Fatal interstitial lung disease associated with a series of tyrosine kinase inhibitors treatment in a non-small cell lung cancer patient: a case report
Introduction
Tyrosine kinase inhibitors (TKIs) have significantly improved outcomes for non-small cell lung cancer patients (NSCLC) (1). However, notwithstanding the favorable clinical effects, clinical adverse effects (AEs) inevitably appeared. The impacts on safety and degree of toxicity of these promising therapies are not well characterized in patients only with targeted therapy without any chemo- or radiotherapy. We report a NSCLC patient developed into an explosive interstitial pneumonia and died, who was treated a series of TKIs, gefitinib, osimertinib, cabozantinib and anlotinib.
Case presentation
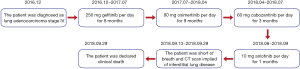
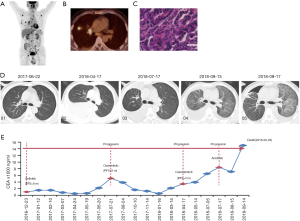
The patient who was in good health was found with elevated CEA (>1,000 ng/mL) in physical examination on December 2016. He had no history of medical illness. The members in the family have no history of malignancy. The timeline picture of the patient was as follows (Figure 1). PET-CT showed abnormal higher FDG metabolism (1.4×1.9 cm, SUVmax =7.0) in the right superior lobe, along with mediastinal and right hilar lymph node metastasis (SUVmax =2.8–7.9), multiple intrapulmonary metastases (SUVmax =1.1–1.2) and with systemic bone metastasis (sternum, bilateral scapula, bilateral multiple ribs, multiple vertebrae and pelvic bones, SUVmax =3.3–7.7) (Figure 2A,B). Lung lesion puncture verified of adenocarcinoma by pathology (Figure 2C). The tumor staging was cTxNxM1 (stage IV) with poor prognosis. EGFR mutation showed of 19 exon mutation. Gefitinib was sensitive for the NSCLC patients with EGFR 19 exon mutation. So, the patients started 250 mg gefitinib per day from December 23, 2016 to July 21, 2017 (progression-free survival, PFS1=8 months). The imaging evaluated of progressive disease (PD) with right hydrothorax (Figure 2D). CEA increased to 5,090 ng/mL (Figure 2E). The patient was detected of T790M mutation and started 80 mg osimertinib per day from July 21, 2017 to April 17, 2018 (PFS2=9 months). CEA gradually decreased to 518 ng/mL. However, CEA rebounded to 3,396 ng/mL with increased pleural effusion and RET rearrangement on April 17, 2018. The patients refused chemotherapy and began 60 mg cabozantinib per day from April 17, 2018 to July 17, 2018 (PFS3=3 months) because of the detection of RET rearrangement. CEA gradually increased to 8,403 ng/mL with increased pleural effusion on July 17, 2018. Thoracocentesis and intrapleural injection (endostar + cisplatin) was administrated repeatedly. In view of uncontrolled hydrothorax, the patient was evaluated of PD and anlotinib was recommended for the third-line or forth-line treatment. So 10 mg anlotinib per day was used from August 10, 2018. All TKI treatments were well tolerated. Side effects included bone marrow suppression, gastrointestinal reactions, liver and kidney dysfunction, etc., are mostly mild and tolerable, and do not require medication. In September 13, 2018, CEA gradually increased to over 15,000 ng/mL. The patient was short of breath and CT scan implied of interstitial lung disease (ILD) (Figure 2D). Oxygen inhalation was given and 500 mg methylprednisolone per day for 3 days and gradually reduced the dosage and 2 g cefepime hydrochloride q12 h was used. However, the patient was progressively aggravated with shortness of breath and dyspnea, and declared clinical death (September 29, 2018).
