
Clinical significance of serum cystatin C in early evaluation of renal impairment caused by chemotherapy in elderly patients
Introduction
Chemotherapy is one of the main treatment approaches for malignant tumors. However, the chemotherapy can lead to a series of toxic reactions. Renal toxicity is a common serious toxic side effect of chemotherapy drugs. Since the renal function reserve of elderly patients is reduced, renal function would more likely be damaged during chemotherapy. Therefore, more attention should be given to the monitoring of renal function during chemotherapy in elderly patients with tumors. The role of serum cystatin C (Cys C), as an intrinsic marker of glomerular filtration rate (GFR), is gaining more and more attention. This has been studied more in the monitoring of early renal impairment caused by chronic nephritis, diabetic nephropathy and hypertensive nephropathy (1). However, few studies have been conducted on application value of Cys C, as a marker of GFR in evaluating renal impairment induced by chemotherapy, and its effect remains uncertain. Furthermore, fewer studies have been conducted on the monitoring of renal function after chemotherapy in elderly patients with tumors. In the present study, the evaluation function of Cys C on renal impairment during postoperative adjuvant chemotherapy in elderly patients with malignant tumors was investigated via the dynamic observation of the postoperative changes in serum Cys C, serum creatinine (SCr), blood urea nitrogen (BUN) and endogenous creatinine clearance rate (CCr) in elderly patients with malignant tumor before and after chemotherapy.
Methods
This study was conducted in accordance with the Declaration of Helsinki and it was conducted with approval from the Ethics Committee of Qingdao Cancer Hospital. Written informed consent was obtained from the participants.
Research object
A total of 124 elderly patients with malignant tumors were selected as study subjects. These patients were diagnosed and received postoperative chemotherapy in Qilu Hospital from October 2010 to October 2011. Among these patients, 76 patients were male and 48 patients were female, with a median age of 68 years old (range, 65–75 years old). Furthermore, among these patients, 28 patients had lung cancer, 45 patients had gastrointestinal tumors, 26 patients had breast cancer, and 25 patients had other malignant tumors. Meanwhile, 69 healthy subjects of the same age were randomly selected and assigned into the control group, in order to exclude the influence of tumor factors. Among these subjects, 42 subjects were male and 27 subjects were female. All chemotherapy regimens were performed according to the National Comprehensive Cancer Network (NCCN) Guidelines. The 124 patients were divided into two groups: platinum-based (cisplatin for injection or carboplatin for injection, Qilu Pharmaceutical Co., Ltd. Jinan, Shandong) chemotherapy group (n=77) and non-platinum-based chemotherapy group (n=47).
Specimen collection and preservation
Five mL of peripheral venous blood was collected from each elderly patient with adjuvant chemotherapy on an empty stomach, before the first chemotherapy after the operation, after two cycles of chemotherapy, and after four cycles of chemotherapy, respectively. Then the samples were placed in a disposable biochemical vacuum blood collection tube. In the control group, 5 mL of peripheral venous blood was also collected on an empty stomach from each subject during the physical examination, and the samples were placed in a disposable biochemical vacuum blood collection tube.
Main instruments and reagents
Japanese Roche Cobas 8000 automatic biochemical analyzer; Roche kit (Shanghai Roche Pharmaceutical Co., Ltd.); Sichuan Xincheng Kit (Shandong Misaite Medical Device Co., Ltd.).
Testing methods
Basic information, such as the age, gender, height and weight of all subjects in the chemotherapy groups and control group, was measured and recorded, respectively. The Cys C, SCr and BUN tests were all completed in an automatic biochemical analyzer. CCr was calculated according to the Cockcroft-Gault formula (2), and corrected based on the standard body surface area of 1.73 m2.
CCr =(140− age) ×2.12× weight × K/BSA × SCr
Remarks: in the formula, the unit of body weight is kg. BSA is the body surface area, and the unit is m2. The unit of SCr is umol/L. K is 1 (male) or 0.85 (female).
