
Evaluation for clinical and prognostic implications of glypican-3 and α-fetoprotein in hepatocellular carcinoma: a new subtype?
Introduction
Globally, liver cancer ranked sixth for cancer incidence and fourth for cancer deaths in 2015 (1). Most primary liver cancers (70–90%) occurring worldwide are hepatocellular carcinoma (HCC) (2). Hepatitis B virus (HBV) and hepatitis C virus (HCV) account for an estimated 32% of infection-related cancer cases, mostly liver cancer, in less-developed countries and 19% in more developed countries (3). Until now, different studies have identified on the molecular level that the expressions of some proteins, such as CD133, OV6, CD44, CD47, CK19, EpCAM, and especially α-fetoprotein (AFP) and glypican-3 (GPC3), are closely associated with the recurrence and survival of HCC patients (4-8). Although great progress has been made in HCC treatment, including curative surgery and nonsurgical treatment, the HCC prognosis remains poor (9). This may be due to the subtype classification based on only one protein, which may be not sufficient to clarify the diagnosis and prognosis of malignant HCC. Consequently, a more precise subtype classification is required to assess HCC patients.
To this goal, several clinical studies have been carried out. For instance, Yamashita et al. (10) defined a new subtype based on the expression of EpCAM and AFP. They found that the EpCAM+AFP+ group showed the poorest prognosis, while the EpCAM–AFP– group had a more favorable outcome. Another study based on the immunohistochemical staining for CK19 and GPC3 proved that CK19+/GPC3+ HCC has the shortest recurrence time compared with other HCC immunophenotypes, such as CK19−/GPC3+ and CK19–/GPC3– (11). However, to the best of our knowledge, HCC classification based on serum AFP levels and GPC3 expression for assessing the prognosis in HCC patients has not been reported to date.
In the present study, we classified an HCC group based on serum AFP levels and GPC3 expression to study the difference between their clinical pathological characteristics and prognosis.
Methods
Study design
Our research was approved by the Ethical Committee of Tongji Hospital, Huazhong University of Science and Technology (Number: S810), and more than 3,000 HCC patients had been subjected to hepatectomy at Tongji Hospital between January 2014 and November 2016.
We carefully screened over the 3,000 medical records. The following entry criteria were applied: (I) all patients were diagnosed for the first time with HCC and had not received any anticancer therapy before the hepatectomy; (II) liver function was classified as Child-Pugh grade A; (III) the surgical resection margin was tumor negative; (IV) all patients were checked for their serum AFP levels and GPC3 expression; (V) patients with other malignancies, serious organ failures, and immunodeficiency diseases were excluded. Finally, only 229 of them were recruited into our study.
The HCC patients’ clinicopathological features—lesion, tumor size, histological grade, vascular invasion, regional lymph node involvement, distant metastasis, and clinical stage—were collected by two independent pathologists. Edmondson-Steiner grade was used for subdividing the groups of differentiation: G3 (poorly differentiated) and G4 (undifferentiated) were both classified into high grade group. Clinical stage was evaluated by the sixth edition tumor-node-metastasis (TNM) system (UICC/AJCC, 2010).
Serum AFP levels were evaluated by an electrochemiluminescence immunoassay (E170 Analytics; Roche Diagnostics, Indianapolis, IN, USA). And we used a well-defined serum AFP cutoff (400 ng/mL) to classify HCC into AFP-positive and AFP-negative HCC, as described previously (12).
The immunohistochemical procedures for GPC3, including antigen recovery, antibody incubation, and antibody detection, were performed by College of American Pathologist (CAP) and Clinical Laboratory Improvement Act (CLIA) accredited certified laboratory. We used anti-GPC3 mouse monoclonal primary antibody (clone GC33, Ventana Medical Systems, Inc. Tucson, AZ, USA) on BenchMark ULTRA to do tissue section staining to detect membrane and cytoplasmic expression. Antigen retrieval followed a primary antibody incubation for 32 minutes at 36 degrees Celsius. Immunoassays were performed with the UltraView Universal DAB Assay Kit. Additionally, each stain contained the appropriate positive and negative controls.
