
Establishment of a stable hepatic metastasis mouse model of murine colorectal cancer by microsurgical orthotopic implantation
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors today. The incidence and fatality rates of CRC have increased rapidly over recent years, likely attributable to dietary changes in both eastern and western countries (1,2). The most common cause of death from CRC is distant metastasis and invasion into other organs, such as liver, lung, bone and brain. Among distant metastatic sites, the liver is the most frequently affected organ, making hepatic metastasis one of the most common causes of death attributable to primary CRC (3,4). Some clinical reports have shown that, according to postmortem examination results, about 60% to 70% of patients in the late stage of CRC develop liver metastasis. Their median survival term is approximately 5 to 6 months, and five years survival rate drops to zero if these patients do not receive medical treatment (5). If, however, liver metastasis can be inhibited, the prognosis of CRC would be more favorable than it is currently. It is necessary to establish a liver metastasis of colon cancer mouse model so that researchers are able to illuminate the disease progression and to develop effective therapies and drugs.
Several techniques for building a metastatic CRC mouse model have been described in recent literature. Methods investigated have included orthotopic cell injection, spontaneous metastasis model, intraabdominal spleen transplantation (spleen removal group and spleen reservation group), rectum transplantation, portal injection and tail vein injection. However, spontaneous metastasis models have yielded uneven results. Spleen transplantation and tail vein injection to establish metastatic CRC mouse model were first selected by our team because of the ease of operation; however, liver metastasis appeared too early among established mouse models to be suitable for drug research. Orthotopic cell injection is an effective technique for developing modified mouse models of CRC metastasis. However, the actual volume of cells injected is not easy controlled since the mesenteric interspace is small, usually resulting in local tumor volume and dissemination sites being too widely variable. Shortcomings of other techniques include not producing metastasis at all or producing only singular metastatic foci (6,7).
Surgical orthotopic implantation (SOI) is an efficient technique to establish a viable metastatic animal model. A number of studies reported that metastatic xenograft mouse models were established with orthotopic patient-derived tissue, such as melanoma, breast cancer or hepatoma (8-10). Building on the success in principle of SOI, we achieved modest improvement using microscopic surgical techniques [microsurgical orthotopic implantation (MSOI)] to develop a liver metastasis of orthotopic CRC mouse model with mouse-derived tissue consisting of transformed CT-26 cells.
Methods
Materials and reagents
Cell culture medium Dulbecco’s modified Eagle medium (DMEM) and fetal bovine serum (FBS) were both purchased from Invitrogen (Thermo Fisher Scientific, MA, USA). Penicillin 100 Units/mL streptomycin 100 µg/mL and 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) were also purchased from Invitrogen.
Cell culture
Murine colon carcinoma CT-26 cells were obtained from Shanghai cell bank of Chinese Academy of Sciences (Shanghai, China) and maintained in DMEM containing 10% (v/v) FBS in 5% CO2 at 37 °C. The cells were subcultured at 80% to 90% confluency.
Animals
BALB/c mice (with an initial body weight of 20–22 g) were obtained from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and housed under pathogen-free conditions with controlled temperature (22 °C) and humidity and a 12-hour light/dark cycle. Food and water were provided ad libitum throughout the experiment. All animal treatments were performed strictly in accordance with international ethical guidelines and the National Institutes of Health Guide concerning the Care and Use of Laboratory Animals. The experiments were approved by the Institutional Animal Care and Use Committee of Fujian University of Traditional Chinese Medicine.
Subcutaneous tumor implantation in nude mice
Mice were subcutaneously injected in the right flank with 2×107 CT-26 cell suspension in DMEM. After 16 days of xenograft implantation, tumors were implanted to different nude mice by 20-gauge inoculating needle at an average size of 1 cm3. Tumor growth among four generations would be suitable for MSOI into the cecum surface.
Metastasis of CRC mouse model with MSOI (11)
Subcutaneous tumors were excised, and then centrally located tumor tissue was cut into pieces (volume about 1 mm3) and placed in normal saline for later use. Mice were anesthetized by intraperitoneal injection of 1% pentobarbital sodium (Abbott Laboratories, 70 mg/kg weight) and fixed in the prone position on the operating table. After the hair was shaved with an electric razor, a tiny incision was made in the skin with surgical scissors. The blind end of the cecum was elevated out with forceps and placed on a sterile gauze alongside the incision. The operating table was moved under the microscope, and microsurgical techniques were used. With stretching and leveling of the blind end of the cecum using smooth forceps, a small rent was made in the serous membrane to create a double-walled pouch in the wall of the cecum. A piece of tumor tissue derived from CT-26 was sutured into the pouch with a single Maxon 11-0 suture (Figure S1). After tumor implantation, the abdominal wall was closed in two layers with Dexon 5-0. Food and water were given ad libitum. Following recovery from cecal implantation of the colon tumor, the mice were randomly assigned between model and control groups (n=18 per group). At 7, 14, 21 and 35 d from tumor implantation, the animals were sacrificed as follows: three mice on the 7th and 14th days, six mice on the 21st and 35th days. Subsequently, the tumor tissue was removed and weighed. Then we recorded all macroscopic tumor deposits or abnormalities in the liver, and the number of liver metastases was calculated. All neoplasms were identified with hematoxylin and eosin (HE) staining.
