
Corilagin decreases insulin resistance in polycystic ovary syndrome rat model through regulating AMPK/GSK3β pathway
Introduction
Polycystic ovary syndrome (PCOS) is a common gynecological endocrine disease affecting 9–21% of women of reproductive age, which is characterized by hyperandrogenism and oligo-anovulation accompany with several comorbidities including dyslipidemia, obesity, hypertension, metabolic syndrome (MS), etc. (1). More recently, PCOS has been recognized as an important metabolic aberration, and 50–60% of PCOS patients suffer from insulin resistance (IR) (2). IR characterized by a decreased respond to insulin signaling, plays a major role in the pathogenesis of most metabolic complications of PCOS (3,4). In most patients with PCOS, IR is independent of obesity but aggravated by obesity (5). Therefore, a proper treatment strategy to reduce IR remains a huge challenge for PCOS research.
Adenosine monophosphate-activated protein kinase (AMPK) is a cellular energy sensor and participates in a variety of physiopathological processes including food intake, fatty acid and glucose uptake, insulin secretion, and hepatic gluconeogenesis. Previous studies have shown that transient reduction of cellular ATP can lead to AMPK activation. AMPK activation inhibits phosphorylation of insulin receptor substrate 1 (Ser636/639) and activates the PI3K/Akt signaling pathway. Under physiological conditions, insulin inhibits hepatic glycolysis by promoting a protein kinase-dependent glycogen synthase kinase 3β (GSK3β) phosphorylation. Therefore, AMPK may be an important target for the prevention and treatment of IR and PCOS.
Corilagin, a gallotannin first isolated in 1951 by Schmidt et al. (6), is the major active component of many herbs such as Phyllanthus niruri L. and Phyllanthus urinaria L. Previous studies indicated that Corilagin exhibits anti-tumor, anti-inflammatory and hepato-protective activities, etc. (7). For example, Li et al. found that Corilagin can inhibit the production of pro-inflammatory cytokines and ameliorates the extreme inflammatory status in sepsis through TLR4 signaling pathway (8). In streptozotocin-induced diabetic rats, Corilagin could reduce hyperglycemia, hyperlipidemia and oxidative stress (7). In addition, Corilagin could also interfere AMPK/GSK3β signaling pathway to alleviate acetaminophen-induced hepatotoxicity. However, it is still unclear whether Corilagin can regulate the IR of PCOS through the AMPK pathway. This study aimed to explore the effects of Corilagin on PCOS and the detail mechanisms.
Methods
Animals and treatment
Sprague-Dawley rats (female, SPF grade, age 21 days) were purchased from the Vitalriver (Beijing, China) and housed at a constant temperature of 25 °C with free access to drinking water and food. The rats were randomly divided into two groups: PCOS model and normal control. The PCOS rats were subcutaneously injected with dehydroepiandrosterone (DHEA, dissolved in soybean oil) of 6 mg/kg/day, while the control rats were injected with the same volume of soybean oil. Corilagin (20 or 50 mg/kg/day) were orally administered simultaneously to control or PCOS rats. Body weight and each ovary weight were recorded. All the animal experiments were performed in strict accordance with Guide for the Care and Use of Laboratory Animals (National Research Council) (9) and approved by the Ethics Committee of Obstetrics and Gynecology Hospital, Fudan University (Approval no. 2018-26).
Identification of estrous cycle stage
Stages of the estrous cycle (diestrus, proestrus, estrus and metestrus) were monitored by daily vaginal smears collected between 9:00 a.m. according to a standard protocol (10). Briefly, vaginal secretion was collected with a plastic pipette filled with 10 µL of saline. The glass slides were coated with collected vaginal fluid. Unstained material was observed under a light microscope with objective lenses. Three types of cells were found in microscope field: epithelial cells (round and nucleated), cornified cells (irregular ones without nucleus), leukocytes (little and round). The proportion of each type of cells was used for the determination of the estrous cycle phases.
Biochemical assessments
Serum glucose was analyzed by Hitachi 7020 automatic biochemical analyzer (Hitachi, Tokyo, Japan) with commercial kit. Fasting serum insulin concentration was assessed with chemiluminescent immunoassay kits according to the manufactory’s suggestion.