The body surface area (BSA) was calculated according to the Stevenson formula. BSA =0.0061× height (cm) +0.0128× weight (kg) −0.1529.
Statistical analysis
The statistical analysis was conducted using SPSS 17.0 statistical software. All data were presented as standard deviation (SD). The tests performed included t-test, Mann Whitney U-test, Spearman’s rank correlation test, F-test and q-test. The area under the curves (AUCs) of Cys C, BUN, SCr and CCr were compared using receiver operator characteristic (ROC) curves. The difference was statistically significant when the P value was <0.05 in all tests, and this was remarkably and statistically significant when the P value was <0.01.
Results
Basic information and the expression of Cys C, SCr, BUN and CCr in the chemotherapy groups and control group
In the present study, elderly patients with malignant tumors after the operation were enrolled in the chemotherapy groups, and their age ranged within 65–75 years old. Meanwhile, healthy subjects of the same age were selected and enrolled into the control group. The difference in age, gender, height, weight and body surface area was not significant between the chemotherapy group and control group (Table 1). The expression of serum Cys C after the operation was 0.97±0.29 mg/L in elderly patients with malignant tumors and 0.95±0.15 mg/L in healthy subjects, but the difference was not statistically significant (P=0.79). Therefore, the influence of tumor factors can be excluded. Furthermore, the difference in SCr, BUN and CCr was not statistically significant (P=0.54, 0.094, 0.39) between the two groups. Therefore, these are well-comparable between the two groups.
Table 1
| Basic information | The chemotherapy group | The control group | P |
|---|---|---|---|
| Age (years old) | 67.64±3.37 | 69.4±3.12 | 0.67 |
| Male/female | 76/48 | 42/27 | 0.42 |
| Height (cm) | 165.3±8.2 | 65.8±7.47 | 0.88 |
| Weight (kg) | 70.16±9.6 | 72.25±12.71 | 0.43 |
| Body surface area (m2) | 1.69±0.23 | 1.73±0.42 | 0.57 |
| SCr (umol/L) | 62.73±11.61 | 67.3±11.23 | 0.54 |
| Cys C (mg/L) | 0.95±0.15 | 0.97±0.29 | 0.79 |
| BUN (mmol/L) | 4.62±1.24 | 5.03±0.32 | 0.094 |
| CCr (mL/min/1.73 m2) | 81.99±17.28 | 78.25±12.71 | 0.39 |
Mann-Whitney U test was applied to sex, and sample t-test was applied to the others. Cys C, serum cystatin C; CCr, creatinine clearance rate; SCr, serum creatinine; BUN, blood urea nitrogen.
Expression of Cys C, SCr, BUN and CCr in the chemotherapy group before chemotherapy, after two cycles of chemotherapy and after four cycles of chemotherapy
Table 2 showed that there were no significant changes (P=0.11, 0.86, 0.23, 0.64) in Cys C, SCr, BUN and CCr in elderly patients after two cycles of adjuvant chemotherapy. However, Cys C significantly increased after four cycles of adjuvant chemotherapy (P=0.01), while CCr significantly decreased (P=0.027), when compared to that before chemotherapy. There were no significant changes (P=0.07, 0.068) in SCr and BUN, when compared to that before chemotherapy.
Table 2
| Index | Before chemotherapy | After two cycles of chemotherapy | After four cycles of chemotherapy |
|---|---|---|---|
| Cys C (mg/L) | 0.95±0.15 | 0.97±0.14 (P=0.11) | 1.03±0.17 (P=0.01) |
| SCr (umol/L) | 62.73±11.61 | 62.9±12.1 (P=0.86) | 64.95±14.89 (P=0.07) |
| BUN (mmol/L) | 4.62±1.24 | 4.83±1.37 (P=0.23) | 5.88±1.13 (P=0.068) |
| CCr (mL/min/1.73 m2) | 81.99±17.28 | 80.97±18.15 (P=0.64) | 79.09±24.49 (P=0.027) |
Paired t-test was used. Cys C, serum cystatin C; CCr, creatinine clearance rate; SCr, serum creatinine; BUN, blood urea nitrogen.