Immunohistochemical analysis
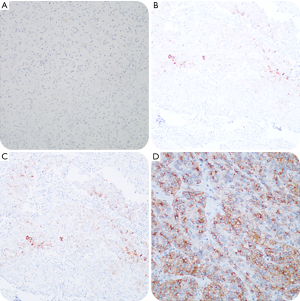
To accurately evaluate the expression of tissue protein GPC3, we adapted the comprehensive scoring scale proposed by Takai et al. (13). The scoring system is composed of three elements, including positive cell rate, staining intensity, and staining pattern. The positive cell rate is graded on a scale of 0 to 3: 0 (<5%), 1+ (5–10%), 2+ (10–50%), and 3+ (>50%). Staining scores of 2 and 3 were defined as positive staining, whereas 0 and 1 were defined as negative staining. All pathological sections were reviewed by two independent pathologists according to the guidelines of the World Health Organization (WHO) criteria, 2010 (14). Groups with cytoplasmic/membranous staining for GPC3 are shown in Figure 1.
Follow-up
This study used a telephone follow-up. After surgery, we contacted the patients every 3 months in the first year, every 4 months in the second year, and every 6 months in the third year. The follow-up schedule ended on September the 1st, 2017, unless a patient died earlier. Patients who died of other causes were excluded.
Statistical analysis
All analyses were performed using the SPSS 19.0 software (IBM, Armonk, NY, USA). Continuous data are expressed as median (range), distributions of categorical variables were studied by χ2 tests, and groups were compared by Kruskal-Wallis one-way ANOVA. Overall survival (OS) was defined as the time between surgery and death or September the 1st, 2017 (the last date of follow-up). Survival was estimated by the Kaplan-Meier method, and differences between the curves were evaluated with a stratified log-rank test. Multivariable analyses performed with the Cox proportional hazards model were used to estimate factors associated with the HCC survival time. Two-tailed P values of P<0.05 were considered statistically significant.
Results
General patient characteristics and clinicopathological features
In this study, 229 eligible HCC patients were included. The general patient characteristics are summarized in Table 1 and Table 2.
Table 1
| Variable | Unit | Value |
|---|---|---|
| Age | Years | 50 [16–77] |
| ALT | U/L | 30 [7–215] |
| AST | U/L | 30 [9–297] |
| Total bilirubin | mmol/L | 6.5 (1.2–36.5) |
| Albumin | g/L | 39.3 (27.4–50.8) |
| AFP | ng/mL | 180.00 (1.03–80,000.00) |
| Follow-up | months | 16.7 (0.3–48.4) |
Data are expressed as median value (range). HCC, hepatocellular carcinoma; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AFP, α-fetoprotein.
Table 2
| HCC type (n) | AFP | GPC3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AFP+ (n=108) | AFP− (n=121) | χ2 | P | GPC3+ (n=196) | GPC3− (n=33) | χ2 | P | ||
| Cohort | 3.008 | 0.087 | 0.835 | 0.451 | |||||
| ≥50 years | 51 | 71 | 102 | 20 | |||||
| <50 years | 57 | 50 | 94 | 13 | |||||
| Gender | 7.226 | 0.010* | 5.812 | 0.021* | |||||
| Male | 87 | 112 | 166 | 33 | |||||
| Female | 21 | 9 | 30 | 0 | |||||
| Lesion | 0.529 | 0.557 | 0.263 | 0.680 | |||||
| Solitary | 75 | 90 | 140 | 25 | |||||
| Multiple | 33 | 31 | 56 | 8 | |||||
| Cirrhosis | 0.073 | 0.879 | 3.955 | 0.059 | |||||
| No | 26 | 90 | 94 | 22 | |||||
| Yes | 82 | 31 | 102 | 11 | |||||
| Tumor size | 0.070 | 0.892 | 1.189 | 0.336 | |||||
| ≥5 cm | 67 | 73 | 117 | 23 | |||||
| <5 cm | 41 | 48 | 79 | 10 | |||||
| Histological grade | 10.164 | 0.002* | 0.232 | 0.708 | |||||
| Well and moderate | 46 | 77 | 104 | 19 | |||||
| High | 62 | 44 | 92 | 14 | |||||
| Vascular invasion | 1.252 | 0.327 | 0.010 | 0.921 | |||||
| No | 91 | 108 | 171 | 28 | |||||
| Yes | 17 | 13 | 25 | 5 | |||||
| Regional lymph node involvement | 0.706 | 0.627 | 1.760 | ||||||
| No | 107 | 118 | 194 | 31 | |||||
| Yes | 1 | 3 | 2 | 2 | |||||
| Distant metastasis | 3.524 | 0.088 | 0.000 | 1.000 | |||||
| No | 101 | 119 | 188 | 32 | |||||
| Yes | 7 | 2 | 8 | 1 | |||||
*, P<0.05. HCC, hepatocellular carcinoma; AFP, α-fetoprotein; GPC3, glypican-3.