Western blot
Protein collection and Western blot procedure were performed as described previously (11). Briefly, proteins were separated by SDS-polyacrylamide gels and transferred onto a PVDF membrane and incubated with the respective antibodies. The primary antibodies were diluted at 1:1,000, which include anti-AMPKα (#ab32047), anti-phospho-AMPKα Thr172 & Thr 183 (#ab23875), anti-phospho-GSK3β (#ab93926), anti-phospho-GSK3β Ser9 (#ab75814), anti-β-actin (#ab8226) antibodies and all the antibodies were purchased from Abcam (Cambridge, MA, USA). The secondary antibodies were diluted at 1:10000, including goat anti-rabbit (#7074) and goat anti-mouse (#7076) secondary antibodies purchased from Cell Signaling Technology (Beverly, MA, USA).
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Statistical analyses were evaluated using the unpaired two-tailed t-test between two groups and one-way ANOVA among more than two groups by SPSS. Differences were considered significant at P<0.05.
Results
DHEA induced ovarian dysfunction in rats
To confirm the successful establishment of DHEA-induced PCOS model, mean body weight, ovary weight and estrus cycle were monitored. The body weight of PCOS rats was significantly increased compared with control group (P<0.0001) (Figure 1A). The tendency of ovaries quotiety was similar with body weight (Figure 1B). In addition, the estrus cycle of control group was regular (with 100% recovery rate), while, estrous cycle of the DHEA model group was disordered (with only 16.67% recovery rate) (Table 1), which indicated that the DHEA treated rats was often in the diestrus. These results indicated that DHEA induced the ovarian dysfunction in rats.
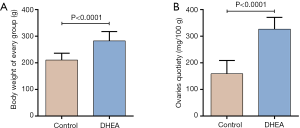
Table 1
| Group | Total number | Recovery number | Recovery rate |
|---|---|---|---|
| Control | 12 | 12 | 100% |
| DHEA | 12 | 2 | 16.67% |
Corilagin alleviated DHEA-induced ovarian dysfunction of rats
The effects of Corilagin on DHEA-induced ovarian dysfunction were explored and the results were shown in Figure 2 and Table 2. Corilagin alone (20 or 50 mg/kg/day) didn’t change the body weight and ovarian function of normal rats (control group). While in DHEA treated rats, Corilagin significantly inhibited the increase in body weight (Figure 2A) and ovaries quotiety (Figure 2B) in a dose-dependent manner. Furthermore, the recovery rate of estrous cycle of DHEA treated rats were also increased after Corilagin treatment, 41.67% for 20 mg/kg/day and 66.67% for 50 mg/kg/day respectively (Table 2). To sum up, Corilagin could alleviate DHEA-induced ovarian dysfunction.
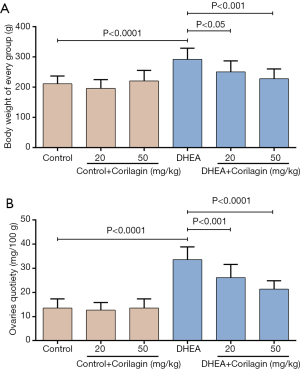
Table 2
| Group | Total number | Recovery number | Recovery rate |
|---|---|---|---|
| Control | 12 | 12 | 100% |
| Control + Corilagin (20 mg/kg) | 12 | 12 | 100% |
| Control + Corilagin (50 mg/kg) | 12 | 12 | 100% |
| DHEA | 12 | 1 | 8.33% |
| DHEA + Corilagin (20 mg/kg) | 12 | 5 | 41.67% |
| DHEA + Corilagin (50 mg/kg) | 12 | 8 | 66.67% |
DHEA, dehydroepiandrosterone.
Corilagin reduced DHEA-induced islet injury of rats
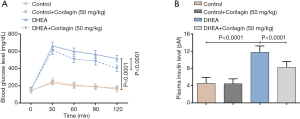
To investigate the changes of islet injury after Corilagin treatment, the levels of blood glucose and plasma insulin were monitored. DHEA induced the increase in blood glucose and the glucose concentration peaked at 30 minutes after DHEA treatment (P<0.0001) (Figure 3A). Corilagin could significantly inhibited the glucose increase induced by DHEA (P<0.0001), while Corilagin alone could not decrease the blood glucose in normal rats. The plasma insulin increased about 3-fold after DHEA treatment compared with control group (P<0.0001), Corilagin could significantly inhibited the DHEA-induced insulin increase (Figure 3B) (P<0.0001).