Expression levels of the four parameters from the increase in Cys C level after two cycles of adjuvant chemotherapy to the completion of four cycles of adjuvant chemotherapy
In the present study, the expression of Cys C, SCr, BUN and CCr before chemotherapy was all within the normal range in 20 elderly patients with malignant tumors. However, the experience of Cys C was higher than the normal range (>1.09 mg/L) after two cycles of adjuvant chemotherapy, while the expression of SCr, BUN and CCr remained within the normal range (P=0.14, 0.07, 0.14). In addition, it was observed that the level of Cys C was significantly higher (P=0.007) after four cycles of adjuvant chemotherapy in elderly patients, while the level of CCr was significantly lower (P=0.009) than that before the adjuvant chemotherapy. That is, CCr was <75 mL/min/1.73 m2. Furthermore, the levels of SCr and BUN were also significantly higher (P=0.015, 0.023) than those before the adjuvant chemotherapy (Table 3).
Table 3
| Index | Before chemotherapy | After two cycles of chemotherapy | After four cycles of chemotherapy |
|---|---|---|---|
| Cys C (mg/L) | 1.05±0.17 | 1.16±0.09 (P=0.09) | 1.20±0.14 (P=0.007) |
| SCr (umol/L) | 64.24±11.37 | 66.71±12.24 (P=0.14) | 68.21±11.96 (P=0.015) |
| BUN (mmol/L) | 4.47±1.03 | 5.35±1.39 (P=0.07) | 5.8±2.05 (P=0.023) |
| CCr (mL/min/1.73 m2) | 80.72±17.3 | 77.6±17.08 (P=0.14) | 70.07±19.58 (P=0.009) |
Paired t-test was used. Cys C, serum cystatin C; CCr, creatinine clearance rate; SCr, serum creatinine; BUN, blood urea nitrogen.
Expression of Cys C, SCr, BUN and CCr in the platinum-based chemotherapy group and non-platinum-based chemotherapy group
According to the chemotherapy regimen, the elderly patients who received adjuvant chemotherapy were divided into two groups: platinum-based chemotherapy group (n=77) and non-platinum-based chemotherapy group (n=47). After two cycles of adjuvant chemotherapy, the difference in expression of Cys C, SCr, BUN and CCr in the platinum-based chemotherapy group was not significantly, when compared to that before chemotherapy. After four cycles of adjuvant chemotherapy, the expression of Cys C increased, while the expression CCr decreased. The two GFR markers of SCr and BUN remained unchanged (Table 4). In the non-platinum chemotherapy group, there were no significant changes in Cys C, SCr, BUN and CCr after two cycles and four cycles of adjuvant chemotherapy (Table 5).
Table 4
| Index | Before chemotherapy | After two cycles of chemotherapy | After four cycles of chemotherapy |
|---|---|---|---|
| Cys C (mg/L) | 0.98±0.15 | 1.00±0.14 (P=0.15) | 1.07±0.17 (P=0.003) |
| SCr (umol/L) | 63.44±11.04 | 64.56±10.89 (P=0.37) | 67.81±15.71 (P=0.087) |
| BUN (mmol/L) | 4.65±1.28 | 4.89±1.54 (P=0.34) | 6.29±7.5 (P=0.12) |
| CCr (mL/min/1.73 m2) | 81.6±16.3 | 80.59±16.44 (P=0.54) | 78.57±26.94 (P=0.037) |
Paired t-test was used. Cys C, serum cystatin C; CCr, creatinine clearance rate; SCr, serum creatinine; BUN, blood urea nitrogen.