We found that the AFP-positive group (AFP ≥400 ng/mL), which contained more females than males (P=0.01), showed a lower histological grade (P=0.002). However, no difference was present in the patients’ age (≥50 or <50 years), tumor number (solitary or multiple), cirrhosis (yes or no), tumor size (≥5 or <5 cm), regional lymph node involvement (yes or no), distant metastasis (yes or no) (Table 2).
In addition, as shown in Table 2, GPC3-positive HCC patients, as identified by immunohistochemistry, exhibited a larger female proportion, whereas no statistical difference was found in other characteristics or clinicopathological features (Table 2).
In contrast to the general data and clinicopathological features, statistically significant differences (P<0.05) among the four groups were only found in their histological grade, such as the sex ratio (P<0.05) and the incidence of cirrhosis (P<0.05) (Table 3). The AFP+/GPC3+ group contained more females (9.2%) than the AFP–/GPC3– group (0%; P=0.031) (Table 3). In addition, AFP+/GPC3+ HCC patients had a higher incidence of cirrhosis (33.6%) than the AFP–/GPC3+ (10.9%) and AFP–/GPC3– groups (2.6%). AFP+/GPC3+ group exhibited a relatively higher percentages of poorly differentiation (25.3%) than AFP–GPC3+ HCC patients (14.8%) (Table 3).
Table 3
| HCC type (n) | AFP+/GPC3+ (n=101) | AFP+/GPC3− (n=7) | AFP−/GPC3+ (n=95) | AFP−/GPC3− (n=26) | χ2 | P |
|---|---|---|---|---|---|---|
| Cohort | 4.695 | 0.193 | ||||
| ≥50 years | 46 | 5 | 56 | 15 | ||
| <50 years | 55 | 2 | 39 | 11 | ||
| Gender | 10.612 | 0.009* | ||||
| Male | 80 | 7 | 86 | 26 | ||
| Female | 21 | 0 | 9 | 0 | ||
| Lesion | 0.811 | 0.866 | ||||
| Solitary | 70 | 5 | 70 | 20 | ||
| Multiple | 31 | 2 | 25 | 6 | ||
| Cirrhosis | 59.704 | 0.000* | ||||
| No | 24 | 2 | 70 | 20 | ||
| Yes | 77 | 5 | 25 | 6 | ||
| Tumor size | 1.370 | 0.726 | ||||
| ≥5 cm | 62 | 5 | 55 | 18 | ||
| <5 cm | 39 | 2 | 40 | 8 | ||
| Histological grade | 10.237 | 0.015* | ||||
| Well and moderate | 43 | 3 | 61 | 16 | ||
| High | 58 | 4 | 34 | 10 | ||
| Vascular invasion | 2.266 | 0.497 | ||||
| No | 85 | 6 | 86 | 22 | ||
| Yes | 16 | 1 | 9 | 4 | ||
| Regional lymph node involvement | 4.898 | 4.898 | ||||
| No | 100 | 7 | 94 | 24 | ||
| Yes | 1 | 0 | 1 | 2 | ||
| Distant metastasis | 4.502 | 0.176 | ||||
| No | 94 | 7 | 94 | 25 | ||
| Yes | 7 | 0 | 1 | 1 | ||
*, P<0.05. There was a significant difference between AFP+/GPC3+ and AFP−GPC3− group in the analysis of gender comparison (P=0.031). For histological grade, there was a statistically significant difference between AFP+/GPC3+ and AFP−/GPC3+ group (P=0.015). Cirrhosis: AFP+/GPC3+ compared to AFP−/GPC3+ group (P=0.001), AFP+/GPC3+ compared to AFP−/GPC3− group (P=0.001). AFP, α-fetoprotein; GPC3, glypican-3.
Prognosis of HCC patients
Among all 229 HCC patients, there were 4 (1.7%) patients died for other reasons were excluded: suicide or encountered a fortuitous accident. Fifty-two (22.7%) of the patients rejected the phone call for various reasons or just lost contact (the censored patients). Eventually, all the followed-up HCC patients included: 98 (42.8%) patients in AFP+/GPC3+ group, 7 (3.1%) patients in AFP+/GPC3− group, 94 (41.0%) patients in AFP–/GPC3+ group, 26 (11.4%) patients in AFP–/GPC3− group.