Corilagin regulated AMPK/GSK3β signaling pathway of rat ovarian
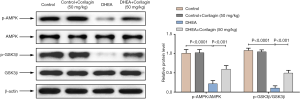
As AMPK/GSK3β plays an important role in the IR and the PCOS, we investigate the regulatory effects of Corilagin on AMPK/GSK3β signaling pathway. As shown in Figure 4, the expression of p-AMPK and p-GSK3β and the ratio of p-AMPK to AMPK and p-GSK3β to GSK3β were significantly decreased after DHEA treatment of ovarian tissue (P<0.0001). Corilagin inhibited the decreased expression of p-AMPK and p-GSK3β (P<0.001). These results indicated that Corilagin could inhibit DHEA-induced activation of AMPK/GSK3β signaling pathway.
Discussion
PCOS is a complex reproductive endocrine disease, which is related to many metabolic symptoms including IR. IR is a hallmark and one of the significant aberrations of PCOS (12). Previous study demonstrated that insulin sensitizers (e.g., metformin), could not only reduce IR but also several other symptoms of PCOS (e.g., reproductive dysfunctions). Tao et al. found the protective effects of AMPKα and SIRT1 on IR of PCOS rats, and both metformin and exenatide can improve the reproductive and endocrine functions of PCOS rats through AMPKα-SIRT1 pathway (13). Our study showed that rats with PCOS lost regular estrous cycle, which suggested the ovulatory disorders in PCOS. In addition, the body weight, serum insulin and glucose in PCOS group were significantly higher than normal rats, suggesting the IR status and the obesity of PCOS rats. These results were in accordance with previous reports.
Corilagin, a gallotannin, has been identified in 53 plants and was reported to have many pharmacological effects for various diseases, including type II diabetes, cardiovascular diseases, PCOS, etc. (7). For example, researchers demonstrated that Corilagin has a potential to regulate diabetes through regulating hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats (14). Corilagin also exerts anti-inflammatory effects in vivo and in vitro. In a rat model of acute cholestasis, Corilagin intervention notably decreased the levels of myeloperoxidase, malondialdehyde and translocation of NF-κB, which indicated that Corilagin could relieve cholestasis through inflammation-related and oxidation-related pathway (15). In this study, the protective effect of Corilagin on PCOS was discovered for the first time, and the molecular mechanism was related to the regulation of IR.
AMPK is a key energy sensor for cellular metabolism and is activated when the cellular AMP/ATP ratio is high, including neurodegeneration, inflammation, and oxidative stress (16). AMPK could regulate glucose uptake and insulin receptor substrate-1 phosphorylation through PI3K/Akt signaling pathway. Also, AMPK activation leads to the phosphorylation of GSK3β, which participates in the process of insulin inhibited glycogenolysis (17,18). Li et al. found that hepatic activation of AMPK could attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice (19). Previous reports indicated that AMPK/GSK3β was important in the IR, which was confirmed in our results. In DHEA-induced PCOS model, the expression of p-AMPK and p-GSK3β was significantly decreased in ovarian tissue. In addition, Corilagin could inhibit the decreased expression of p-AMPK and p-GSK3β, as well as the increase of blood glucose and insulin of PCOS rats. Moreover, previous research has demonstrated that Corilagin could affect on AMPK/GSK3β signaling pathway to attenuate oxidative stress. In acetaminophen overdose-induced acute liver failure model, Corilagin efficiently decreased acetaminophen-induced production of reactive oxygen species (ROS) and several antioxidant enzymes production and cell death of HepG2 cells via the up-regulation of the AMPK/GSK3β-Nrf2 signaling pathway (20). Therefore, combining previous research and our findings, we further speculate that, as the role of GSK3β in the oxidative stress, the AMPK/GSK-3β-Nrf2 (an important transcription factor to protect against oxidative stress) pathway may be a underlying mechanism of the effects of Corilagin on IR. On the other hand, Corilagin induced an increase in the phosphorylation of AMPK and GSK3β, may be even activation of acetyl CoA carboxylase, which can reduce lipid accumulation.