Table 5
| Index | BEFORE chemotherapy | After two cycles of chemotherapy | After four cycles of chemotherapy |
|---|---|---|---|
| Cys C (mg/L) | 0.90±0.17 | 0.92±0.14 (P=0.44) | 0.96±0.14 (P=0.14) |
| SCr (umol/L) | 61.57±12.17 | 61.18±12.89 (P=0.32) | 61.26±11.96 (P=0.3) |
| BUN (mmol/L) | 4.57±1.18 | 4.74±1.01 (P=0.46) | 5.20±1.13 (P=0.093) |
| CCr (mL/min/1.73 m2) | 82.63±19.27 | 81.59±20.78 (P=0.2) | 79.94±19.58 (P=0.27) |
Paired t-test was used. Cys C, serum cystatin C; CCr, creatinine clearance rate; SCr, serum creatinine; BUN, blood urea nitrogen.
The correlation among Cys C, SCr, BUN and CCr
Table 6 showed that there was a certain correlation among Cys C, SCr, BUN and CCr in the control group (r=−0.78, −0.41, −0.27). The correlation between Cys C and CCr was significantly better than the latter two, showing a high correlation. Furthermore, there was a correlation (r=−0.82, −0.49, −0.25) among Cys C, SCr, BUN and CCr in the chemotherapy group. However, the correlation coefficient between Cys C and CCr was remained the highest, showing a high correlation.
Table 6
| Index | CCr(mL/min) | ||||
|---|---|---|---|---|---|
| The control group | The chemotherapy group | ||||
| r | P | r | P | ||
| Cys C(mg/L) | −0.78 | <0.01 | −0.82 | <0.01 | |
| SCr (umol/L) | −0.41 | <0.01 | −0.49 | <0.01 | |
| BUN (mmol/L) | −0.27 | <0.01 | −0.25 | <0.05 | |
Cys C, serum cystatin C; CCr, creatinine clearance rate; SCr, serum creatinine; BUN, blood urea nitrogen.
ROC curve comparison
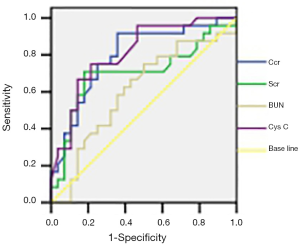
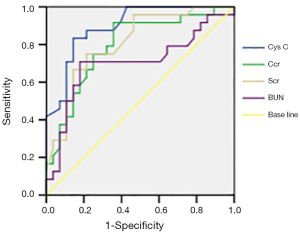
The area under the curve (AUC) of Cys C, CCr, SCr and BUN were 0.82, 0.81, 0.72 and 0.65, respectively, and Cys C and CCr had the largest values (P<0.01). After chemotherapy, the AUCROC of Cys C, CCr, SCr and BUN was 0.93, 0.91, 0.85 and 0.73, respectively, and Cys C had the largest value (P<0.01) (Figures 1 and 2).
Discussion
Chemotherapy will inevitably damage normal tissues and organs, in which renal impairment is more common (3), and most chemotherapy drugs have varying degrees of renal toxicity. As a broad-spectrum and effective drug for chemotherapy, platinum-based drugs have been widely used in the treatment of various solid tumors and malignant tumors of the blood system (4). However, it is also the main chemotherapy drug that results in renal impairment (5). Elderly cancer patients with decreased renal reserve function is a high risk group for renal impairment. Therefore, with respect to elderly cancer patients receiving chemotherapy, especially those using platinum-based chemotherapy regimens, attention should be given on the changes in renal function (6).