Prognosis of HCC subtypes defined by GPC3 or AFP
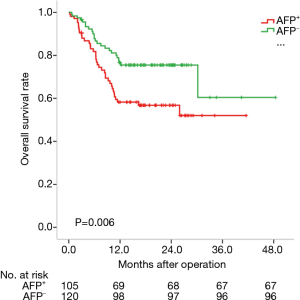
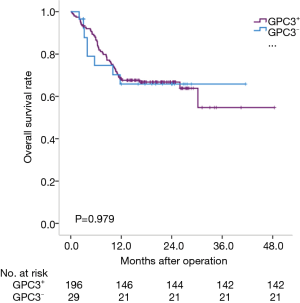
We analyzed the prognosis of HCC subtypes defined by GPC3 or AFP by the Kaplan-Meier method. The average survival times of these four subtypes were: 25.7 months for AFP-positive HCC (95% CI: 21.8–29.5 months); 35.5 months for AFP-negative HCC (95% CI: 29.6–41.3 months); 32.3 months for GPC3-positive HCC (95% CI: 28.0–36.6 months); and 29.2 months for GPC3-negative HCC (95% CI: 22.2–26.2 months). Actually, AFP-negative HCC patients lived longer than AFP-positive HCC patients (P=0.006) (Figure 2). However, there was no statistical difference among the GPC subtypes (P=0.979) (Figure 3).
Prognosis of HCC subtypes defined by GPC3 and AFP
For the four groups, the following average survival times were determined by the Kaplan-Meier method: 21.4 months for AFP+GPC3+ (95% CI: 18.4–24.5 months); 33.8 months for AFP+GPC3– (95% CI: 20.3–47.3 months); 36.5 months for AFP–GPC3+ (95% CI: 30.4–42.6 months); and 21.2 months for AFP–GPC3– (95% CI: 16.6–25.8 months). Only the AFP+GPC3+ and AFP–GPC3+ groups were statistically different (P=0.002) (Figure 4).

Multivariable Cox proportional hazards regression analysis of risk factors associated with death
According to the analysis above, several factors were entered in the multivariate analysis as shown in Table 4. Only age ≥50 years [hazard ratio (HR) =1.987, 95% confidence interval (CI): 1.096–3.262, P=0.011], was independent factors associated with the death to HCC patients.
Table 4
| Characteristic | HR | 95.0% CI | P value |
|---|---|---|---|
| AFP positive | 0.476 | 0.056–4.031 | 0.496 |
| GPC3 positive | 0.520 | 0.207–1.311 | 0.166 |
| AFP+ and GPC3+vs. AFP– and GPC3– | 1.561 | 0.673–3.622 | 0.299 |
| Gender: male | 0.862 | 0.408–1.821 | 0.698 |
| Age ≥50 years | 1.987 | 1.096–3.262 | 0.011* |
| Cirrhosis | 1.174 | 0.613–2.249 | 0.629 |
*, P<0.05. We analyzed all the single variables, and then made a comprehensive analysis of meaningful variables. AFP, α-fetoprotein; GPC3, glypican-3; HR, hazard ratio; CI, confidence interval.
Discussion
HCC is a highly heterogeneous tumor. It has been found that the expression of some proteins, including AFP and GPC3, is closely associated with the recurrence and survival of HCC patients (4-8). However, AFP is not thought to be the most optimal diagnostic marker for HCC, as only a small proportion of HCC patients presents with an elevated serum AFP (15). Therefore, better markers for diagnosis as well as prognosis have been investigated (16-19).
Recently, Zhang et al. detected GPC3 in the liver of fetuses at 18–30 weeks of gestational age, but could not identify GPC3 in any normal adult hepatic tissue (16), in contrast to AFP. These findings attracted our interest, and we conducted a meta-analysis, which indicated that HCC patients had a higher serum GPC3 level when compared with healthy individuals, but whether GPC3 is an optimal diagnostic marker for HCC and liver cirrhosis remained uncertain (17). Furthermore, Li et al. (18) realized that GPC3 might be a serum marker for HCC and could be used to distinguish AFP-negative HCC from cirrhotic nodules. Another study indicated that high GPC3 immunohistochemical expression is associated with poor OS in HCC patients (19). Consequently, we followed the approach to combine GPC3 with AFP to define a new subtype for the analysis of HCC.
Our previous study has proven that an AFP cut-off value of 400 ng/mL was a more optimal level for estimating the relationship between AFP levels and survival than a value of 300 ng/mL (12), as recommended in literature (20).