HE staining
Tissues were fixed with 10% buffered formalin for 24 h. Samples were then paraffin-embedded, sectioned and stained with HE. Histopathological changes were observed under a light microscope.
Statistical analysis
All data are the means of six mice, analyzed using SPSS Package for Windows (Version 18). Statistical data analysis was performed using Student’s t-test. Differences where P<0.05 were considered statistically significant.
Results
BALB/c mice growth after transplanted CT-26 tumor tissue by MSOI
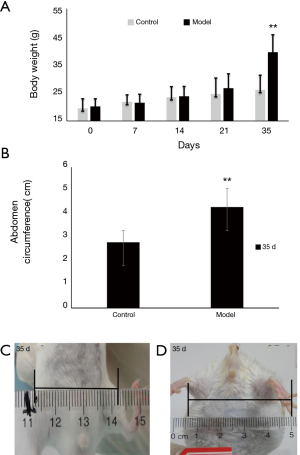
The BALB/c mice showed normal activity in the first 21 d after MSOI of murine CT-26 colon carcinoma tissue. Gradually over the ensuing 14 days, enlarged abdomen, slow-going, weight gain, food intake decrease and hair drying. There was no difference in body weight between control and model groups in the first 21 days. After 28 days the abdomen began to enlarge and body weight rapidly increased, reaching 39.5±6.2 g on the 35th day. Compared with control group, the body weight of model group increased 14.9 g (Figure 1A,B, P<0.01). The abdomen circumference was about 4.3±0.8 cm in model group but only 2.8±0.5 cm in control group on the 35th day (Figure 1C,D, P<0.01). The volume of ascites of the model group mice was about 3.9±0.7 mL.
Local tumor growth and take rates on the 21st day and 35th day
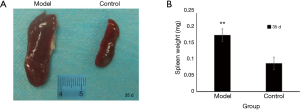
Implanted tissue began to form solid tumor on the 7th day with the volume of tumor about 2 mm × 1 mm. On the 14th day, local tumor volume had doubled. After 14 days, local tumor began increasing in volume more rapidly. On the 21st and 35th day, the local tumor weights were 0.5±0.2 and 1.1±0.4 g respectively (Figure 2, P<0.01). The murine colon carcinoma cell line CT-26 showed high take rates. Among model group mice, five out of six mice generated local tumor (83.3%) on the 21st day, and all six mice (100%) were observed to have local tumor growth on the 35th day (Table 1). The spleen volume of the model group was twice that of the control group, and the weight of spleen in the model group had increased 0.09 g more than the control group (Figure 3, P<0.01).
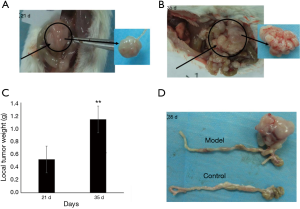
Table 1
| Days | Local tumor take rate | Liver | Stomach | Abdominal wall | Retroperitoneal lymph node |
|---|---|---|---|---|---|
| 21 d | 6/6 | 5/6 | 0 | 0 | 0 |
| 35 d | 6/6 | 6/6 | 3/6 | 6/6 | 6/6 |
†, 6 mice on 21 and 35 d differently for observed.