GFR is one of the most important indexes that reflect the renal function. The “gold standard” for the clinical detection of GFR is inulin clearance. The inulin is filtered through the glomerulus without being reabsorbed and secreted by renal tubules. Hence, it can accurately reflect GFR. Nonetheless, as an exogenous substance, the inulin requires intravenous infusion, which is not convenient to use. The clearance rate represented by radioisotope markers (such as 51Cr-EDTA or 99mTc-DTPA) is time-consuming, expensive and radioactive, and its clinical application is limited (7). BUN and SCr are the common indexes used to evaluate the renal function. However, the levels of BUN and SCr are associated with gender, muscle content, protein intake and metabolic level. Furthermore, the creatinine excretion from the body is through renal filtration and renal tubule secretion. When GFR decreases to 50% of the normal range, SCr can still remain within the normal range. However, since the specificity and sensitivity are relatively low, the value in the monitoring of early renal impairment is limited. CCr is a common approach to evaluate the renal function in clinic. However, it takes time to collect the urine. Furthermore, the operation process is tedious, inaccurate and inconvenient, and the results cannot be reported in time. Therefore, it is also limited in clinical application.
Cys C (also known as cysteinase inhibitor C) has been considered to be an ideal endogenous marker for CCr. Its structure and function were identified in the 1980s. It is a non-glycosylated elliptical small molecule protein, which comprises of 120 amino acids with a molecular weight of 13.3 KD. Furthermore, the pH value of Cys C is 8.6, and the isoelectric point is 9.3. In addition, it has a positive charge under physiological conditions (8). As a member of cystine protease inhibitor superfamily 2, the Cys C is the most important cysteinase inhibitor. Its main physiological function is to manage cell secretion or necrosis, since cysteine proteinase leaks from lysosome during apoptosis (9). The human Cys C gene is located at the chromosome 20 (10) and belongs to a “housekeeping gene”. It is continuously expressed in most tissues, and is widely distributed in vivo, with the highest concentration in seminal plasma and spinal cord fluid, and the lowest concentration in urine (11). The molecular weight of Cys C is small and positively charged, which can be completely filtered through the kidney. Furthermore, it is not secreted by the renal tubule, but is completely reabsorbed by the proximal tubule, which subsequently decomposes and blocks it from returning to the blood. In addition, the Cys C is not affected by gender, age, inflammation, muscle and other factors. Hence, the Cys C is theoretically a reliable endogenous marker for GFR. Recent studies have also confirmed that the Cys C is an ideal endogenous marker for monitoring GFR, and has high sensitivity for detecting early renal impairment (12). Serum Cys C is of great value in monitoring the renal function of patients with acute renal failure (13), renal transplantation (14) and diabetic nephropathy (15). Other studies have reported that the Cys C can better represent the GFR (16) in the elderly. However, there are few clinical data on the serum Cys C for the evaluation of renal impairment induced by chemotherapy drugs, especially for the monitoring of renal function in elderly patients with malignant tumors after chemotherapy. Wilson et al. (17) compared the accuracy of Cys C, CCr and SCr in the evaluation of GFR in patients with head and neck cancer, who received platinum-based chemotherapy. The results revealed that the Cys C was more sensitive to renal impairment, when compared to SCr, and that it maintained a good correlation with CCr. It has been preliminarily confirmed that the serum Cys C could replace CCr in the monitoring of early renal impairment after chemotherapy.