In our current study, we found some differences in the general characteristics and clinicopathological features between the subgroups. We only found a correlation between the immunohistochemical expression of GPC3 and the sex ratio (P=0.021), while the serum AFP levels was related to the histological grade of HCC, for the reason that there was a statistically significant difference between AFP+/GPC3+ and AFP−/GPC3+ group. Also, the serum AFP levels and the four subtypes exhibited additional differences in their clinicopathological features besides the gender differences. However, Yu et al. (21) found that, in HCC, GPC3 was highly expressed and was positively correlated with tumor size and liver cancer pathological grading, Unfortunately, that is in conflict with our conclusion. As for the discrepancy in conclusion, we can only consider that it may be due to the lack of awareness of physical examination and the inability to diagnose diseases early in Hubei, China, Furthermore, our study showed that in the diagnosis of HCC, the four subtypes were better markers than AFP levels and GPC3 expression in HCC patients who had cirrhosis. We found that different subtypes were associated with differences in the cirrhosis incidence, which was not correlated with AFP levels and GPC3 expression, and the result is consistent with other research (22). In combination with a previous study, which indicated that simultaneous testing of serum GPC3 and AFP could improve the diagnostic accuracy and sensitivity for early HCC (23,24), we conclude that the newly defined subtype, to some extent, could be an optimal marker for the diagnosis of HCC.
In fact, our study was the first research to analyze the prognosis of HCC patients classified by serum AFP levels and GPC3 immunohistochemical expression. At the beginning, we analyzed prognostic data based on the subtypes defined by GPC3 or AFP and found that AFP-negative HCC patients lived longer than the AFP-positive group (P=0.001). The relationship between AFP and the prognosis of HCC patients was consistent with our previous studies (12). However, we observed no statistical difference between the GPC subtypes. Other studies reported two conflicting results. On the one hand, some researchers claimed that positive GPC3 expression contributes to a poorer OS of HCC patients compared to negative GPC3 expression (25,26). On the other hand, Kaseb and Jeon independently supported that the immunohistochemical expression level of GPC3 is not correlated with the OS of HCC patients (27,28), which is consistent with our study.
Then, after we concluded prognostic data based on the subtypes defined by GPC3 and AFP, we found that AFP–GPC3+ group showed the longest survival time, followed by AFP+GPC3– group and group AFP+GPC3+, while group AFP–GPC3– had the poorest prognosis. Unfortunately, Only the AFP+GPC3+ and AFP–GPC3+ groups were statistically different (P=0.001). The result clearly showed that AFP+GPC+ was a relatively qualified subtype and could lead to a worse prognosis.
After the analysis the results of multivariable Cox proportional hazards regression and the survival data, it was apparently that GPC3 immunohistochemical expression cannot affect the prognosis of HCC patients alone. However, interestingly, when we found that there was such a markedly distinction in survival between AFP positive group and AFP negative group, the distinction disappeared when the factor GPC3− was added into the equation. Moreover, the average survival time for AFP−GPC3− group was very similar to group AFP+GPC+. What’s more, most of the published prognostic studies suggest that both AFP− and GPC3− lead to shorter survival in patients with HCC, whereas our study did the opposite. We can assume that AFP−GPC3− subtype could be a special one, and AFP−GPC3− leaded to a relatively poorer prognosis.
In conclusion, even though our study had some limitations. The small sample size, the single-center design, as well as the inaccurate follow-up method and the relatively low follow-up rate affected the reliability of our results to a certain extent. We still defined a new classification system for subclassifying HCC based on AFP and GPC3 into four different prognosis subtypes. And they could be meaningful indicators that are instructive for prognosis to a certain extent. Meanwhile, the new HCC subtype could guide the personalized therapy of HCC patients.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr-19-1803). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our research was approved by the Ethical Committee of Tongji Hospital, Huazhong University of Science and Technology (No. S810).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012;13:607-15. [Crossref] [PubMed]
- Yeh CT, Kuo CJ, Lai MW, et al. CD133-positive hepatocellular carcinoma in an area endemic for hepatitis B virus infection. BMC Cancer 2009;9:324. [Crossref] [PubMed]
- Lo RC, Ng IO. Hepatic progenitor cells: their role and functional significance in the new classification of primary liver cancers. Liver Cancer 2013;2:84-92. [Crossref] [PubMed]
- Yang XR, Xu Y, Yu B, et al. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut 2010;59:953-62. [Crossref] [PubMed]
- Liu H, Li P, Zhai Y, et al. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World J Gastroenterol 2010;16:4410-5. [Crossref] [PubMed]
- A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998;28:751-5. [Crossref] [PubMed]
- Zhu RX, Seto WK, Lai CL, et al. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver 2016;10:332-9. [Crossref] [PubMed]
- Yamashita T, Forgues M, Wang W, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res 2008;68:1451-61. [Crossref] [PubMed]
- Feng J, Zhu R, Chang C, et al. CK19 and Glypican 3 Expression Profiling in the Prognostic Indication for Patients with HCC after Surgical Resection. PLoS One 2016;11:e0151501. [Crossref] [PubMed]
- Yang SL, Liu LP, Yang S, et al. Preoperative serum alpha-fetoprotein and prognosis after hepatectomy for hepatocellular carcinoma. Br J Surg 2016;103:716-24. [Crossref] [PubMed]
- Takai H, Kato A, Ishiguro T, et al. Optimization of tissue processing for immunohistochemistry for the detection of human glypican-3. Acta Histochem 2010;112:240-50. [Crossref] [PubMed]
- Bosman FT, World Health Organization, International Agency for Research on Cancer. WHO classification of tumours of the digestive system. 4th ed. World Health Organization classification of tumours, vol 3. Lyon: International Agency for Research on Cancer, 2010.
