahin M, Sahin E, Gumuslu S. Cyclooxygenase-2 in cancer and angiogenesis. Angiology 2009;60:242-53. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Zhai JS, Li N, Song JG, et al. Expressions of APPL1 and COX-2 in gastric carcinoma and relationship with the prognosis of patient0s. Chinese J Gastroenterol Hepatol 2016;25:16-9.
- Park ES, Do IG, Park CK, et al. Cyclooxygenase-2 is an independent prognostic factor in gastric carcinoma patients receiving adjuvant chemotherapy and is not associated with EBV infection. Clin Cancer Res 2009;15:291-8. [Crossref] [PubMed]
- Okano H, Shinohara H, Miyamoto A, et al. Concomitant overexpression of cyclooxygenase-2 in HER-2-positive on Smad4-reduced human gastric carcinomas is associated with a poor patient outcome. Clin Cancer Res 2004;10:6938. [Crossref] [PubMed]
- Yu JR, Wu YJ, Qin Q, et al. Expression of cyclooxygenase-2 in gastric cancer and its relation to liver metastasis and long-term prognosis. World J Gastroenterol 2005;11:4908-11. [Crossref] [PubMed]
- Joo YE, Chung IJ, Park YK, et al. Expression of cyclooxygenase-2, p53 and Ki-67 in gastric cancer. J Korean Med Sci 2006;21:871-6. [Crossref] [PubMed]
- Zhang JJ, Zou JP, Ma XY, et al. Expression of COX-2,NF-κB and VEGF in gastric carcinoma tissues and its relationship with angiogenesis. Modern Journal of Integrated Traditional Chinese and Western Medicine 2018;27:1263-6.
- Liu Y, Li KF, Gu H. Correlation analysis of the COX-2, CD31 and CD105 expression and the staging or prognosis of gastric cancer. Chinese Journal of Operative Procedures of General Surgery 2018;12:500-2. (Electronic Version).
- Zhang YH, Yan W, Qiao BJ, et al. Expression and significance of COX-2 and VEGF-C protein in gastric cancer and precancerous lesions. J Clin Experimental Med 2018;17:1040-3.
- Lv XL, Guo M. Significance of COX-2, EGFR and E-cad in evaluation of prognosis of gastric cancer. Chinese J Clin Res 2018;31:20-4.
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Kosaka T, Miyata A, Ihara H, et al. Characterization of the human gene (PTGS2) encoding prostaglandin-endoperoxide synthase 2. Eur J Biochem 1994;221:889-97. [Crossref] [PubMed]
- Wang JX, Zhang KG. Advance in relationship between COX-2 and gastric cancer. World Chinese J Digestol 2009;17:378-83. [Crossref]
- Luo MX, Long BB, Li F, et al. Roles of Cyclooxygenase-2 gene -765G > C (rs20417) and -1195G>A (rs689466) polymorphisms in gastric cancer: A systematic review and meta-analysis. Gene 2019;685:125-35. [Crossref] [PubMed]
- Wang Z, Chen JQ, Liu JL. COX-2 Inhibitors and Gastric Cancer. Gastroenterol Res Pract 2014;2014:132320.
- Guo Q, Li Q, Wang J, et al. A comprehensive evaluation of clinical efficacy and safety of celecoxib in combination with chemotherapy in metastatic or postoperative recurrent gastric cancer patients: A preliminary, three-center, clinical trial study. Medicine (Baltimore) 2019;98:e16234. [Crossref] [PubMed]
- Hamy AS, Tury S, Wang X, et al. Celecoxib With Neoadjuvant Chemotherapy for Breast Cancer Might Worsen Outcomes Differentially by COX-2 Expression and ER Status: Exploratory Analysis of the REMAGUS02 Trial. J Clin Oncol 2019;37:624-35. [Crossref] [PubMed]
- Edelman MJ, Wang X, Hodgson L, et al. Phase III Randomized, Placebo-Controlled, Double-Blind Trial of Celecoxib in Addition to Standard Chemotherapy for Advanced Non-Small-Cell Lung Cancer With Cyclooxygenase-2 Overexpression: CALGB 30801 (Alliance). J Clin Oncol 2017;35:2184-92. [Crossref] [PubMed]
- Zhang Y, Li Y, Li H, et al. Clostridium difficile toxin B recombinant protein inhibits tumor growth and induces apoptosis through inhibiting Bcl-2 expression, triggering inflammatory responses and activating C-erbB-2 and Cox-2 expression in breast cancer mouse model. Biomed Pharmacother 2018;101:391-8. [Crossref] [PubMed]
- Bonhin RG, de Carvalho GM, Guimarães AC, et al. Histologic correlation of VEGF and COX-2 expression with tumor size in squamous cell carcinoma of the larynx and hypopharynx. Ear Nose Throat J 2017;96:176-82. [PubMed]
- Nardone G, Rocco A, Vaira D, et al. Expression of COX-2, mPGE-synthase1, MDR-1 (P-gp), and Bcl-xL: a molecular pathway of H pylori-related gastric carcinogenesis. J Pathol 2004;202:305-12. [Crossref] [PubMed]
- Zhuo Q, Ni M. Relationship between COX-2 expression and gastric carcinoma: a meta-analysis. Chinese J Evidence-Based Med 2017;17:1276-82.
- Zeng JY. A Meta-analysis of relationship between COX-2 Expression and LymphNode metastasis in gastric cancer. Canc Res Prev Treat 2011;38:584-7.
- Song J, Su H, Zhou YY, et al. Cyclooxygenase-2 expression is associated with poor overall survival of patients with gastric cancer: a meta-analysis. Dig Dis Sci 2014;59:436-45. [Crossref] [PubMed]