In the present study, the Cys C, SCr, BUN and CCr were selected as renal function markers during adjuvant chemotherapy in elderly patients with malignant tumors, and these four markers were analyzed and compared. There was no significant difference in Cys C, SCr, BUN and CCr before chemotherapy among elderly patients between the chemotherapy group and healthy subjects of the same age. Furthermore, there was no significant change in the four parameters after two cycles of adjuvant chemotherapy. However, the Cys C increased and CCr decreased after four cycles of adjuvant chemotherapy, but SCr and BUN still did not significantly change. Thus, it can be observed that Cys C could be used as a sensitive marker for renal impairment in elderly patients with malignant tumors during the adjuvant chemotherapy, while SCr and BUN have limited value in determining renal impairment caused by chemotherapy in elderly patients. Kawakami et al. (18) investigated 41 tumor patients. Among these patients, 35 patients were treated with platinum-based chemotherapy regimen. The blood samples were collected before and after chemotherapy, and the clearance rates of Cys C, SCr and inulin were measured. The results revealed that the serum level of Cys C increased by 21% and the inulin clearance decreased by 23%. In contrast, there was no significant change in the level of SCr before and after the chemotherapy. In addition, it also revealed that Cys C was more sensitive than SCr. In combining these with the area under the receiver operating characteristics (AUROC) curves of subjects, it could be inferred that Cys C could be used as a sensitive index for GFR in the course of the postoperative adjuvant chemotherapy of elderly patients with malignant tumors, and that this could more sensitively reflect the renal impairment, when compared to SCr and BUN. The Cys C can replace CCr in monitoring renal function damage during the chemotherapy in elderly cancer patients due to its simplicity, stability, sensitivity and accuracy. However, there are some differences in the reports between the present study and some previous studies. The study results of Kawakami et al. (18) indicated that the expression of Cys C immediately increased after chemotherapy. However, the expression of Cys C did not increase after two cycles of adjuvant chemotherapy, but increased after four cycles of adjuvant chemotherapy, when compared to that before chemotherapy. The increase in Cys C level was slow and failed to monitor the renal function damage induced by the first chemotherapy. Hence, it was generally considered that there was no significant difference in serum Cys C level between the tumor patients and healthy subjects. Nevertheless, in recent years, it has been reported that the expression of Cys C may be elevated (19) in tumor tissues. In the present study, there was no significant difference in the expression of Cys C after chemotherapy between elderly patients with malignant tumors and healthy subjects, thereby excluding the impacts of tumor factors on the concentration of Cys C in blood. The increase in Cys C concentration after chemotherapy was caused by renal function damage.
Among the 124 patients in the present study, the Cys C was higher than the normal range (>1.09 mg/L) in 20 elderly patients after two cycles of adjuvant chemotherapy, while the SCr, BUN and CCr of these patients were still within the normal range. A separate study of these patients revealed that the level of Cys C was significantly higher, when compared to that before (P=0.007) the completion of four cycles of adjuvant chemotherapy, and the average level reached 1.20 mg/L, while CCr was significantly lower than that before the chemotherapy (P=0.009), which dropped below 75 mL/min/1.73 m2. At this time, SCr and BUN levels were also significantly higher (P=0.015 and 0.023, respectively), when compared to those before the chemotherapy. This indicates that these elderly patients have obvious renal impairment at the end of chemotherapy. Therefore, it is recommended when the age is ≥65 years old and the expression of Cys C is higher than the normal range, more attention should be given to the changes in renal function. Furthermore, if necessary, the dosage of chemotherapy drugs with high toxicity should be reduced, as appropriate, in order to avoid further aggravating the renal impairment.
According to the chemotherapy regimen, patients were divided into two groups for subgroup analysis: the platinum-based chemotherapy group (n=77) and non-platinum-based chemotherapy group (n=47). It was found that there was no significant change in Cys C, SCr, BUN and CCr in the non-platinum-based chemotherapy group after two cycles of chemotherapy and four cycles of chemotherapy. This indicates that there is no obvious renal impairment in elderly patients with malignant tumors during the non-platinum-based adjuvant chemotherapy. In the platinum-based chemotherapy group, there was no significant change in the four indexes of GFR after two cycles of adjuvant chemotherapy, but the level of Cys C significantly decreased, when compared to that before after four cycles of adjuvant chemotherapy. This indicates that elderly patients in the platinum chemotherapy group has obvious renal impairment at the end of the four cycles of adjuvant chemotherapy. It has been reported that the incidence of nephrotoxicity in elderly patients with malignant tumors is low during combined chemotherapy with platinum-based chemotherapy (20,21). On the contrary, the present study shows that the possibility of renal impairment in elderly cancer patients with platinum-based chemotherapy increases, and that that these patents are only well-tolerant to non-platinum-based chemotherapy regimens. The present study also shows that platinum is the main chemotherapy drug that causes renal impairment in elderly patients with malignant tumors. Hence, it can be observed that more attention should be given to the changes in renal function when elderly patients are treated with platinum-based chemotherapy regimen.