Discussion
The AEs of TKIs associated with ILD during their clinical trials are EGFR-TKIs (2), such as erlotinib, gefitinib and osimertinib, and non EGFR-TKIs, such as sorafenib (3) and pazopanib (4). Anlotinib-induced ILD in NSCLC patients has not been systematically characterized before, and the factors contributing to anlotinib-induced ILD in NSCLC patients remain unclear, whereas risk factors significantly correlated with the development of ILD in gefitinib have been described and include male sex, a history of smoking, and coincidence of interstitial pneumonia (5). In this case, a compromised performance status, multiple metastasis loci and pleural effusions were observed in the chest and, hence, could be the key factors contributing to the development of ILD. The exact mechanism of TKI-induced ILD is unclear, possibly due to pulmonary toxic damage caused by drug metabolism in the lungs, or the immune response induced by drugs. EGF is an important regulatory peptide to maintain airway epithelial regeneration and repair, inhibition of alveolar macrophages chemotaxis, regulating immune and inflammatory response (6). Thus, it is possible that TKI induced EGFR inhibition will at least partly impair the ability of pneumocytes to respond to lung injury. Shi et al. systematically reviewed 15,618 patients who have showed that relative risk of developing all grades of ILD and fatal interstitial pneumonitis is significantly higher in patients with EGFR-TKI’s upfront (7). However, some investigations implied that ILD is not linked with inhibition of the EGFR pathway. Togashi et al. reviewed and concluded that of the EGFR-signaling pathway blockade by EGFR TKIs is not necessarily associated with the occurrence of ILD and that EGFR-TKI dose reduction plays only a limited role in preventing recurrence (8). Interestingly, the AEs of TKIs known to be associated with ILD during their clinical trials are EGFR-TKIs, such as afatinib, erlotinib, gefitinib, lapatinib, osimertinib, icotinib, rociletinib, and non-EGFR-TKIs, such as pazopanib. In particular, only pazopanib inhibits PDGFR, FGFR, VEGFR and KIT, with no activity at EGFR, just like the targets of anlotinib. Shotaro Ide reported a case of ILD induced by pazopanib treatment (4). But the mechanisms and risk factors of ILD due to VEGFR/PDGFR/KIT-TKI are controversial. Though our current understanding of the role of TKIs in pathogenesis of ILD remains anecdotal, the association of ILD with non-EGFR-TKI has been proven beyond doubt.
In our case, the eye-catching point is that the patient only receives a series of TKIs, without any chemoradiotherapy. Gefitinib and osimertinib both can induce ILD. Though in the clinic course, the patient did not co-exist with ILD, or induced ILD during first and second-line treatment, yet there still might combined the pre-existing ILD status. Anlotinib, as multiple targets inhibitor, might further aggravated lung injury, and caused an uncontrollable and fatal ILD. Previous studies showed that 16% of advanced NSCLC patients with pre-existing ILD developed pneumonitis, and it was found to be a risk factor of ILD later on (9,10). It is therefore necessary to monitor NSCLC patients with TKIs or with pre-existing ILD very closely as they are at high risk of developing fatal ILD. However, this report has the following limitations. Due to the limited sample size with one case, the study only offers some caution in the clinical settings. Thus, multi-institutional or multi-regional studies were further needed.
Conclusions
The present case suggests fatal ILD developed in a NSCLC patient with a series TKIs treatment. Physicians should consider the indications of TKIs for these patients, and carefully observe their respiratory symptoms during TKIs treatment.
Acknowledgments
Funding: This study was supported in part by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.76). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient’s daughter for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhu VW, Klempner SJ, Ou SI. Receptor Tyrosine Kinase Fusions as an Actionable Resistance Mechanism to EGFR TKIs in EGFR-Mutant Non-Small-Cell Lung Cancer. Trends Cancer 2019;5:677-92. [Crossref] [PubMed]
- Shah RR. Tyrosine Kinase Inhibitor-Induced Interstitial Lung Disease: Clinical Features, Diagnostic Challenges, and Therapeutic Dilemmas. Drug Saf 2016;39:1073-91. [Crossref] [PubMed]
- Ide S, Soda H, Hakariya T, et al. Interstitial pneumonia probably associated with sorafenib treatment: An alert of an adverse event. Lung Cancer 2010;67:248-50. [Crossref] [PubMed]
- Ide S, Sakamoto N, Hara S, et al. Interstitial Lung Disease Induced by Pazopanib Treatment. Intern Med 2017;56:79-83. [Crossref] [PubMed]
- Ando M, Okamoto I, Yamamoto N, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol 2006;24:2549-56. [Crossref] [PubMed]
- Aida S, Tamai S, Sekiguchi S, et al. Distribution of epidermal growth factor and epidermal growth factor receptor in human lung: immunohistochemical and immunoelectron-microscopic studies. Respiration 1994;61:161-6. [Crossref] [PubMed]
- Shi L, Tang J, Tong L, et al. Risk of interstitial lung disease with gefitinib and erlotinib in advanced non-small cell lung cancer: a systematic review and meta-analysis of clinical trials. Lung Cancer 2014;83:231-9. [Crossref] [PubMed]
- Togashi Y, Masago K, Hamatani Y, et al. Successful erlotinib rechallenge for leptomeningeal metastases of lung adenocarcinoma after erlotinib-induced interstitial lung disease: a case report and review of the literature. Lung Cancer 2012;77:464-8. [Crossref] [PubMed]
- Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med 2008;177:1348-57. [Crossref] [PubMed]
- Sakurada T, Kakiuchi S, Tajima S, et al. Characteristics of and risk factors for interstitial lung disease induced by chemotherapy for lung cancer. Ann Pharmacother 2015;49:398-404. [Crossref] [PubMed]