- Zhang Z, Zhang Y, Wang Y, et al. Alpha-fetoprotein-L3 and Golgi protein 73 may serve as candidate biomarkers for diagnosing alpha-fetoprotein-negative hepatocellular carcinoma. Onco Targets Ther 2015;9:123-9. [PubMed]
- Zhang L, Liu H, Sun L, et al. Glypican-3 as a potential differential diagnosis marker for hepatocellular carcinoma: a tissue microarray-based study. Acta Histochem 2012;114:547-52. [Crossref] [PubMed]
- Yang SL, Fang X, Huang ZZ, et al. Can serum glypican-3 be a biomarker for effective diagnosis of hepatocellular carcinoma? A meta-analysis of the literature. Dis Markers 2014;2014:127831.
- Li B, Liu H, Shang HW, et al. Diagnostic value of glypican-3 in alpha fetoprotein negative hepatocellular carcinoma patients. Afr Health Sci 2013;13:703-9. [PubMed]
- Shirakawa H, Suzuki H, Shimomura M, et al. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci 2009;100:1403-7. [Crossref] [PubMed]
- Lee JS, Chu IS, Heo J, et al. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology 2004;40:667-76. [Crossref] [PubMed]
- Yu JP, Xu XG, Ma RJ, et al. Development of a Clinical Chemiluminescent Immunoassay for Serum GPC3 and Simultaneous Measurements Alone With AFP and CK19 in Diagnosis of Hepatocellular Carcinoma. J Clin Lab Anal 2015;29:85-93. [Crossref] [PubMed]
- Xu D, Su C, Sun L, et al. Performance of Serum Glypican 3 in Diagnosis of Hepatocellular Carcinoma: A meta-analysis. Ann Hepatol 2019;18:58-67. [Crossref] [PubMed]
- Tangkijvanich P, Chanmee T, Komtong S, et al. Diagnostic role of serum glypican-3 in differentiating hepatocellular carcinoma from non-malignant chronic liver disease and other liver cancers. J Gastroenterol Hepatol 2010;25:129-37. [Crossref] [PubMed]
- Ozkan H, Erdal H, Kocak E, et al. Diagnostic and prognostic role of serum glypican 3 in patients with hepatocellular carcinoma. J Clin Lab Anal 2011;25:350-3. [Crossref] [PubMed]
- Fu SJ, Qi CY, Xiao WK, et al. Glypican-3 is a potential prognostic biomarker for hepatocellular carcinoma after curative resection. Surgery 2013;154:536-44. [Crossref] [PubMed]
- Wang L, Pan L, Yao M, et al. Expression of oncofetal antigen glypican-3 associates significantly with poor prognosis in HBV-related hepatocellular carcinoma. Oncotarget 2016;7:42150-8. [Crossref] [PubMed]
- Kaseb AO, Hassan M, Lacin S, et al. Evaluating clinical and prognostic implications of Glypican-3 in hepatocellular carcinoma. Oncotarget 2016;7:69916-26. [Crossref] [PubMed]
- Jeon Y, Kim H, Jang ES, et al. Expression profile and prognostic value of glypican-3 in post-operative South Korean hepatocellular carcinoma patients. APMIS 2016;124:208-15. [Crossref] [PubMed]