In the present study, there was a significant negative correlation (r=−0.78, −0.82) between Cys C and CCr in either healthy subjects or elderly patients with malignant tumors. Furthermore, there was a significant correlation at the test level of 0.01. The present results were in accordance with the reported correlation coefficient range (22) (0.71–0.87) of Cys C and 51Cr-EDTA, or inulin clearance. Furthermore, there was also a certain correlation among SCr, BUN and CCr. The correlation coefficients for SCr, BUN and CCr were −0.41 and −0.27, while the correlation coefficients for SCr, BUN and CCr in elderly cancer patients with postoperative chemotherapy were −0.49, and −0.25. All these were lower than the correlation between Cys C and CCr. The present results were consistent with the results reported by Torres da Costa E Silva et al. (23) There was a better correlation between Cys C and CCr, showing a high correlation. Therefore, the investigators consider that serum Cys C can replace CCr in monitoring renal function injury caused by chemotherapy drugs in elderly patients with malignant tumors.
Conclusions
(I) Although SCr and BUN do not significantly change during the postoperative adjuvant chemotherapy in elderly patients with malignant tumors, the GFR may have significantly decreased. Serum Cys C level can be more sensitive to monitor the renal impairment during the chemotherapy, which can replace the CCr in monitoring renal impairment in elderly cancer patients during the chemotherapy. (II) If the expression of serum Cys C in elderly patients with malignant tumors is higher than normal, more attention should be given to the changes in renal function, and if necessary, the dosage of chemotherapy drugs with high toxicity should be reduced, as appropriate, in order to avoid further aggravating the renal impairment. (III) Platinum is the main chemotherapy drug that causes renal impairment during the postoperative adjuvant chemotherapy in elderly patients with malignant tumors. The changes in renal function should be closely monitored when a platinum-based adjuvant chemotherapy regimen is used. (IV) Elderly patients with malignant tumors have good tolerance to non-platinum-based adjuvant chemotherapy and no obvious renal impairment occurs during the postoperative adjuvant chemotherapy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.30). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and it was conducted with approval from the Ethics Committee of Qingdao Cancer Hospital (No. SD20190106). Written informed consent was obtained from the participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Larki RA, Jamali B, Meidani M, et al. Serum Cystatin C for Evaluation of Acute Kidney Injury in Adults Treated with Colistin. J Res Pharm Pract 2018;7:178-81. [Crossref] [PubMed]
- Mian AN, Schwartz GJ. Measurement and Estimation of Glomerular Filtration Rate in Children. Adv Chronic Kidney Dis 2017;24:348-56. [Crossref] [PubMed]
- Pokrivčák T, Poprach A. Cancer Treatment-induced Changes in Renal Function in Patients with Tumors - Update on Current Knowledge. Klin Onkol 2017;31:28-34. [PubMed]
- Potočnjak I, Šimić L, Vukelić I, et al. Oleanolic acid attenuates cisplatin-induced nephrotoxicity in mice and chemosensitizes human cervical cancer cells to cisplatin cytotoxicity. Food Chem Toxicol 2019;132:110676. [Crossref] [PubMed]
- Tan Z, Guo F, Huang Z, et al. Pharmacological and genetic inhibition of fatty acid-binding protein 4 alleviated cisplatin-induced acute kidney injury. J Cell Mol Med 2019;23:6260-70. [Crossref] [PubMed]