In summary, the present research reported the protective effects of Corilagin on PCOS, and demonstrated that this effect was related to reduced IR. Corilagin could inhibit IR in DHEA-induced PCOS through regulating AMPK/GSK3β pathway. These findings suggested that Corilagin could be a promising candidate for PCOS treatment, and AMPK/GSK3β signaling pathway could be an important target for attenuating IR and PCOS.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2020.04.27). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the animal experiments were performed in strict accordance with Guide for the Care and Use of Laboratory Animals (National Research Council) and approved by the Ethics Committee of Obstetrics and Gynecology Hospital, Fudan University (Approval no. 2018-26).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tauchert S, Ludwig AK, Diedrich K, et al. Treatment strategies in PCOS patients. Reprod Biomed Online 2005;10:67-74. [Crossref] [PubMed]
- Azziz R. Polycystic ovary syndrome, insulin resistance, and molecular defects of insulin signaling. J Clin Endocrinol Metab 2002;87:4085-7. [Crossref] [PubMed]
- Zhong WY, Peng H, Li H, et al. Effect of Thiazolidinedione Amide on Insulin Resistance, Creactive Protein and Endothelial Function in Young Women with Polycystic Ovary Syndrome. Trop J Pharm Res 2015;14:2287-92. [Crossref]
- Rojas J, Chávez M, Olivar L, et al. Polycystic ovary syndrome, insulin resistance, and obesity: navigating the pathophysiologic labyrinth. Int J Reprod Med 2014;2014:719050.
- Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012;33:981-1030. [Crossref] [PubMed]
- Schmidt OT, Lademann R. Corilagin, ein weiterer kristallisierter Gerbstoff aus Dividivi. X. Mitteilung über natürliche Gerbstoffe. Justus Liebigs Ann Chem 1951;571:232-7. [Crossref]
- Li X, Deng Y, Zheng Z, et al. Corilagin, a promising medicinal herbal agent. Biomed Pharmacother 2018;99:43-50. [Crossref] [PubMed]
- Li HR, Liu J, Zhang SL, et al. Corilagin ameliorates the extreme inflammatory status in sepsis through TLR4 signaling pathways. BMC Complement Altern Med 2017;17:18. [Crossref] [PubMed]
- Guide for the Care and Use of Laboratory Animals. Washington, D.C: National Academy Press; 1996.
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 2002;62:609-14. [Crossref] [PubMed]
- Xie M, Zhang G, Yin W, et al. Cognitive enhancing and antioxidant effects of tetrahydroxystilbene glucoside in Aβ1-42-induced neurodegeneration in mice. J Integr Neurosci 2018;17:355-65. [Crossref] [PubMed]
- Belani M, Deo A, Shah P, Banker M, Singal P, Gupta S. Differential insulin and steroidogenic signaling in insulin resistant and non-insulin resistant human luteinized granulosa cells-A study in PCOS patients. J Steroid Biochem Mol Biol 2018;178:283-92. [Crossref] [PubMed]
- Tao X, Cai L, Chen L, et al. Effects of metformin and Exenatide on insulin resistance and AMPKα-SIRT1 molecular pathway in PCOS rats. J Ovarian Res 2019;12:86. [Crossref] [PubMed]
- Nandini HS, Naik PR. Action of corilagin on hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. Chem Biol Interact 2019;299:186-93. [Crossref] [PubMed]
- Jin F, Cheng D, Tao JY, et al. Anti-inflammatory and anti-oxidative effects of corilagin in a rat model of acute cholestasis. BMC Gastroenterol 2013;13:79. [Crossref] [PubMed]
- Carling D, Thornton C, Woods A, et al. AMP-activated protein kinase: new regulation, new roles? Biochem J 2012;445:11-27. [Crossref] [PubMed]
- Choi SH, Kim YW, Kim SG. AMPK-mediated GSK3beta inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress. Biochem Pharmacol 2010;79:1352-62. [Crossref] [PubMed]
- Horike N, Sakoda H, Kushiyama A, et al. AMP-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3beta and thereby reduces cAMP-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver. J Biol Chem 2008;283:33902-10. [Crossref] [PubMed]
- Li Y, Xu S, Mihaylova MM, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 2011;13:376-88. [Crossref] [PubMed]
- Lv H, Hong L, Tian Y, et al. Corilagin alleviates acetaminophen-induced hepatotoxicity via enhancing the AMPK/GSK3β-Nrf2 signaling pathway. Cell Commun Signal 2019;17:2. [Crossref] [PubMed]