Tumor dissemination sites on the 21st day and 35th day
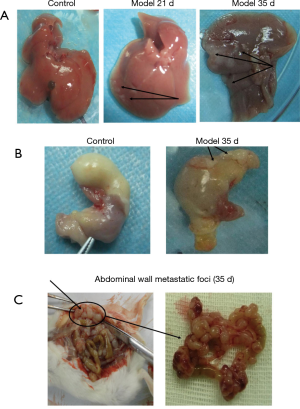
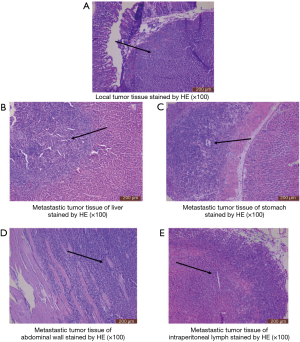
After 21 days, liver metastatic foci were observed, but there was no ascites. Some nodules appeared in liver lobe locations but no in hepatic lobules. The size of metastatic nodules ranged from needle-tip to 2 mm × 1 mm, and they were scattered over the surface of the large liver lobe and hepatic lobules. Liver color and shape remained normal (Figure 4A). CT-26-generated metastatic tumor in liver was present in about five out of six mice (83%) on the 21st day (Table 1). After 28 days, model mice begin to produce ascites with subsequent gradual tumor dissemination to other organs. On the 35th day there were multiple organ metastases, including to liver, stomach and abdominal wall. Liver lobe and hepatic lobules of mice that generated hepatic metastasis were covered with tumor nodules of different sizes, the largest one about peanut-sized or larger (5 mm × 5 mm), the smallest nodules about rice-sized in a diffuse distribution, with almost no normal liver tissue remaining. Liver color and shape were abnormal compared control group (Figure 4A). CT-26-generated metastatic tumor in liver was about six out of six (100%) on the 35th day (Table 1). Liver function values (AST and ALT, Table S1) of the model group on the 21st day and the 35th day were all normal compared with control group. On the 35th day the metastatic sites included retroperitoneal lymph node, abdominal wall and stomach. Metastatic rates were six out of six (100%), three out of six (50%) and six out of six (100%), respectively (Figure 4B,C, Table 1).
Local and metastatic tumor pathologic analysis
Pathological changes of local tumor and tumor dissemination foci, such as liver, stomach and abdominal wall, were analyze by immunohistochemical staining. The results showed that local tumors invaded the normal cecum in both tangential and transverse directions to yield tumors. Liver tumor foci were also detected because there are so many sites for blood-borne metastases in human CRC. The results showed liver foci with tumor cells within and around blood vessels invading liver parenchyma. Stomach tissue and abdominal wall tissue assayed showed that tumor cells invade the mucous layer and placenta percreta (Figure 5).
Discussion
With cancer morbidity increasing rapidly over the last 10 years, mice models of many kinds of cancer, including CRC, have been established. For the purpose of illuminating cancer progression and metastasis, cancer mice model research has turned from subcutaneous transplantation to in situ (12). A stable metastatic mouse model is essential to developing anti-cancer drugs and studying tumor progression mechanisms. This study established a stable hepatic metastasis of CRC mouse model with MSOI that can observe body conditions, tumor growth and metastasis at different time intervals. The method of sewing murine CRC tissue into the mucosa of the cecal wall of BALB/c mice generates models with dissemination patterns that closely replicate relevant metastatic sites observed in humans (13). However, the preliminary experiment results showed tumor tissue from CT-26 cell subcutaneous transplantation nude mice can produce CRC along with metastatic nodes on different organ sites only within six generations. When CT-26 cell line was transplanted to nude mice in excess of seven generations, tumor tissue would not develop orthotopic colorectal tumor. Until now the reason is not clear but probably due to CT-26 cells growth and invasion ability reduction too low to produce orthotopic colorectal tumor with subcutaneous transplantation generations accumulation.
CT-26 cell local tumor growth showed relatively high take rates, showing 100% take on the 21st 35th days. Other researchers have reported local tumor take rates of human CRC cell lines (HCT-116, SW-620, and DLD-1) in the 75% to 88% range with orthotopic microinjection transplantation. According to the results of HE staining, the local tumor pathological characteristics are very similar to those in human disease. For this reason, our orthotopic colorectal tumor mouse model of CRC in situ is a good animal model for new drug research.
Moreover, a key important site of colorectal tumor dissemination is liver and hepatic metastasis usually is a vital reason of patients death. Some papers have reported that human colorectal cell lines which express epidermal growth factor receptor can generate tumor foci in liver (14,15). Our results showed that on the 21st d and 35th d CT-26 tumor tissue successfully developed hepatic metastasis by MSOI and metastatic rates were high than reported before (16-18). CT-26 mouse model of MSOI on the 21st d is adaptive for early liver metastasis because the metastatic nodes were relative small and no systemic metastases. Our team used CT-26 mouse model of MSOI on the 21st d to evaluate the effect of anti-liver metastasis of Pien Tze (11). And on the 35th d except liver metastasis, mouse models also showed ascitic fluid and metastasis nodes on stomach and abdominal wall. That characteristic mouse model can be used to detected advanced metastasis of colorectal tumor. However stomach metastases are rare and less reported. The feasible reason was that stomach adhered with peritoneal metastasis and will be studied further in the future.
In conclusion, the MSOI technique is a useful method to generate early- as well as advanced-stage liver metastasis of CRC with high take rates that can be achieved within a short time interval (one to two months). Therefore, this technique complements and expands the set of animal models currently available to CRC disease and treatment researchers.