- He G, Xiao X, Zou M, et al. Pemetrexed/cisplatin as first-line chemotherapy for advanced lung cancer with brain metastases: A case report and literature review. Medicine (Baltimore) 2016;95:e4401. [Crossref] [PubMed]
- Stefani M, Singer RF, Roberts DM. How to adjust drug doses in chronic kidney disease. Aust Prescr 2019;42:163-7. [Crossref] [PubMed]
- Jurczak P, Groves P, Szymanska A, et al. Human cystatin C monomer, dimer, oligomer, and amyloid structures are related to health and disease. FEBS Lett 2016;590:4192-201. [Crossref] [PubMed]
- Dickinson DP, Zhao Y, Thiesse M, et al. Direct mapping of seven genes encoding human type 2 cystatins to a single site located 20p11.2. Genomics 1994;24:172-5. [Crossref] [PubMed]
- Fu L, Liu K, Ferreira RB, et al. Proteome-Wide Analysis of Cysteine S-Sulfenylation Using a Benzothiazine-Based Probe. Curr Protoc Protein Sci 2019;95:e76. [Crossref] [PubMed]
- Perlenfein TJ, Murphy RM. Expression, purification, and characterization of human cystatin C monomers and oligomers. Protein Expr Purif. 2016;117:35-43. [Crossref] [PubMed]
- Kozan R, Şare M, Yılmaz TU, et al. Effectiveness of new parameters in the evaluation of pneumoperitoneum-related acute kidney injury in rats. Turk J Med Sci 2018;48:1278-84. [Crossref] [PubMed]
- Sorkhi H, Behzadi R, Joghtaei N, et al. Glomerular filtration rate determination by creatinine and cystatin-C in patients with acute pyelonephritis. Caspian J Intern Med 2018;9:290-5. [PubMed]
- Mao W, Liu S, Wang K, et al. Cystatin C in Evaluating Renal Function in Ureteral Calculi Hydronephrosis in Adults. Kidney Blood Press Res 2020;45:109-21. [Crossref] [PubMed]
- Dardashti A, Nozohoor S, Algotsson L, et al. The predictive value of s-cystatin C for mortality after coronary artery bypass surgery. J Thorac Cardiovasc Surg 2016;152:139-46. [Crossref] [PubMed]
- Nozawa Y, Sato H, Wakamatsu A, et al. Utility of estimated glomerular filtration rate using cystatin C and its interpretation in patients with rheumatoid arthritis under glucocorticoid therapy. Clin Chim Acta 2018;487:299-305. [Crossref] [PubMed]
- Wilson TG, d'Udekem Y, Winlaw DS, et al. Creatinine-based estimation of glomerular filtration rate in patients with a Fontan circulation. Congenit Heart Dis 2019;14:454-63. [Crossref] [PubMed]
- Kawakami M, Hirata S, Mizuta M, et al. Modified serum creatinine-derived equations with muscle mass-adjusted estimation of renal function and serum cystatin C-derived estimated glomerular filtration rate in elderly individuals. Int J Clin Pharmacol Ther 2019;57:229-39. [Crossref] [PubMed]
- Dohchin A, Suzuki JI, Seki H, et al. Immunostained cathepsins B and L correlate with depth of invasion and different metastatic pathways in early stage gastric carcinoma. Cancer 2000;89:482-7. [Crossref] [PubMed]
- Ma G, Cheng W, Ma M. Effect of Docetaxel Combined with Cisplatin Preoperative Neoadjuvant Chemotherapy for Stage III NSCLC. J Coll Physicians Surg Pak 2019;29:1230-31. [Crossref] [PubMed]
- Yue D, Xu S, Wang Q, et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): a randomised, open-label, phase 2 trial. Lancet Respir Med 2018;6:863-73. [Crossref] [PubMed]
- Scarr D, Bjornstad P, Lovblom LE, et al. Estimating GFR by Serum Creatinine, Cystatin C, and β2-Microglobulin in Older Adults: Results From the Canadian Study of Longevity in Type 1 Diabetes. Kidney Int Rep 2019;4:786-96. [Crossref] [PubMed]
- Torres da Costa E. Assessment of Kidney Function in Patients With Cancer. Adv Chronic Kidney Dis 2018;25:49-56. [Crossref] [PubMed]