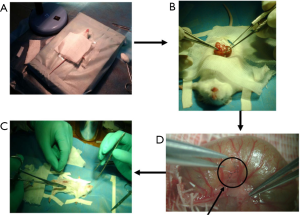
Table S1
| Days | Control group (U/L) | Model group (U/L) |
|---|---|---|
| 21 d | ||
| ALT | 19.35±2.18 | 17.40±0.44* |
| AST | 17.61±3.06 | 19.71±0.71* |
| 35 d | ||
| ALT | 18.21±0.64 | 17.23±1.37* |
| AST | 16.14±3.55 | 25.31±5.82* |
†, 6 mice every group for observed and data represent mean ± SD; *, P>0.05 control
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.03.61). The authors have no conflicts of interest to declare.
Ethical Statement: We are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal treatments were performed strictly in accordance with international ethical guidelines and the National Institutes of Health Guide concerning the Care and Use of Laboratory Animals. The experiments were approved by the Institutional Animal Care and Use Committee of Fujian University of Traditional Chinese Medicine.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics. CA Cancer J Clin 2014;64:104-17. [Crossref] [PubMed]
- Weinberg BA, Marshall JL, Salem ME. The Growing Challenge of Young Adults with Colorectal Cancer. Oncology (Williston Park) 2017;31:381-9. [PubMed]
- Azeem S, Gillani SW, Siddiqui A, et al. Diet and Colorectal Cancer Risk in Asia—a Systematic Review. Asian Pac J Cancer Prev 2015;16:5389-96. [Crossref] [PubMed]
- Rodrigues RV, Pereira da Silva J, Rosa I, et al. Intensive follow-up after curative surgery for colorectal cancer. Acta Med Port 2017;30:633-41. [Crossref] [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013;14:1208-15. [Crossref] [PubMed]
- Huang XD, Zheng YB, Yang YJ, et al. Mouse models for human colorectal cancer with liver metastasis. Zhonghua Yi Xue Za Zhi 2019;99:2701-05. [PubMed]
- Céspedes MV, Espina C, García-Cabezas MA, et al. Orthotopic microinjection of human colon cancer cells in nude mice induces tumor foci in all clinically relevant metastatic sites. Am J Pathol 2007;170:1077-85. [Crossref] [PubMed]
- Kageyama K, O’Hara M, Saito K, et al. Establishment of an orthotopic patient-derived xenograft mouse model using uveal melanoma hepatic metastasis. J Transl Med 2017;15:145-74. [Crossref] [PubMed]
- Hoffman RM. Patient-Derived Orthotopic Xenograft (PDOX) Models of Melanoma. Int J Mol Sci 2017;18:1875-89. [Crossref] [PubMed]
- Suarez CD, Littlepage LE. Patient-Derived Tumor Xenograft Models of Breast Cancer. Methods Mol Biol 2016;1406:211-23. [Crossref] [PubMed]
- Lin W, Zhuang Q, Zheng L, et al. Pien Tze Huang inhibits liver metastasis by targeting TGF-β signaling in an orthotopic model of colorectal cancer. Oncol Rep 2015;33:1922-8. [Crossref] [PubMed]
- Hoffman RM. Patient derived orthotopic xenografts: Better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer 2015;15:451-52. [Crossref] [PubMed]
- Puig I, Chicote I, Tenbaum SP, et al. A personalized preclinical model to evaluate the metastatic potential of patient-derived colon cancer initiating cells. Clin Cancer Res 2013;19:6787-801. [Crossref] [PubMed]
- Piawah S, Venook AP. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer 2019;125:4139-47. [Crossref] [PubMed]
- Khan K, Valeri N, Dearman C, et al. Targeting EGFR pathway in metastatic colorectal cancer-tumour heterogeneity and convergent evolution. Crit Rev Oncol Hematol 2019;143:153-163. [Crossref] [PubMed]
- Chen YL, Wei PK, Xu L, et al. Nude mouse model of human gastric carcinoma metastasis constructed by orthotopic transplantation using organism glue paste technique. Ai Zheng 2005;24:246-8. [PubMed]
- He X, Shi W, Wen S, et al. Establishment and evaluation of a novel mouse model of orthotopic colon cancer in the mesenteric triangle of the cecum. Zhonghua Zhong Liu Za Zhi 2015;37:418-21. [PubMed]
- Huang XD, Zheng YB, Yang YJ, et al. Mouse models for human colorectal cancer with liver metastasis. Zhonghua Yi Xue Za Zhi 2019;99:2701-5. [PubMed]


